Abstract
The essence of One Health is an interdisciplinary approach combined with some degree of intersectoral integration that is aimed at mitigation of human and animal health risks, taking account of environmental, ecological, social and economic factors. While a large number of international stakeholders now consider the One Health approach necessary for more effective protection of the global community against health threats, there is still no systematic allocation of resources to integrated national or multinational programmes, partly due to the inertia of existing sectoral systems and the lack of convincing economic arguments in support of the approach. We propose different degrees of sectoral integration depending on system types and associated economic efficiency gains to be expected from a One Health approach. International and regional organisations have an important role in facilitating the adoption of the approach, since the costs and the benefits are often of a regional or even a global nature, such as in the case of avian influenza.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
One Health is often presented as an approach to mitigate risks to human health arising from microorganisms harboured in non-human animal species, which have the potential to infect and cause disease in humans. These microorganisms include known pathogens as well as hitherto unknown existing microorganisms capable of infecting humans or capable of evolving into human-infective forms under favourable circumstances. For the purpose of this chapter, emerging diseases are defined as a previously undefined (unknown) condition, which may result from the evolution or change in an existing infectious agent causing a change of strain, host range, vector or increase in virulence, or may be the occurrence of any other previously undefined condition (Hoinville et al. 2011). Given the alarming number and serious consequences of human pathogen emergence from animal reservoirs over the past decades (bovine spongiform encephalopathy, BSE; Nipah virus; severe acute respiratory syndrome, SARS; highly pathogenic avian influenza, HPAI H5N1; and influenza A H1N1), the rationale behind the concept of One Health, namely promotion of a more harmonised and integrated approach to investigate, plan for, and react to mitigate zoonotic disease risks, is appealing. During the last five years there has been a growing momentum, particularly from the international community, requesting that health research, systems and services implement a One Health approach. The reaction from people working in the health professions has been polarised, the majority of those working in the human health sector have not engaged with One Health,Footnote 1 whereas the majority of professionals working in animal and environmental health are interested in the approach, and some recognise its value. One Health advocates assume, a priori, that a more holistic management of microbial health hazards would result in a more efficient use of the scarce resources available for mitigation of disease risk. This paper aims to contribute to the One Health debate by using an economics assessment framework, in order to find ways of moving forward the agenda and engagement. In this exploration, we will limit ourselves largely to the mitigationFootnote 2 of zoonotic disease risks, recognising that a similar approach should be extended to non-communicable diseases of humans and to production diseases in animals.
1.1 The Use of an Economics Framework
One Health advocates are challenging a strong and existing health paradigm based on the separation of, and specialisation within, human and animal health systems. In order to achieve a paradigm shift in health service provision there has to be convincing arguments that the costs of a major shift will generate substantial net benefits.
Economics, a discipline that examines trade-offs in scarce resource allocation, has two important aspects for thinking on One Health:
-
Efficiency of resource use.
-
The marginal value of a change in approach.
The aim of activities to mitigate zoonotic disease risks, delivered by public, non-government organisations (NGOs) and/or private providers, is the promotion of health by avoiding, containing, reducing or removing zoonotic pathogens. A change from a traditional sectoral approach of health management to a holistic One Health approach needs to compare the marginal benefits against the marginal costs of such a change. The final outcome of One Health initiatives is the avoidance or reduction of disease in humans and animals—an outcome that can be measured in technical terms (e.g. number of human cases averted, reduction of prevalence in animal population) and translated into values using established economic quantification methods. The value of an activity or its impact may be reflected by market prices, e.g. health treatment expenditures or production losses avoided. But often, One Health risk mitigation activities result in non-market outcomes such as avoidance of human distress or death, feelings of consumer confidence, improved animal welfare or conservation of an animal species, which do not have a market value but nevertheless can be measured using one of the many approaches available in economic theory, such as contingent valuation.
1.2 The Limitations of an Economics Approach
Economics is a necessary discipline for understanding the dynamics of resource use and presenting arguments for change. However neoclassical economics, a dominant school of thought in economic theory that investigates resource allocation by means of supply and demand models in relation to subjective preferences of producers and consumers, has some important limitations. It tends to oversimplify the fluid nature of resources, by assuming that resources are completely divisible and available timelessly. It also underplays that market prices for resources are often distorted through public and private rule structures.
