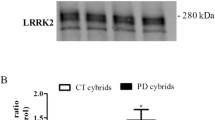
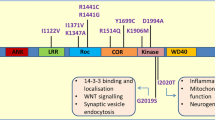
Abstract
Parkinson’s disease (PD) is a progressively debilitating neurodegenerative syndrome. It is best described as a movement disorder characterized by motor dysfunctions, progressive degeneration of dopaminergic neurons of the substantia nigra pars compacta, and abnormal intraneuronal protein aggregates, named Lewy bodies and Lewy neurites. Nevertheless, knowledge of the molecular events leading to this pathophysiology is incomplete. To date, only mutations in the α-synuclein and LRRK2-encoding genes have been associated with typical findings of clinical and pathologic PD. LRRK2 appears to have a central role in the pathogenesis of PD as it is associated with α-synuclein pathology and other proteins implicated in neurodegeneration. Thus, LRRK2 dysfunction may influence the accumulation of α-synuclein and its pathology through diverse pathomechanisms altering cellular functions and signaling pathways, including immune system, autophagy, vesicle trafficking, and retromer complex modulation. Consequently, development of novel LRRK2 inhibitors can be justified to treat the neurodegeneration associated with abnormal α-synuclein accumulation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lang AE, Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med 339(15):1044–1053
Lang AE, Lozano AM (1998) Parkinson’s disease. Second of two parts. N Engl J Med 339(16):1130–1143
Spillantini MG et al (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
Gasser T (2009) Mendelian forms of Parkinson’s disease. Biochim Biophys Acta 1792(7):587–596
Hardy J et al (2006) Genetics of Parkinson’s disease and parkinsonism. Ann Neurol 60(4):389–398
Polymeropoulos MH (1997) Mutation in the -synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047
Kruger R et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108
Zarranz JJ et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173
Chartier-Harlin MC et al (2004) alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364(9440):1167–1169
Singleton AB et al (2003) α-Synuclein locus triplication causes Parkinson’s disease. Science 302(5646):841
Ross OA et al (2008) Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol 63(6):743–750
Paisan-Ruiz C et al (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44(4):595–600
Zimprich A et al (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44(4):601–607
Healy DG et al (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 7(7):583–590
International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M et al (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377(9766):641–649
Santpere G, Ferrer I (2009) LRRK2 and neurodegeneration. Acta Neuropathol 117(3):227–246
Wider C et al (2010) Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener Dis 7(1–3):175–179
Ruffmann C et al (2012) Atypical tauopathy in a patient with LRRK2-G2019S mutation and tremor-dominant Parkinsonism. Neuropathol Appl Neurobiol 38(4):382–386
Ujiie S et al (2012) LRRK2 I2020T mutation is associated with tau pathology. Parkinsonism Relat Disord 18(7):819–823
Khan NL et al (2005) Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain 128(Pt 12):2786–2796
Wszolek ZK et al (2004) Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62(9):1619–1622
Marti-Masso JF et al (2009) Neuropathology of Parkinson’s disease with the R1441G mutation in LRRK2. Mov Disord 24(13):1998–2001
Puschmann A et al (2012) First neuropathological description of a patient with Parkinson’s disease and LRRK2 p.N1437H mutation. Parkinsonism Relat Disord 18(4):332–338
Iwai A et al (1995) The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14(2):467–475
Jakes R et al (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345(1):27–32
Withers GS et al (1997) Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Dev Brain Res 99(1):87–94
Lee SJ et al (2008) alpha-Synuclein is localized in a subpopulation of rat brain synaptic vesicles. Acta Neurobiol Exp 68(4):509–515
Cabin DE et al (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci 22(20):8797–8807
Abeliovich A et al (2000) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25(1):239–252
Yavich L et al (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24(49):11165–11170
Scott DA et al (2010) A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci 30(24):8083–8095
Nemani VM et al (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79
Lee HJ et al (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40(9):1835–1849
Lee HJ et al (2008) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 372(3):423–428
Conway KA et al (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4(11):1318–1320
Tsigelny IF et al (2008) Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS One 3(9), e3135
Oueslati A et al (2010) Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein. Prog Brain Res 183:115–145
Taschenberger G et al (2012) Aggregation of alphaSynuclein promotes progressive in vivo neurotoxicity in adult rat dopaminergic neurons. Acta Neuropathol 123(5):671–683
Galvin JE et al (2001) Synucleinopathies. Arch Neurol 58(2):186
Conway KA et al (2000) Accelerated oligomerization by Parkinson’s disease linked α-synuclein mutants. Ann N Y Acad Sci 920(1):42–45
Lashuel HA et al (2013) The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48
Braak H et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Kordower JH et al (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14(5):504–506
Li JY et al (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14(5):501–503
Desplats P et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A 106(31):13010–13015
Lee HJ et al (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25(25):6016–6024
Hansen C et al (2011) alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121(2):715–725
Greggio E et al (2008) The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem 283(24):16906–16914
Stafa K et al (2012) GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet 8(2), e1002526
Sheng Z et al (2012) Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med 4(164):164ra161
Greggio E et al (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23(2):329–341
Smith WW et al (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9(10):1231–1233
Smith WW et al (2005) Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A 102(51):18676–18681
West AB et al (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 102(46):16842–16847
West AB et al (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16(2):223–232
Lee BD et al (2010) Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat Med 16(9):998–1000
Lee S et al (2010) LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J Neurosci 30(50):16959–16969
