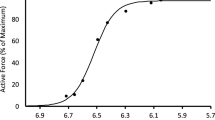
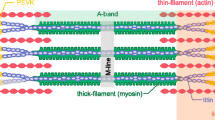
Abstract
With a single activation, a skeletal muscle fiber, motor unit or whole muscle will yield a twitch contraction. The twitch is not an “all-or-none” response, but a submaximal response that can vary from one time to another. Prior activation causes myosin regulatory light chain (RLC) phosphorylation, by an enzyme called myosin light chain kinase. This phosphorylation dissipates slowly over the next several minutes due to a slow activity of light chain phosphatase. Phosphorylation of the RLC increases the mobility of the S1 head of myosin, bringing the S1 head in closer proximity to the myosin binding sites on actin. This increased mobility increases the rate of engagement of cross-bridges and increases the rate of force development and contraction magnitude on subsequent twitch or other submaximal contractions. We call this increased contractile response “activity dependent potentiation”. With sequential twitches or incompletely fused tetanic contractions, the term staircase is used to describe the progressive increase in amplitude of contraction. If a twitch is elicited after a tetanic contraction, we call the enhanced response posttetanic potentiation. Stretching a muscle fiber to a longer length will also bring the actin filaments close to the myosin heads. This increases the Ca2+ sensitivity, independent of RLC phosphorylation. At long sarcomere lengths, the impact of RLC phosphorylation is diminished, because Ca2+ sensitivity is already increased. Similarly, lowering the temperature at which the muscle is tested increases Ca2+ sensitivity. At low temperatures, staircase and posttetanic potentiation are diminished, but RLC phosphorylation still occurs. Activity dependent potentiation is an important functional modulator of contractile response.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbate F, Bruton JD, de Haan A, Westerblad H (2002) Prolonged force increase following a high-frequency burst is not due to a sustained elevation of [Ca2+]i. Am J Physiol Cell Physiol 283:C42–C47
Allen DG, Lee JA, Westerblad H (1989) Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol 415:433–458
Balnave CD, Allen DG (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol 492:705–713
Barsotti RJ, Butler TM (1984) Chemical energy usage and myosin light chain phosphorylation in mammalian skeletal muscle. J Muscle Res Cell Motil 5:45–64
Baudry S, Klass M, Duchateau J (2005) Postactivation potentiation influences differently the nonlinear summation of contractions in young and elderly adults. J Appl Physiol 98:1243–1250
Billeter R, Heizmann CW, Howald H, Jenny E (1981) Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem 116:389–395
Blumenthal DK, Stull JT (1980) Activation of skeletal muscle myosin light chain kinase by Ca2+ and calmodulin. Biochemistry 19:5608–5614
Bozzo C, Spolaore B, Toniolo L, Stevens L, Bastide B, Cieniewski-Bernard C, Fontana A, Mounier Y, Reggiani C (2005) Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles. FEBS J 272:5771–5785
Burke RE, Rudomin P, Zajac FE 3rd (1976) The effect of activation history on tension production by individual muscle units. Brain Res 109:515–529
Carlsen RC, Walsh DA (1987) Decrease in force potentiation and appearance of alpha-adrenergic mediated contracture in aging rat skeletal muscle. Pflügers Archiv 408:224–230
Celio MR, Heizmann CW (1982) Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature 297:504–506
Chin ER, Balnave CD, Allen DG (1997) Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol Cell Physiol 272:C550–C559
Close R, Hoh JFY (1968) The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol 197:461–477
Close RI, Luff AR (1974) Dynamic properties of inferior rectus muscle of the rat. J Physiol 236:259–270
Crow MT, Kushmerick MJ (1982) Myosin light chain phosphorylation is associated with a decrease in the energy cost for concentration in fast twitch mouse muscle. J Biol Chem 257:2121–2124
Decostre V, Gillis JM, Gailly P (2000) Effect of adrenaline on the post-tetanic potentiation in mouse skeletal muscle. J Muscle Res Cell Motil 21:247–254
Desmedt JE, Hainaut K (1968) Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature 217:529–532