With regard to One Health, resources are not divisible and instantly available. Time will be required to develop human, institutional and infrastructure capacities; these investmentsFootnote 3 are so substantial that it is unlikely that the private sector or even governments of relatively small countriesFootnote 4 will be able to afford such an initial fixed capital investment. Without such investments, One Health field level initiatives are likely to remain interesting projects rather than paradigm shifts, and economic analysis may well undervalue the larger scale benefits of a more comprehensive paradigm shift.
In terms of the pricing mechanisms for resources, more careful assessment and where necessary research is required around the public and private institutions that set and influence prices. This requires a mix of sociological, cultural and psychological skills to supplement the economics and the science.
1.3 Structure
This paper will examine what evidence is available to support the assumption that a One Health approach leads to efficiency gains and generates net benefits to society. The examples where this approach has been tested are small-scale modifications of health systems, simply because no country has taken a decision towards major funding of institutions whose main activity is One Health.Footnote 5 This chapter is split in two core sections: (1) context, and (2) conceptual considerations about the economic assessment of One Health and evidence from the literature illustrating the economic value of One Health. The evidence presented is summarised in a section of discussion and conclusions with the intention of identifying where economics in the future can improve understanding of societal health management and what factors in One Health need to be strengthened to achieve this.
2 Context
All countries, developed and developing, have limited resources for human and animal health service provision, but there is a growing need to improve human and animal health services to protect global health and food security.
There are increasing demands on disease surveillance, emergency response and disease control due to an apparent increase in the emergence of new diseases or the re-emergence of existing diseases, many of which are zoonotic (Harper and Armelagos 2010; Jones et al. 2008; Taylor et al. 2001). The factors reported to be influencing emergence include human population and behaviour changes, increasing livestock production, intensification of production, trade, habitat change, loss of biodiversity, and globalisation (McMichael 2004; Morse 2004). In the next sections, disease emergence is described in more detail.
2.1 Risk of Zoonotic Disease Emergence
Human population growth combined with economic development has resulted in increased demand for livestock-derived food products, which has led to larger livestock populations, increased production intensity and changes in trade volumes and patterns. This in turn has provided an environment that facilitates the evolution and spread of infectious zoonotic pathogens, including those with antibiotic resistance genes (Daszak et al. 2000; Dobson and Carper 1996; Greger 2007; Jones et al. 2008; McMichael 2004; Morse 1995; Palumbi 2001; Pearce-Duvet 2006; Woolhouse and Gaunt 2007).
In such circumstances, emergence of an infectious disease may be the result of a pathogen increasing frequency of transmission within a given population (often accompanied by an increase in virulence), expanding its host range or expanding its geographical distribution. Pathogens which require a vector for transmission may emerge as a consequence of the vector changing its geographical range or by becoming more abundant within a particular geographical area due to environmental factors (Smolinski et al. 2003).
These changes have been accompanied by a reduction in the diversity of livestock species and the genetic variation within these species, which provides an environment for increased disease transmission once these populations have been exposed to a new pathogen to which they are susceptible. The source of such new pathogens can be wildlife populations, which in comparison to livestock are still highly biodiverse, and more likely to harbour new pathogens than domestic species. Given exposure to such new pathogens, high densities of domestic animal host species or humans will increase the likelihood of these pathogens becoming successfully established in their new host population (Keesing et al. 2010).
In terms of the development of resistant pathogens, reduced microbial diversity within host populations as a result of preventive or curative treatment combined with increased host density will increase the risk of the emergence of resistant genetic variants (Altizer et al. 2003; Davies and Davies 2010; zur Wiesch et al. 2011).
2.2 Types of Pathogens
Emerging zoonotic disease can be caused by a wide range of infectious agents, including viruses, bacteria, prions, helminths and fungi. These vary in the likelihood of genetic change which can result in the ability to infect a new host species or to develop resistance to treatments or in the case of vaccination a genetic drift away from protection by the vaccine. In addition, viruses and bacteria are able to exchange genetic information and change gene expression in response to environmental factors.
Viruses, in particular RNA viruses, are most likely to be candidates for zoonotic infectious disease emergence due to their high mutation rates. The probability of emergence as a new human pathogen increases if the pathogen already has wide host range (Woolhouse and Gaunt 2007).
