Abstract
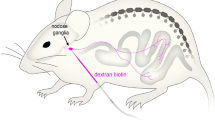
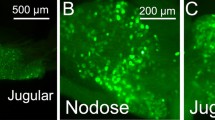
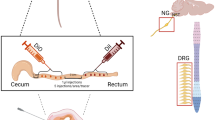
Results from functional studies point to the importance of chemoreceptive endings in the duodenum innervated by vagal afferents in the regulation of gastrointestinal functions such as gastric emptying and acid secretion, as well as in the process of satiation. In order to visualize the vagal sensory innervation of this gut segment, vagal afferents were selectively labeled in vivo by injecting the lipophilic carbocyanine dye DiI into either the left or the right nodose ganglion of young adult rats. Thick cryostat sections or whole-mounted peels of muscularis externa or submucosa of formalinfixed tissue were analyzed with conventional and/or confocal microscopy. In the mucosa, many DiI-labeled vagal afferent fibers were found with terminal arborizations mainly between the crypts and the villous lamina propria. In both areas, vagal terminal branches came in close contact with the basal lamina, but did not appear to penetrate it so as to make direct contact with epithelial cells. Labeled vagal afferent fibers in the villous and cryptic lamina propria were found to be in intimate anatomical contact with fibrocyte-like cells that may belong to the class of interstitial cells of Cajal, and with small granular cells that might be granulocytes or histiocytes. Although our analysis was not quantitative, and considering that labeling was unilateral and not complete, it appears that the overall density of vagal afferent mucosal innervation was variable; many villi showed no evidence for innervation while other areas had quite dense networks of arborizing terminal fibers in several neighboring villi. Analysis of separate whole-mounted muscularis externa and submucosa peels revealed the presence of large bundles of labeled afferent fibers running within the myenteric plexus along the mesenteric attachment primarily in an aboral direction, with individual fibers turning towards the antimesenteric pole, and either penetrating into the submucosa or forming the characteristic intraganglionic laminar endings (IGLEs). Although the possibility of individual fibers issuing collaterals to myenteric IGLEs and at the same time to mucosal terminals was not demonstrated, it cannot be ruled out. These anatomical findings are discussed in the context of absorptive mechanisms for the different macronutrients and the implication of enteroendocrine cells such as CCK-containing cells that may function as intestinal “taste cells”.
Similar content being viewed by others
References
Anderson CR, Edwards SL (1993) Subunit b of cholera toxin labels interstitial cells of Cajal in the gut of rat and mouse. Histochemistry 100:457–464
Baluk P, Gabella G (1987) Scanning electron microscopy of the muscle coat of the guinea-pig small intestine. Cell Tissue Res 250:551
Berthoud H-R, Neuhuber WL (1994) Distribution and morphology of vagal afferents and efferents supplying the digestive system. In: Taché Y, Wingate DL (eds) Brain-gut interactions. CRC Press, Boca Raton, Ann Arbor, Boston, pp 43–65
Berthoud H-R, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319:261–276
Berthoud H-R, Kressel M, Neuhuber W (1992) An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol 186:431–442
Blackshaw LA, Grundy D (1990) Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal afferent fiber. J Auton Nerv Syst 31:191–202
Carey MC, Small DM, Bliss CM (1983) Lipid digestion and absorption. Annu Rev Physiol 45:651–677
Clarke GD, Davison JS (1978) Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol (Lond) 284:55
Clerc N, Condamin M (1987) Selective labeling of vagal sensory nerve fibers in the lower esophageal sphincter with anterogradely transported WGA-HRP. Brain Res 424:216
Davison JS, Clarke GD (1988) Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol 255: G55-G61
El Ouazzani T, Mei N (1981) Acidoét glucorecepteurs vagaux de la région gastro-duodénale. Exp Brain Res 42:442
Ewart WR, Wingate DL (1984) Central representation of arrival of nutrient in the duodenum. Am J Physiol 246: G750-G756
Fujita T, Kobayashi S (1971) Experimentally induced granule release in the endocrine cells of dog pyloric antrum. Z Zellforsch Mikrosk Anat 116:52–60
Fujita T, Kobayashi S (1978) Paraneuronal cells in the GEP endocrine system. In: Bloom SR (ed) Gut hormones. Churchill Livingstone, Edinburgh, pp 414–422
Greenberg D, Smith GP, Gibbs J (1990) Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am J Physiol 259: R110-R118
Grundy D, Scratcherd T (1989) Sensory afferents in the gastrointestinal tract. In: Handbook of physiology, sect 6. The gastrointestinal system, vol 1, part 2. Motility and circulation. American Physiological Society, Bethesda, Md, pp 593–620
Hardcastle J, Hardcastle PT, Sanford PA (1978) Effect of actively transported hexoses on afferent nerve discharge from rat small intestine. J Physiol (Lond) 285:71–84
