Abstract
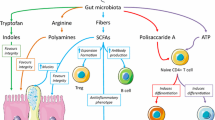
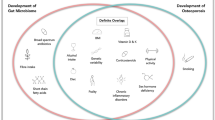
The past decade has seen an explosion of research in the area of how the bacteria that inhabit the human body impact health and disease. One of the more surprising concepts to emerge from this work is the ability of the intestinal microbiota to impact virtually all systems in the body. Recently, the role of gut bacteria in bone health and disease has received more significant attention. In this chapter, we review what has been learned about how the gut microbiome impacts bone health and discuss possible mechanisms of how the gut-bone axis may be connected. We also discuss the use of therapeutic microbes in the modulation of bone health. Finally, we propose an emerging field of the gut-brain-bone axis, in which the gut drives bone physiology via regulation of key hormones that are originally synthesized in the brain.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Riggs BL, Melton Iii LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–54.
Martin GHKQR. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–50.
Pappalardo A, Thompson K. Activated γδ T cells inhibit osteoclast differentiation and resorptive activity in vitro. Clin Exp Immunol. 2013;174(2):281–91.
Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29(5):555–67.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–64.
Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor- mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271(46):28890–7.
Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005;350(1):1–13.
Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104(4):503–13.
Weitzmann MN. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica (Cairo). 2013;2013:125705.
Most W, Van der Wee-Pals L, Ederveen A, Papapoulos S, Löwik C. Ovariectomy and orchidectomy induce a transient increase in the osteoclastogenic potential of bone marrow cells in the mouse. Bone. 1997;20(1):27–30.
Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens: the role of the androgen receptor. J Clin Invest. 1995;95(6):2886–95.
Karieb S, Fox SW. Suppression of T cell-induced osteoclast formation. Biochem Biophys Res Commun. Elsevier Inc. 2013;436(4):619–24.
Salamanna F, Maglio M, Borsari V, Giavaresi G, Aldini NN, Fini M. Peripheral blood mononuclear cells spontaneous osteoclastogenesis: mechanisms driving the process and clinical relevance in skeletal disease. J Cell Physiol. 2016;231(3):521–30.
D’Amelio P, Sassi F. Osteoimmunology: from mice to humans. Bonekey Rep. 2016;5:802.
Helander HF, Fändriks L. Surface area of the digestive tract – revisited. Scand J Gastroenterol. 2014;49(6):681–9.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. Nature Publishing Group. 2013;19:576–85.
Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410.
Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. Elsevier Ltd. 2015;29(4):621–31.
Sharan K, Yadav VK. Hypothalamic control of bone metabolism. Best Pract Res Clin Endocrinol Metab. Elsevier Ltd. 2014;28(5):713–23.
Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7(1):1–19.
Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, Mccabe LD, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem (ACS Publ). 2011;59:6501–10.
Whisner CM, Martin BR, Nakatsu CH, Story JA, MacDonald-Clarke CJ, McCabe LD, et al. Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: a randomized dose-response trial in free-living pubertal females. J Nutr. 2016;146(7):1298–306.
Zafar TA, Weaver CM, Zhao YD, Martin BR, Wastney ME. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr. 2004;134(2):399–402.
McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13(6):363–71.
Morelli L, Capurso L. FAO/WHO guidelines on probiotics. J Clin Gastroenterol. 2012;46:S1–2.
Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J-I, Mizumachi K. Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr. 2007;98(6):1178–86.
Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, et al. Effects of long-term intervention with lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab. 2004;48(4):228–34.
Narva M, Rissanen J, Halleen J, Vapaatalo H, Väänänen K, Korpela R. Effects of bioactive peptide, Valyl-Prolyl-Proline (VPP), and Lactobacillus helveticus fermented milk containing VPP on bone loss in ovariectomized rats. Ann Nutr Metab. 2007;51(1):65–74.
Narva M, Nevala R, Poussa T, Korpela R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr. 2004;43(2):61–8.
McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228(8):1793–8.
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. Wiley-Liss Inc. 2014;229(11):1822–30.
Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, et al. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11(4):e0153180.
Jones SE, Whitehead K, Saulnier D, Thomas CM, Versalovic J, Britton RA. Cyclopropane fatty acid synthase mutants of probiotic human-derived lactobacillus reuteri are defective in TNF inhibition. Gut Microbes. 2011;2(2):69–79.
Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic lactobacillus reuteri suppresses tnf via modulation of pka and erk signaling. PLoS One. 2012;7(2):e31951.
Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26(2):69–74.
Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252(1–2):68–80.
Pacifici R. Role of T cells in ovariectomy induced bone loss-revisited. J Bone Miner Res. 2012;27(2):231–9.
Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300.
Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe [Internet]. 2014;15(3):374–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24629343
Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67.
Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6.
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21.
Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, et al. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19(6):637–44.
Schwarzer M, Makki K, Storelli G, Machuca-gayet I, Hudcovic T, Heddi A, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–7.
Li J-Y, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–63.
Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS One. 2015;10(2):1–19.
Weitzmann MN, Pacifici R. Estrogen regulation of immune cell bone interactions. Ann N Y Acad Sci. 2006;1068(1):256–74.
Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford). 2002;41(11):1232–9.
Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol. Elsevier Ireland Ltd. 2017;3:68–78.
Adachi T, Kakuta S, Aihara Y, Kamiya T, Watanabe Y, Osakabe N, et al. Visualization of probiotic-mediated Ca(2+) signaling in intestinal epithelial cells in vivo. Front Immunol. 2016;7:601.
Zofková I, Matucha P. New insights into the physiology of bone regulation: the role of neurohormones. Physiol Res. 2014;63(4):421–7.
Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci. 2013;70(1):55–69.
Hyland NP, Cryan JF. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016;417(2):182–7.
Das UN. Obesity: genes, brain, gut, and environment. Nutrition. Elsevier Ltd. 2010;26(5):459–73.
Cluny NL, Reimer RA, Sharkey KA. Cannabinoid signalling regulates inflammation and energy balance: the importance of the brain-gut axis. Brain Behav Immun. Elsevier Inc. 2012;26(5):691–8.
Han C, Rice MW, Cai D. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. Am J Physiol Endocrinol Metab. 2016;311(1):E32–41.
Mooney-Leber SM, Brummelte S. Neonatal pain and reduced maternal care: early-life stressors interacting to impact brain and behavioral development. Neurosci IBRO. 2017;342:21–36.
Cavadas C, Aveleira CA, Souza GFP, Velloso LA. The pathophysiology of defective proteostasis in the hypothalamus – from obesity to ageing. Nat Rev Endocrinol. Nature Publishing Group. 2016;12(12):723–33.
Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One. 2009;4(12):e8415.
Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, et al. Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res. 2006;21(10):1600–7.
Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92(1–3):73–8.
Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25(2):423–39.
Quiros-Gonzalez I, Yadav VK. Central genes, pathways and modules that regulate bone mass. Arch Biochem Biophys. Elsevier Inc. 2014;561:130–9.
Schéle E, Grahnemo L, Anesten F, Halleń A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154(10):3643–51.
Sun H, Nie X, Wang N, Cang Z, Zhu C, Zhao L, et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes Facts. 2016;9:365–78.
Federico A, Dallio M, Tolone S, Gravina AG, Patrone V, Romano M, et al. Gastrointestinal hormones, intestinal microbiota and metabolic homeostasis in obese patients: effect of bariatric surgery. In Vivo. 2016;30(3):321–30.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–5.
Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8(10):e78898.
Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9(1):e84877.
Ferrini M, Wang C, Swerdloff RS, Sinha Hikim AP, Rajfer J, Gonzalez-Cadavid NF. Aging-related increased expression of inducible nitric oxide synthase and cytotoxicity markers in rat hypothalamic regions associated with male reproductive function. Neuroendocrinology. 2001;74(1):1–11.
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. Elsevier Inc. 2016;165(7):1762–75.
Beranger GE, Pisani DF, Castel J, Djedaini M, Battaglia S, Amiaud J, et al. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology. 2014;155(4):1340–52.
Breuil V, Panaia-Ferrari P, Fontas E, Roux C, Kolta S, Eastell R, et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. J Clin Endocrinol Metab. 2014;99(4):634–41.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Quach, D., Britton, R.A. (2017). Gut Microbiota and Bone Health. In: McCabe, L., Parameswaran, N. (eds) Understanding the Gut-Bone Signaling Axis. Advances in Experimental Medicine and Biology, vol 1033. Springer, Cham. https://doi.org/10.1007/978-3-319-66653-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-66653-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66651-8
Online ISBN: 978-3-319-66653-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)