Based on past experience, drug-resistant strains are most likely to develop among bacterial and rickettsial pathogens (Jones et al. 2008). While the emergence of treatment-resistant human pathogens is currently still primarily associated with usage of antimicrobials among humans, the increasing intensity of food production together with lack of effective regulation of antimicrobial usage in many parts of the world has increased the risk of emergence of genetic variants of zoonotic pathogens that cannot be treated effectively (Anonymous 2012).
It has recently been pointed out that the potential for fungal diseases to become an emerging threat has been underestimated (Olsen et al. 2011).
2.3 Most Important Animal Species
The importance of various animal species as sources of zoonotic pathogens is influenced by many factors, including the number of different microorganisms present in a species, the prevalence of a particular microorganism within a given species reservoir, the number of host species, the density of a particular host species, the contact opportunity between reservoir species and humans and the phylogenetic distance to humans.
Pigs and poultry are likely to have a key role as potential source of new zoonotic diseases among domestic animals due to their large populations which are often kept in high densities with high turnover rates. While the pig and poultry production systems have a reduced number of people working with animals, the intensity of the contact of these people is greater than before (Graham et al. 2008). In the case of influenza virus infections, the similarity in respiratory epithelium receptors between humans and pigs increases the likelihood of cross-infectivity (Drew 2011; Greger 2007; Ma et al. 2009).
Among wild animals, non-human primates are of particular importance as reservoirs of potential zoonotic pathogens due to their short phylogenetic distance to humans, which compensate for their relatively low density and limited opportunity of contact with humans compared with domestic animals (Greger 2007; Smith et al. 2011; Wolfe et al. 2007). Rodents have a more distant phylogenetic relationship to humans but have high abundance and density, several species are peri-domestic, and are a reservoir for several re-emerging zoonoses, e.g. leptospirosis, plague, typhus. In recent years, bats have been linked to the emergence of SARS, lyssaviruses, Ebola, Hendra and Nipah viruses. Bats represent almost 20 % of all mammalian biodiversity, are found worldwide apart from Antarctica and tend to live in large colonies in close contact with each other (Bennett 2006). Habitat changes have led to changes in bat foraging patterns and closer proximity of colonies to human settlements and domestic animals, increasing the opportunity for transmission of microorganisms to new hosts and the emergence of new zoonotic diseases (Breed et al. 2006; Calisher et al. 2006; Drexler et al. 2012; Field 2009).
3 Economic Assessments of One Health Disease Risk Mitigation Programmes
3.1 The Economic Logic
One Health programmes are commonly promoted because they are believed to enhance the effectiveness and efficiency of disease risk mitigation compared to measures already in place under traditional, sectoral approaches. Consequently, to investigate the added value of One Health compared to what is already in place, economic assessments of One Health must contrast the resource use and outcomes of the proposed holistic approach to current practices; in other words an incremental analysis. However, such analyses assume the baseline or counterfactual is the traditional approach and do not demonstrate whether traditional approaches are efficient in a first place. Only the comparison of One Health and traditional approaches as independent options in relation to the same baseline allow the demonstration of their economic efficiency. The difficulty in applying this approach is the need for a baseline or counterfactual that equates to no coordinated societal intervention.
Economic assessments can be performed at three stages:
-
In the planning stage of a programme ex ante assessments provide information for decision makers regarding the selection of the most efficient option. Such studies focus on alternative strategies and make predictions about possible outcomes, i.e. they deal with expected costs and values. They provide information about the technical feasibility and economic viability and flag up potential challenges for implementation.
-
During implementation, interim assessments are done to review, and if necessary modify, the assumptions used in the ex ante study to see if the probability of a successful outcome has changed, for example if significant technological progress occurs or participants’ compliance is lower than foreseen. Alternatively, if no ex ante analysis has been conducted, interim evaluation may be used to assess the value of a running programme. If the programme is deviating from expectations, funds can be redirected to achieve the envisaged outcomes.
-
After completion of a programme ex post evaluations are performed to assess whether it produced a positive net value. They never fully inform a resource allocation decision, because they only look back at decisions already taken, but provide important information for the ex ante assessments of future strategies, i.e. allow lessons to be learnt about factors relevant to the success or failure of a programme and its economic value.
