Abstract
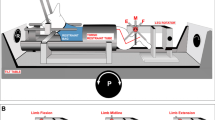
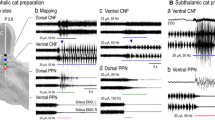
The present study was aimed at elucidating the pontomedullary and spinal cord mechanisms of postural atonia induced by microinjection of carbachol and restored by microinjections of serotonin or atropine sulfate into the nucleus reticularis pontis oralis (NRPo). Medullary reticulospinal neurons (n=132) antidromically activated by stimulating the L1 spinal cord segment were recorded extracellularly. Seventy-eight of them were orthodromically activated with mono- or disynaptic latencies by stimulating the NRPo area at the site where carbachol injections effectively induced postural atonia. Most of these reticulospinal neurons (71 of 78) were located in the nucleus reticularis gigantocellularis (NRGc). Following carbachol injection into the NRPo, discharge rates of the NRGc reticulospinal neurons (29 of 34) increased, while the activity of soleus muscles decreased bilaterally. Serotonin or atropine injections into the same NRPo area resulted in a decrease in the discharge rates of the reticulospinal neurons with a concomitant increase in the levels of hindlimb muscle tone. Membrane potentials of hindlimb extensor and flexor alpha motoneurons (MNs) were hyperpolarized and depolarized by carbachol and serotonin or atropine injections, respectively. In all pairs of reticulospinal neurons and MNs (n=11), there was a high correlation between the increase in the discharge rates and the degree of membrane hyperpolarization of the MNs. Spike-triggered averaging during carbachol-induced atonia revealed that inhibitory postsynaptic potentials (IPSPs) were evoked in 15 MNs by the discharges of nine reticulospinal neurons. Four of them evoked IPSPs in more than one MN. The mean segmental delay and the mean time to the peak of IPSPs were 1.6 ms and 2.0 ms, respectively. Axonal trajectories of reticulospinal neurons (n=6), which evoked IPSPs in MNs, were investigated in the lumbosacral segments (L1-S1) by antidromic threshold mapping. The stem axons descended through the ventral (n=2) and ventrolateral (n=4) funiculi in the lumbar segments. All axons projected their collaterals to the intermediate region (laminae V, VI) and ventromedial part (laminae VII, VIII) of the gray matter. All these results suggest that the reticulospinal pathway originating from the NRGc is involved in postural atonia induced by pontine microinjection of carbachol, and that the pathway is inactivated during the postural restoration induced by subsequent injections of serotonin or atropine. It is further suggested that the pontine inhibitory effect is mediated via segmental inhibitory interneurons projecting to MNs.
Similar content being viewed by others
References
Amatruda T, Black D, McKenna T, McCarley RW, Hobson JA (1974) The effects of carbachol injections at two brain stem sites. Sleep Res 3:38
Berman AL (1968) The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin Press, Madison, WI
Brock LG, Coombs JS, Eccles JC (1953) Intracellular recording from antidromically activated motoneurons in the cat. J Physiol (Lond) 122:429–461
Brodal A (1981) Neurological anatomy, 3rd edn., Oxford University Press, New York, pp 394–447
Chase MH (1980) The motor functions of the reticular formation are multifaceted and state-determined. In: Hobson JA, Brazier MAB (eds) The reticular formation revisited. Raven, New York, pp 449–472
Chase MH, Willis NK (1979) Brainstem control of masseteric reflex activity during sleep and wakefulness: Medulla. Exp Neurol 64:118–131
Chase MH, Morales FR, Boxer P Fung SJ, Soja PJ (1986) Effect of stimulation of the nucleus reticularis gigantocellularis on the membrane potential of cat lumbar motoneurons during sleep and wakefulness. Brain Res 386:237–244
Greene RW, Carpenter DO (1985) Actions of neurotransmitters on pontine medial reticular formation neurons of the cat. J Neurophysiol 54:520–531
Hobson JA, Goldberg M, Vivaldi E, Riew D (1983) Enhancement of desynchronized sleep signs after pontine microinjection of the muscarinic agonist bethanechol. Brain Res 275:127–136
Holstege G, Kuypers HGJM (1982) The anatomy of of brain stem pathways to the spinal cord in cat: a labeled amino acid tracing study. Prog Brain Res 57:145–175
Hoshino K, Pompeiano O (1976) Selective discharge of pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cats. Arch Ital Biol 114:244–277
Houdouin F, Cespuglio R, Jouvet M (1991) Effects induced by the electrical stimulation of the nucleus raphe dorsalis upon hypothalamic release of 5-hydroxyindole compounds and sleep parameters in the rat. Brain Res 565:48–56