Ito G et al (2007) GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry 46(5):1380–1388
Guo L et al (2007) The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res 313(16):3658–3670
Xiong Y et al (2010) GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet 6(4), e1000902
Taymans JM et al (2011) LRRK2 kinase activity is dependent on LRRK2 GTP binding capacity but independent of LRRK2 GTP binding. PLoS One 6(8), e23207
Biosa A et al (2013) GTPase activity regulates kinase activity and cellular phenotypes of Parkinson’s disease-associated LRRK2. Hum Mol Genet 22(6):1140–1156
MacLeod D et al (2006) The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron 52(4):587–593
Plowey ED et al (2008) Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem 105(3):1048–1056
Moehle MS et al (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci 32(5):1602–1611
Thevenet J et al (2011) Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS One 6(6), e21519
Maekawa T et al (2010) Age-dependent and cell-population-restricted LRRK2 expression in normal mouse spleen. Biochem Biophys Res Commun 392(3):431–435
Moehle MS et al (2015) The G2019S LRRK2 mutation increases myeloid cell chemotactic responses and enhances LRRK2 binding to actin-regulatory proteins. Hum Mol Genet 24(15):4250–4267
Choi I et al (2015) LRRK2 G2019S mutation attenuates microglial motility by inhibiting focal adhesion kinase. Nat Commun 6:8255
Saha S et al (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29(29):9210–9218
Wang D et al (2008) Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol Neurodegener 3:3
Andres-Mateos E et al (2009) Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). J Neurosci 29(50):15846–15850
Daher JP et al (2014) Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A 111(25):9289–9294
Liu Z et al (2008) A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A 105(7):2693–2698
Ng CH et al (2009) Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci 29(36):11257–11262
Li Y et al (2009) Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci 12(7):826–828
Li X et al (2010) Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J Neurosci 30(5):1788–1797
Melrose HL et al (2010) Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis 40(3):503–517
Tong Y et al (2009) R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A 106(34):14622–14627
Ramonet D et al (2011) Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One 6(4), e18568
Dusonchet J et al (2011) A rat model of progressive nigral neurodegeneration induced by the Parkinson’s disease-associated G2019S mutation in LRRK2. J Neurosci 31(3):907–912
Tsika E et al (2015) Adenoviral-mediated expression of G2019S LRRK2 induces striatal pathology in a kinase-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis 77:49–61
Walker MD et al (2014) Behavioral deficits and striatal DA signaling in LRRK2 p.G2019S transgenic rats: a multimodal investigation including PET neuroimaging. J Parkinsons Dis 4(3):483–498
Lee JW et al (2015) Behavioral, neurochemical, and pathologic alterations in bacterial artificial chromosome transgenic G2019S leucine-rich repeated kinase 2 rats. Neurobiol Aging 36(1):505–518
Sloan M et al (2016) LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function. Hum Mol Genet 25(5):951–963
Lin X et al (2009) Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron 64(6):807–827
Daher JP et al (2012) Neurodegenerative phenotypes in an A53T alpha-synuclein transgenic mouse model are independent of LRRK2. Hum Mol Genet 21(11):2420–2431
Herzig MC et al (2012) High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS One 7(5), e36581
Daher JP et al (2015) Leucine-rich Repeat Kinase 2 (LRRK2) pharmacological inhibition abates alpha-synuclein gene-induced neurodegeneration. J Biol Chem 290(32):19433–19444
Russo I et al (2015) Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-kappaB p50 signaling in cultured microglia cells. J Neuroinflammation 12(1):230
Boddu R et al (2015) Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Hum Mol Genet 24(14):4078–4093
Fuji RN et al (2015) Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med 7(273):273ra215
Alegre-Abarrategui J et al (2009) LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet 18(21):4022–4034
Tong Y et al (2010) Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A 107(21):9879–9884
Tong Y et al (2012) Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener 7:2
Bravo-San Pedro JM et al (2013) The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell Mol Life Sci 70(1):121–136
Manzoni C et al (2013) Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim Biophys Acta 1833(12):2900–2910
Orenstein SJ et al (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 16(4):394–406
Dodson MW et al (2014) Novel ethyl methanesulfonate (EMS)-induced null alleles of the Drosophila homolog of LRRK2 reveal a crucial role in endolysosomal functions and autophagy in vivo. Dis Model Mech 7(12):1351–1363
Schapansky J et al (2014) Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet 23(16):4201–4214
Saez-Atienzar S et al (2014) The LRRK2 inhibitor GSK2578215A induces protective autophagy in SH-SY5Y cells: involvement of Drp-1-mediated mitochondrial fission and mitochondrial-derived ROS signaling. Cell Death Dis 5, e1368
Pfeffer S, Aivazian D (2004) Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 5(11):886–896
Shin N et al (2008) LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res 314(10):2055–2065
Kondo K et al (2011) alpha-Synuclein aggregation and transmission are enhanced by leucine-rich repeat kinase 2 in human neuroblastoma SH-SY5Y cells. Biol Pharm Bull 34(7):1078–1083
Matta S et al (2012) LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75(6):1008–1021
Guerreiro PS et al (2013) LRRK2 interactions with alpha-synuclein in Parkinson’s disease brains and in cell models. J Mol Med (Berl) 91(4):513–522
MacLeod DA et al (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 77(3):425–439
Beilina A et al (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A 111(7):2626–2631
Luk KC et al (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338(6109):949–953
Acknowledgments
I thank Dr. Andrew West for a critical review of this manuscript.
Conflict of Interest
The author declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Daher, J.P.L. (2017). Interaction of LRRK2 and α-Synuclein in Parkinson’s Disease. In: Rideout, H. (eds) Leucine-Rich Repeat Kinase 2 (LRRK2). Advances in Neurobiology, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-319-49969-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-49969-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49967-3
Online ISBN: 978-3-319-49969-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)