These basic considerations apply equally to One Health. Standardised economic evaluations help to avoid misallocation of funds and increase the likelihood of cost-effective mitigation success. The value of the resources used for One Health activities should not be higher than the resulting economic, social and environmental benefits in relation to the baseline or counterfactual. However, there are important One Health characteristics that need to be taken into account when aiming to assess its economic benefits:
-
(A)
One Health is considered as an approach, which can guide and improve mitigation of communicable zoonotic disease risk, drawing attention to the intimate connection between human and animal health. For empirical analysis or modelling, a system is often broken down into its parts that are more manageable and allow individual investigation. Only after each component is assessed and modelled will the outcomes be combined. However, such disaggregation, analysis and reconstruction in many cases lose detail of how the whole system functions, i.e. the ability with which feedback mechanisms and their importance can be evaluated is reduced.
-
(B)
A wide range of One Health projects aim to detect, combat and/or prevent emerging disease, which by definition is unknown. It is impossible to collect data about something that has not occurred yet and no empirical data will be available about the type of a pathogen emerging, nor its geographic location, host preference, virulence, infectivity or transmission pathways, and of greatest importance whether such a pathogen will establish itself in humans. Therefore, any model attempting to predict what might happen will have to draw on expert assessment, educated guesswork and bio-prospecting/screening of putative sources of pathogens and exposed human population groups.
-
(C)
Anecdotal evidence suggests that One Health and other interdisciplinary or intersectoral strategies may be initiated because other strategies failed to produce the desired effect. Hence, certain initiatives may be driven by beliefs and convictions rather than scientific evidence. While broad goals may be formulated, specific activities and cause-effect patterns may be less clear, i.e. it can be seen as an evolving learning process taking advantage of opportunities and integrating new knowledge as it becomes available.
Consequently, there is no “one size fits all” blueprint for the economic evaluation of One Health programmes, but appropriate evaluation criteria must be selected on a case-by-case basis using robust economic concepts and criteria while acknowledging particular One Health challenges.
3.2 Evidence for the Economic Value of the One Health Approach
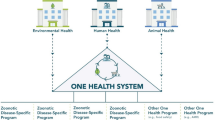
Increases in technical and/or economic efficiency of One Health risk mitigation programmes are anticipated to result from four types of intersectoral collaboration that are not clearly demarcated and may therefore overlap (Fig. 1):
-
(1)
One Health to share and save operational costs. In some countries and remote locations, governments are struggling to provide basic animal and public health services due to a lack of resources. Consequently, there is a financial incentive for animal and public health services to combine their resources to either attain a critical mass that allows the establishment of some minimal infrastructure and service provision or to enhance the delivery of services by sharing cost-structures. The economic logic for integrating the delivery of animal and public health services at the operational level is obvious. Where similar structures in terms of logistics, personnel and materials are required by multiple programmes running concurrently, resources can be shared and in some cases saved by intersectoral partnering. Such cost-sharing initiatives serve to reduce the investment required in individual programmes, thus increasing the efficiency of a programme.
-
(2)
One Health risk mitigation programmes for endemic zoonotic diseases. Strategic higher level budgetary and resource allocation provides sufficient financing to control disease along the livestock value chain and leads to benefits in humans. This requires a proactive and preventative approach to disease management and recognition that disease can be managed further upstream, which requires significant shifts in resource allocation. Mitigation activities are introduced at multiple points encompassing multiple sectors or in one specific population only with financial contribution from the other sector.
-
(3)
One Health surveillance and response for early detection of emerging, re-emerging or exotic zoonotic pathogens. Recent experiences with emerging zoonotic diseases have demonstrated their potential for major impact on human and animal populations, either directly due to morbidity and mortality in animals or humans, or indirectly due to response to disease, control measures, effect on trade or fear of disease and associated behaviour change. This has stimulated the creation of interdisciplinary partnerships for integrated surveillance and/or rapid response for future emerging disease threats (King et al. 2004). If such partnerships are well coordinated, a (re-)emerging or exotic disease may be detected early before it has spread widely, making it easier and less costly to contain.
-
(4)
One Health activities to prevent zoonotic disease emergence and establishment. The ultimate goal of zoonotic disease risk mitigation is to prevent emergence and subsequent establishment through One Health or Ecohealth approaches. Ecohealth takes One Health further by examining changes in the biological, physical, social and economic environments and relates these changes to human health. To prevent disease emergence, risky environments, contacts and behaviours must be modified in a way to decrease the probability of zoonotic disease emergence. This requires not only knowledge about factors for disease emergence, but also a willingness of service providers to invest resources in activities that have a highly uncertain outcome. If successful, the benefits from avoiding zoonotic disease emergence are potentially very large.