Ito K, McCarley RW (1987) Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res 409:97–110
Iwamoto Y, Sasaki S (1990) Monosynaptic excitatory connexions of reticulospinal neurons in the nucleus reticularis pontis caudalis with dorsal neck motoneurons in the cat. Exp Brain Res 80:277–289
Jankowska E, Roberts WJ (1972) Synaptic actions of single interneurons mediating reciprocal Ia inhibition of motoneurons. J Physiol (Lond) 222:623–642
Katayama Y, Dewitt DS, Becker DP, Hayers RL (1984) Behavioral evidence from a cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res 296:241–262
Lipski J (1981) Antidromic activation of neurons as an analytical tool in the study of the central nervous system. J Neurosci Methods 4:1–32
Matsuyama K, Ohta Y, Mori S (1988) Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 460:124–141
Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, Kimura H (1993) Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res 17:9–21
Mendell LM, Henneman E (1971) Terminals of single Ia fibers; location, density and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol 34:171–187
Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH (1987) Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol 57:1118–1129
Mori S, Nishimura H, Aoki M (1980) Brain stem activation of spinal stepping generator. In: Hobson JA, Brazier MAB (eds) The reticular formation revisited. Raven, New York, pp 241–249
Mori S, Kawahara K, Sakamoto T, Aoki M, Tomiyama T (1982) Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of brain stem. J Neurophysiol 48:737–748
Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K (1989) Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res 505:66–74
Ohta Y, Kimura H, Mori S (1988) Neuronal structures of the brainstem participating in postural suppression in cats. Neurosci Res 5:181–202
Pompeiano O (1980) Cholinergic activation of reticular and vestibular mechanisms controlling posture and eye movements. In: Hobson JA, Brazier MAB (eds) The reticular formation revisited. Raven, New York, pp 473–512
Rhines R, Magoun HW (1946) Brain stem facilitation of cortical motor response. J Neurophysiol 9:219–229
Shiromani P, McGinty DJ (1986) Pontine neuronal response to local cholinergic infusion: relation to REM sleep. Brain Res 386:20–31
Snider RS, Niemer WT (1961) A stereotaxic atlas of the cat brain. University of Chicago Press, Chicago
Takakusaki K (1991) Cholinergic and serotonergic control of reticulo-spinal actions of motor activity in decerebrate cats. Jpn J Physiol [Suppl] 41:S9
Takakusaki K, Ohta Y, Mori S (1987) Neuronal mechanisms involved in postural suppression in decerebrate, reflex standing cats. Neurosci Res [Suppl] 5:S132
Takakusaki K, Ohta Y, Mori S (1989) Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res 74:11–23
Takakusaki K, Kohyama J, Matsuyama K, Mori S (1993a) Synaptic mechanisms acting on lumbar motoneurons during postural augmentation induced by serotonin injection into the rostral pontine reticular formation in decerebrate cats. Exp Brain Res 93:471–482
Takakusaki K, Matsuyama K, Kohyama J, Kobayashi Y, Mori S (1993b) Pontine microinjection of carbachol and critical zone for inducing postural atonia in reflexively standing decerebrate cats. Neurosci Lett 153:185–188
Vanni-Mercier G, Sakai K, Lin JS, Jouvet M (1989) Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol 127:133–164
Watt DGD, Stauffer EK, Taylor A, Reinking RM, Stuart DG (1976) Analysis of muscle receptor connections by spike-triggered averaging. I. Spindle primary receptors and tendon organ afferents. J Neurophysiol 39:1375–1392
Willis NK, Chase MH (1979) Brain stem control of masseteric reflex activity during sleep and wakefulness: mesencephalon and pons. Exp Neurol 64:98–117
Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA (1990a) A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: locus of the sensitive region. Neuroscience 39:279–293
Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA (1990b) A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience 39:295–304
Yamuy J, Mancillas JR, Tran RT, Morales FR, Chase MH (1991a) C-fos-like expression in the lumbar spinal cord of the cat during carbachol-induced atonia (abstract). Sleep Res 20A:76
Yamuy J, Mancillas JR, Morales FR, Chase MH (1991b) C-fos-like expression in the brainstem of the cat during carbachol-induced atonia. Soc Neurosci Abstr 17:468
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takakusaki, K., Shimoda, N., Matsuyama, K. et al. Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res 99, 361–374 (1994). https://doi.org/10.1007/BF00228973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228973