Desmedt JE, Hainaut K (1977). Inhibition of the intracellular release of calcium by dantrolene in barnacle giant muscle fibers. J Physiol 265:565–585
Edman KAP, Mattiazzi AR (1981) Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil 2:321–334
Franks-Skiba K, Lardelli R, Goh G, Cooke R (2007) Myosin light chain phosphorylation inhibits muscle fiber shortening velocity in the presence of vanadate. Am J Physiol Reg Int Comp Physiol 292:R1603–R1612
Gordon AM, Regnier M, Homsher E (2001) Skeletal and cardiac muscle contractile activation: tropomyosin “rocks and rolls”. News Physiol Sci 16:49–55
Grange RW, Vandenboom R, Houston ME (1993) Physiological significance of myosin phosphorylation in skeletal muscle. Can J Appl Physiol 18:229–242
Grange RW, Cory CR, Vandenboom R, Houston ME (1995) Myosin phosphorylation augments force–displacement and force–velocity relationships of mouse fast muscle. Am J Physiol Cell Physiol 269:C713–C724
Grange RW, Vandenboom R, Xeni J, Houston ME (1998) Potentiation of in vitro concentric work in mouse fast muscle. J Appl Physiol 84:236–243
Green HJ, Jones SR (1989) Does post-tetanic potentiation compensate for low frequency fatigue? Clin Physiol 9:499–514
Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR (2009) The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol 297:R265–R274
Guttman SA, Horton RG, Wilber DT (1936) Enhancement of muscle contraction after tetanus. In: Proceedings of the Society for Experimental Biology and Medicine 34:219–221
Györke S (1993) Effects of repeated tetanic stimulation on excitation–contraction coupling in cut muscle fibres of the frog. J Physiol 464:699–710
Hicks AL, Cupido CM, Martin J, Dent J (1991) Twitch potentiation during fatiguing exercise in the elderly: the effects of training. Eur J Appl Physiol 63:278–281
Hiraoki T, Vogel HJ (1987) Structure and function of calcium-binding proteins. J Cardiol Pharmacol 10:S14–S31
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Irving TC, Konhilas J, Perry D, Fischetti R, De Tombe PP (2000) Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol 279:H2568–H2573
Karatzaferi C, Franks-Skiba K, Cooke R (2007) Inhibition of shortening velocity of ksinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Reg Int Comp Physiol 294:R948–R955
Klug GA, Botterman BR, Stull JT (1982) The effect of low frequency stimulation on myosin light chain phosphorylation in skeletal muscle. J Biol Chem 257:4688–4690
Konhilas JP, Irving TC, De Tombe PP (2002) Length-dependent activation in three striated muscle types of the rat. J Physiol 544:225–236
Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, De Tombe PP (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547:951–961
Krarup C (1981a) Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:355–372
Krarup C (1981b) Temperature dependence of enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol 331:373–387
Lambert CR, Gladden LB, Stainsby WN (1979) Length-dependent activation of in situ canine skeletal muscle. Am J Physiol Cell Physiol 237:C38–C42
Levine RJC, Kensler RW, Yang ZH, Stull JT, Sweeney HL (1996) Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71:898–907
MacIntosh BR (1991) Skeletal muscle staircase response with fatigue or dantrolene sodium. Med Sci Sports Exer 23:56–63
MacIntosh BR (2003) Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18:222–225
MacIntosh BR, Bryan SN (2002) Potentiation of shortening and velocity of shortening during repeated isotonic tetanic contractions. Pflügers Archiv 443:804–812
MacIntosh BR, Gardiner PF (1987) Posttetanic potentiation and skeletal muscle fatigue: interactions with caffeine. Can J Physiol Pharmacol 65:260–268
MacIntosh BR, Kupsh CC (1987) Staircase, fatigue and caffeine in skeletal muscle in situ. Muscle Nerve 10:717–722
MacIntosh BR, Rassier DE (2002) What is fatigue? Can J Appl Physiol 27:42–55
MacIntosh BR, Willis JC (2000) Force–frequency relationship and potentiation in mammalian skeletal muscle. J Appl Physiol 88:2088–2096
MacIntosh BR, Roberge MC, Gardiner PF (1988) Absence of staircase following disuse in rat gastrocnemius muscle. Can J Physiol Pharmacol 66:707–713
MacIntosh BR, Grange RW, Cory CR, Houston ME (1994) Contractile properties of rat gastrocnemius muscle during staircase, fatigue and recovery. Exp Physiol 79:59–70