Both the magnitude of potential benefits and the uncertainty of accruing them increase as the degree of professional integration expands from ‘sharing operational costs’ to ‘prevention of zoonotic disease emergence and establishment’. Because economic assessments are incomplete without considering the additional resource use required to avert value losses, extra costs such as staff time needed for planning, preparation, data analysis and communication and costs for setting up new structures (e.g. shared databases and communication channels) must be accounted for.
In the following sections, we discuss available evidence in support of economic efficiency of the four types of One Health risk mitigation programmes.
3.3 One Health to Share and Save Operational Costs
It is often assumed that human medicine has a strong public good component, in which it increases the utility of the beneficiaries and therefore their ability to contribute to society. For veterinary medicine the generation of public goods depends on the nature of the intervention (De Haan and Umali 1992) and there is a grey area that relates to externalities (Leonard 2010; Rushton and Leonard 2009). Within the grey area the ability of livestock to provide improved livelihood outcomes to the poor has been recognised (Randolph et al. 2007) and the link between improvements in livelihoods and improvements in health is well established (Smith 1999). Veterinary interventions, which are diagnostic or curative, contain a public good component when considered at the level of the smallholder or pastoralist. People living in such systems are more likely to be living in areas of poor infrastructure, be less well informed about health issues, have a lower capacity to bear the risk presented by disease (McDermott et al. 1999) and are therefore most in need of basic medical and veterinary services. In addition, the proximity in which these people live to their animals puts them at particular risk of zoonotic disease transmission. One Health represents an opportunity to build the capacity of medical and veterinary service provision in such situations.
The extension of primary healthcare provision in developing countries by use of community health workers or community animal health workers is well documented (Lehmann and Sanders 2007; Leyland and Catley 2002; Peeling and Holden 2004). However, such schemes have often proven to be unsustainable when project funding is withdrawn (Lehmann and Sanders 2007) despite achieving significant positive outcomes (Schreuder et al. 1996; Yahya 1990).Footnote 6 In areas of relative isolation or areas of seasonal human occupation, where demand is insufficient to sustain specialised services, integration under a One Health concept may allow provision of such services to be sustainable (Schelling et al. 2005). Further, the availability of human resources restricts the implementation of health interventions in the developing world (Kurowski et al. 2007; Wyss et al. 2003). The coordination and collaboration between human and animal health service providers into an integrated veterinary and medical provider therefore represents a potential saving of critical resources, such as trained personnel, as well as offering possible cost-sharing opportunities. The focus of such initiatives may not necessarily be on zoonotic disease, but a range of human and/or animal health priorities.
A further consideration is the potential for reaching a wider group of the population by integrating services. In Chad, a joint vaccination programme for humans and cattle had a higher human uptake particularly among women and children when animal vaccination was being offered concurrently (Schelling et al. 2007; Schelling et al. 2005). It also provided an opportunity for contact between public health services and nomads, many of whom had never previously visited a health centre. Similar effects have been observed in South Sudan, when polio and rinderpest vaccinations were offered simultaneously (Ward et al. 1993). Since vaccination campaigns are typified by high initial set-up costs but reduced marginal costs as coverage is extended, increasing coverage represents an increase in economic efficiency as the cost per animal vaccinated decreases and the threshold for herd immunity is reached.
Schelling et al. (2007) describe the results of a cost-sharing initiative between medical and veterinary vaccination campaigns in rural Chad. Mobile veterinary vaccination teams already visited pastoral livestock keepers in this area to administer veterinary vaccines; as a result a joint human-livestock campaign was initiated utilising the existing personnel and infrastructure to deliver vaccination for anthrax, blackleg, contagious bovine pleuropneumonia and pasteurellosis for animals, and pertussis, tetanus, diphtheria and polio for humans in a single campaign. An evaluation of costs indicated a 15 % reduction in operational costs compared with separate vaccination campaigns.
Despite compelling logic of integrated veterinary and medical services at the operational level, at least in sparsely populated rural areas where livestock form an important livelihood component, to date no evidence could be found of systematic rather than pragmatic implementation of these principles and related strategic resource allocation.
