Abstract
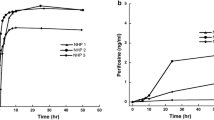
Generally tritiated homoharringtonine ([3H]HHT, 150 μCi, 430 μg) was administered intravenously to seven patients at varying times before surgical resection of malignant brain tumor. Plasma, urine, cerebrospinal fluid (CSF), and tumor specimens were obtained during surgery, and the concentrations of HHT, its major metabolite, and [3H]HHT equivalent were determined chromatographically and radiochemically. For [3H]HHT equivalent, the concentration in tumor ranged from 0.6 to 4.3 ng/g and the ratio of tumor to plasma concentration from 0.5 to 1.8. In one patient who had CSF available for drug determination, the CSF to plasma ratio of total [3H]HHT was 0.3 at 45 minutes after drug administration and less than 0.2 ng/ml was unchanged HHT. For unchanged HHT, drug concentration in tumor ranged from undetectable (4 patients) to 1.8 ng/g. A major metabolite of HHT was detectable in the tumor specimens of all the patients. These results indicate that homoharringtonine can penetrate into brain tumors; in 3 patients with brain tumors, the ratios of HHT concentration in the tumor to that in the concurrent plasma were greater than one.
Similar content being viewed by others
References
Smith CR, Mikolajczak KL, Powell RG: Harringtonine and related cephalotaxine esthers. Anticancer agents based on natural product models. Academic Press Inc Chapter 11 391-416
Ma KE, Lin LT, Chao TY: Studies of the alkaloids of cephalotaxus I. The isolation and characterization of antileukernic alkaloid together with a new alkaloid (+)-acetylcephalotaxinc from cephalotaxus fortunei. Acta Chimica Sinica 35:314, 201–208, 1977
Clinical Brochure. Homoharringtonine (NSC-141633), Bethesda, MD, Investigational Drug Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment, National Cancer Institute, August, 1982
Legha SS, Keating M, Picket S, et al: Phase I clinical investigation of homoharringtonine. Cancer Treat Rep 68:1085–1091, 1984
Whitacre MY, Van Echo DA, Applefeld M, Wiernik PH, Aisner J: Phase I study of homoharringtonine (NSC 141633). Proc Amer Soc Clin Oncol 2:33, 1983
Malomud SC, Ohnuma T, Coffey V, Paincci PA, Wasserman LR, Holland JF: Phase I study of homoharringtonine (HHT) in 10 day schedule: 6 HR infusion daily vs continuous infusion. Proc Amer Assoc Can Res 25:179, 1984
Warrell Jr RP, Connley CJ, Gee TS: Homoharringtonine (HHT): An effective new drug for acute non-lymphoblastic leukemia (ANLL). Proc Amer Soc Clin Oncol 3:198, 1984
Tscherne JS, Pestka S: Inhibition of protein synthesis in intact Hela cells. Antimicrob Agent Chemother 6:479–486, 1975
Savaraj N, Kantarjian H, Lu K, Keating M, Loo TL: In Vitro Prediction of Response to Homoharringtonine Proc Amer Soc Clin Onc 5:51, 1986
Savaraj N, Lu K, Dimery I, Feun LG, Burgess MA, Guo Z, Chawla S, Loo TL: Clinical pharmacology of homoharringtonine (HHT). Proc Amer Soc Clin Oncol 3:38, 1984
Lu K, Savaraj N, Feun LG, Zhangang G, Loo TL: Pharmacokinetics of homoharringtonine (HHT, NSC 141633) in dogs. Proc Amer Assoc Can Res 24:289, 1983
Author information
Authors and Affiliations
Additional information
address for offprints
Rights and permissions
About this article
Cite this article
Savaraj, N., Feun, L.G., Lu, K. et al. Central nervous system (CNS) penetration of homoharringtonine (HHT). J Neuro-Oncol 5, 77–81 (1987). https://doi.org/10.1007/BF00162769
Issue Date:
DOI: https://doi.org/10.1007/BF00162769