Hölzer HH, Turkelson CT, Solomon T, Raybould HE (1994) Intestinal liquid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol 267:G625-G629
Iwai K, Fukuoka SI, Fushiki T, Tsujikawa M, Hirose M, Tsunasawa S, Sakiyama F (1987) Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. J Biol Chem 262:8956–8959
Jeanningros R (1982) Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiol Behav 28:9
Jersild RA (1966) A time sequence study of fat absorption in the rat jejunum. Am J Anat 118:135–162
Kobayashi S (1990) The structure of the autonomie end apparatus in the guinea-pig small intestine and the problem of the interstitial cells of Cajal. Acta Med Biol 38:103–127
Kressel M, Berthoud H-R, Neuhuber WL (1994) Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res 275:109–123
Krstic RV (1991) Human microscopic anatomy. Springer, Berlin Heidelberg New York
Lewis LD, Williams JA (1989) Regulation of cholecystokinin (CCK) secretion (abstract). FASEB J 3:997
Lloyd KCK, Hölzer HH, Zittel TT, Raybould HE (1993) Duodenal lipid inhibits gastric acid secretion by vagal capsaicin-sensitive pathway in rats. Am J Physiol 264: G659-G663
Lundberg JM, Dahlström A, Bylock A, Ahlman H, Pettersson G, Larsson I, Hansson H-A, Kewenter J (1978) Ultrastructural evidence for an innervation of epithelial enterochromaffine cells in the guinea pig duodenum. Acta Physiol Scand 104:3–12
Mei N (1978) Vagal glucoreceptors in the small intestine of the cat. J Physiol (Lond) 282:485
Mei N (1985) Intestinal chemosensitivity. Physiol Rev 65:221
Mélone J (1986) Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst 17:231
Meyer JH (1987) Motility of the stomach and gastroduodenal junction. In: Johnson LR et al (eds) Physiology of the gastrointestinal tract, vol 1, 2nd edn. Raven Press, New York, pp 613–629
Moran TH, Norgren R, Crosby RJ, McHugh PR (1990) Central and peripheral vagal transport of cholecystokinin-binding sites occurs in afferent fibers. Brain Res 526:95–102
Neuhuber WL (1987) Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst 20:243–255
Neuhuber WL, Clerc N (1990) Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL (eds) The primary afferent neuron: a survey of recent morpho-functional aspects. Plenum Press, New York, pp 93–107
Powley TL, Berthoud HR (1991) A fluorescent labeling strategy for staining the enteric nervous system. J Neurosci Methods 36:9–15
Raybould HE, Hölzer HH (1992) Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by carbohydrate. Neurosci Lett 141:236–238
Raybould HE, Hölzer HH (1993) Duodenal acid-induced inhibition of gastric motility and emptying in rats. Am J Physiol 265:G540-G546
Ritter RC, Simon E (1989) Suppression of feeding by intraintestinal maltose is mediated by phloridzin-sensitive mechanism. Soc Neurosci Abstr 16:646
Ritter RC, Brenner L, Yox DP (1992) Participation of vagal sensory neurons in putative satiety signals from upper gastrointestinal tract. In: Ritter S, Ritter RC, Barnes CD (eds) Neuroanatomy and physiology of abdominal vagal afferents. CRC Press, Boca Raton, Ann Arbor, Boston, pp 221–248
Rumessen JJ, Thuneberg L (1982) Plexus muscularis profundus and associated interstitial cells. I. Light microscopical studies of mouse small intestine. Anat Rec 203:115
Sato M, Koyano H (1987) Autoradiographic study on the distri- bution of vagal afferent nerve fibers in the gastroduodenal wall of the rabbit. Brain Res 400:101–109
Schwartz GJ, McHugh PR, Moran TH (1992) Vagal afferent responses to gastric loads and cholecystokinin in the rat. Am J Physiol:262
Sharara AI, Bouras EP, Misukonis MA, Liddle RA (1993) Evidence for indirect dietary regulation of cholecystokinin release in rats. Am J Physiol 265:G107-G112
Smith GP, Gibbs J (1992) The development and proof of the CCK hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD, Iversen LL (eds) Multiple cholecystokinin receptors in the CNS. Oxford University Press, Oxford, pp 166–182
Stach W (1973) Über die Nervengeflechte der Duodenalzotten. Licht- und elektronenmikroskopische Untersuchungen. Acta Anat (Basel) 85:216–231
Stach W (1976) Afferente Nervenendigungen im Dünndarm und Magen. Licht-und elektronenmikroskopische Untersuchrngen. Z Mikrosk Anat Forsch 90:790–800
Tamura C, Ritter RC (1991) Transient, selective attenuation of oleate and CCK-induced suppression of sham feeding by intra-intestinal capsaicin infusion. Soc Neurosci Abstr 17:542
Yox DP, Ritter RC (1988) Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol 255:R569-R574
Yox DP, Brenner L, Ritter RC (1992) CCK receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol 262:R554-R561
Zittel TT, Rothenhöfer I, Meyer JH, Raybould HE (1994) Capsaicin-sensitive afferents innervating the small intestine mediate feedback inhibition of gastric emptying in rats. Am J Physiol (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berthoud, HR., Kressel, M., Raybould, H.E. et al. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol 191, 203–212 (1995). https://doi.org/10.1007/BF00187819
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187819