An example of a cost-sharing initiative in an industrialised nation is provided by the Canadian Science Centre for Human and Animal Health (CSCHAH). This facility houses the National Microbiology Laboratory, operated by the Public Health Agency of Canada and the Canadian Food Inspection Agency’s National Centre for Foreign Animal Disease. Opened in 1999 at a cost of CAD $200 m, this facility accommodates the study of infectious disease of humans and animals at the highest biosafety level (Square 1999). No published assessment of the marginal benefit of sharing the facilities provided by the CSCHAH could be found; however, given the scale of the initial investment, the costs saved are likely to be substantial. Additional benefits may also be generated by collocating disciplines by establishment of new social contact networks and collaborative projects, although such benefits will prove difficult to monetise.
3.4 One Health Risk Mitigation Programmes for Endemic Zoonotic Diseases
Strategic One Health risk mitigation programmes for endemic zoonotic disease allow the allocation of resources to the sector in which they will generate the largest societal benefit. Increasing the benefit gained per resource unit used thus represents an increase in economic efficiency. Implementation may be sectoral or integrated. Sectoral implementation refers to cases where an individual sector implements interventions to accrue benefits which will be seen at the societal rather than the individual sectoral level, while integrated implementation requires the participation of multiple sectors.
The lack of adequate brucellosis control in livestock in Mongolia led to an incidence of 60 cases per 100,000 per year in humans (Roth et al. 2003). As a result, the public and animal health benefits of a potential 10-year vaccination campaign for livestock were assessed. Cost-benefit analysis indicated that as an animal health intervention brucellosis vaccination of animals was not efficient. However, if the costs of the vaccination campaign were attributed to different sectors according to benefits received, from a public health perspective brucellosis control in livestock was a highly efficient intervention with a cost of less than $25 per disability-adjusted life year gained.
Echinococcosis mitigation in La Rioja region of Spain was achieved by education on disease risk in the human population, chemotherapy of all owned dogs in the area, euthanasia of stray dogs, sanitary disposal of offal from slaughterhouses and safe disposal of dead sheep by the construction of pits (Jiménez et al. 2002). Integrated surveillance in all three host populations was conducted throughout the programme allowing data to be collected on which the programme could be evaluated and resources redeployed in a reactive manner. This allowed the redirection of resources from chemotherapy to measures for the sanitary disposal of sheep carcases when chemotherapy was seen to be producing no further reduction in prevalence. Economic analyses found that by year 8 of the programme, the cumulative benefit-cost ratio had exceeded 1, indicating costs had been recouped. Since the benefits accrued annually were proportional to the reduction in canine prevalence relative to a no intervention scenario, reallocating resources between activities in a reactive manner increased the economic efficiency of this mitigation programme.
In China, schistosomiasis control programmes based on chemotherapy of humans and animals, and control of snail populations by environmental management and molluscicide treatment were implemented periodically since the 1950s with considerable progress made. However, in 1992 it was estimated that 11.83 million people and 1.2 million animals were still infected (Chen and Feng 1999). A new mitigation programme integrating case detection and morbidity control in humans, molluscicide treatment, health education, surveillance, environmental management and livestock control initiatives was implemented from 1992 to 1999. Subsequent cost-benefit analysis taking into account human cases avoided indicated that the integrated programme created a net benefit for society of $6.20 per $1 invested.
These examples illustrate the benefits of interdisciplinary collaboration at the planning and evaluation stages of an intervention with multiple activities implemented by single or multiple sectors working in parallel. Depending on the type of delivery, i.e. stand-alone or integrated between sectors, activities may be planned and carried out by existing institutions under coordinated, intersectoral leadership or in some cases by newly founded departments. The creation of new departments would incur considerable transaction costs, which are hard to justify when field activities can be divided between existing institutions.
3.5 One Health Surveillance and Response for Early Detection of Emerging, Re-emerging or Exotic Zoonotic Pathogens
An integrated surveillance and response system involves human health, animal health and wildlife sectors working together to detect unusual disease events in human, domestic and wild animal populations that may indicate the emergence of a new disease or a change in the frequency or geographical distribution of known diseases. The surveillance system then triggers an integrated response to contain the disease and monitor the effectiveness of intervention measures. Such a system requires clear leadership and coordination, common goals and objectives, data collection tools for human, domestic and wild animal diseases, integration of data collation and analysis, integrated contingency plans and good communication from field to central level and between disciplines.