MacIntosh BR, Taub EC, Dormer GN, Tomaras EK (2008) Potentiation of isometric and isotonic contractions during high-frequency stimulation. Pflugers Arch 456:449–458
Manning DR, Stull JT (1982) Myosin light chain phosphorylation–dephosphorylation in mammalian skeletal muscle. Am J Physiol Cell Physiol 242:C234–C241
Metzger JM, Moss RL (1988) Thin filament regulation of shortening velocity in rat skinned skeletal muscle: effects of osmotic compression. J Physiol 398:165–175
Moore RL, Stull JT (1984) Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol Cell Physiol 247:C462–C471
Pate E, Bhimani M, Franks-Skiba K, Cooke R (1995) Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 486:689–694
Perrie WT, Smillie LB, Perry SV (1973) A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J 135:151–164
Persechini A, Stull JT, Cooke R (1985) The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260:7951–7954
Petrella RJ, Cunningham DA, Vandervoort AA, Paterson DH (1989) Comparison of twitch potentiation in the gastrocnemius of young and elderly men. Eur J Appl Physiol 58:395–399
Podlubnaya Z, Kakol I, Moczarska A, Stepkowski D, Udaltsov S (1999) Calcium-induced structural changes in synthetic myosin filaments of vertebrate striated muscles. J Str Biol 127:1–15
Posterino GS, Cellini MA, Lamb GD (2003) Effects of oxidation and cytosolic redox conditions on excitation–contraction coupling in rat skeletal muscle. J Physiol 547:807–823
Rall JA (1996) Role of parvalbumin in skeletal muscle relaxation. News Physiol Sci 11:249–255
Rankin LL, Enoka RM, Volz KA, Stuart DG (1988) Coexistence of twitch potentiation and tetanic force decline in rat hindlimb muscle. J App Physiol 65:2687–2695
Rassier DE, MacIntosh BR (2002) Sarcomere length-dependence of activity-dependent twitch potentiation in mouse skeletal muscle. BMC Physiol 2:1–8
Rassier DE, Tubman LA, MacIntosh BR (1997) Length-dependent potentiation and myosin light chain phosphorylation in rat gastrocnemius muscle. Am J Physiol Cell Physiol 273:C198–C204
Rassier DE, Tubman LA, MacIntosh BR (1999) Staircase in mammalian muscle without light chain phosphorylation. Braz J Med Biol Res 32:121–129
Rosenblueth A, Morison RS (1937) Curarization, fatigue and Wedensky inhibition. Am J Physiol 119:236–256
Roszek B, Huijing PA (1997) Stimulation frequency history alters length-force characteristics of fully recruited rat muscle. J Electromyogr Kinesiol 7:161–177
Staron RS, Pette D (1993) The continuum of pure and hybrid myosin heavy chain-based fibre types in rat skeletal muscle. Histochemistry 100:149–153
Stephenson DG, Williams DA (1985) Temperature-dependent calcium sensitivity changes in skinned muscle fibres of rat and toad. J Physiol 360:1–12
St-Pierre DMM, Gardiner PF (1985) Effect of “disuse” on mammalian fast-twitch muscle: joint fixation compared with neurally applied tetrodotoxin. Exp Neurol 90:635–651
Sweeney HL, Kushmerick MJ (1985) Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol Cell Physiol 249:C362–C365
Sweeney HL, Stull JT (1990) Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci U S A 87:414–418
Sweeney HL, Bowman BF, Stull JT (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol 264:C1085–C1095
Tubman LA, MacIntosh BR, Rassier DE (1996) Absence of myosin light chain phosphorylation and twitch potentiation in atrophied skeletal muscle. Can J Physiol Pharmacol 74:723–728
Walsh MP (1983) Calmodulin and its roles in skeletal muscle function. Can Anaesth Soc J. 30:390–398
Xu S, Offer G, Gu J, White HD, Yu LC (2003) Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry 42:390–401
Yang Z, Stull JT, Levine RJ, Sweeney HL (1998) Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol 122:139–148
Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT (2005) Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci U S A 102:17519–17524
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
MacIntosh, B.R. (2010). Cellular and Whole Muscle Studies of Activity Dependent Potentiation. In: Rassier, D. (eds) Muscle Biophysics. Advances in Experimental Medicine and Biology, vol 682. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6366-6_18
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6366-6_18
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6365-9
Online ISBN: 978-1-4419-6366-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)