The common rationale for establishing early warning surveillance systems is the expectation that the early detection of disease reduces subsequent outbreak response expenditure and disease losses. In other words, surveillance and intervention are, to a large extent, seen as economic substitutes. The technical rate of substitution and their relative costs of provision then determine their least-cost combinations (Howe et al. 2012). that should be compared to the level of value loss avoidance to determine optimal or acceptable levels of resource use. For One Health early warning surveillance, there will be an initial investment to integrate existing surveillance and response systems (or in rare cases to set up a completely new system) and recurring expenditure for the maintenance of the system. For such a system to be efficient from an economic point of view, the set-up and running costs must be equal or smaller than the potential cost savings from averting an epidemic or pandemic.
Potential cost savings are calculated taking into account the probability of a rare event such as zoonotic disease emergence occurring (e.g. one emergence event every 20 years) and its possible consequences (e.g. impact depending on infectivity, virulence and geographic scale of the system affected). Estimated costs of diseases that have emerged in the recent past were for example (1) bovine spongiform encephalopathy: EU Euro 92 billion, USA US $15 billion, Canada US $2.5 billion, and Japan US $990 million (Walsh and Morgan 2005), (2) SARS worldwide US $30-50 billion (Newcomb et al. 2011), (3) HPAI H5N1 worldwide US $50 billion (Newcomb et al. 2011).
To estimate the costs of a zoonotic disease outbreak, data are needed about the effects of the disease in affected humans and animals, as well as the impact of individual human behaviour, market and public responses. For known diseases, contingency plans generally clearly define activities, roles and responsibilities in the case of an outbreak. For emerging disease outbreaks, general structures such as leadership, communication channels and epidemiological investigations may be foreseen, while specific risk mitigation activities need to be tailored according to the hazard. Further, data on disease transmission and spread, such as incidence, the number of humans, holdings and animals affected are needed to estimate disease losses and the magnitude of the response. Data gathered during past outbreaks provide the necessary information for ex post analysis, while mathematical simulation models can be used to make predictions on disease transmission and spread in animal and human populations for ex ante analyses. For known diseases, the consequences can be estimated with sufficient precision as a function of incidence or prevalence. The major challenge lies with collating reliable information and assumptions for emerging, hence unknown, disease events.
The perceived need for integrated surveillance systems has triggered the implementation of such systems worldwide. However, they are rarely linked to effective integrated response capacity as the response remains under national sovereignty. At global level, the Global Early Warning System for Major Animal Diseases including Zoonoses (GLEWS) combines the existing alert mechanisms of the FAO and WHO organisations of the United Nations with the OIE for early warning of animal disease threats, while Connecting Health Organizations for Regional Disease Surveillance (CHORDS) is a One Health global partnership of regional disease surveillance networks concerned with enhancing local capacity for interventions in response to infectious disease threats. At national level, the Human Animal Infections and Risk Surveillance (HAIRS) group in the UK is a multi-agency, cross-disciplinary group for the rapid, early assessment of disease risk in a systematic, objective and transparent manner (Morgan et al. 2009). ArboNET, the national electronic surveillance system for arboviruses in the USA, which was established after the introduction of West Nile Virus into the USA in 1999, collates potentially relevant surveillance data from humans, animals (including dead birds), sentinel chickens and mosquitoes.
While more and more One Health surveillance systems are established, sparse evidence is available about the economic efficiency of such systems, either analysed as independent strategies or incrementally. One rare exception is the analysis of the societal costs and benefits of a surveillance system for identifying E. coli O157:H7 outbreaks in Colorado with recall of contaminated beef as response strategy (Elbasha et al. 2000). It was concluded that by early detection of a single outbreak and averting at least 15 human cases through the recall of 25 million pounds of potentially contaminated beef, the surveillance and response system would recover all costs for the 5 years of start-up and operation.
Often, such systems build on existing surveillance and response structures and aim at adding value by screening, analysing and communicating the gathered data generated by different systems in an integrated way. For incremental economic analysis of such an approach, the additional costs of collating the information, staff time for meetings of working groups, task force and management committee, fees for expert consultants, extra time needed for joint analysis and communication would have to be compared to additional benefits resulting from the integration of these efforts. Potential benefits include timely access to data across species and geographical barriers and sharing of expertise, which allow reduction of uncertainty and more comprehensive and better informed risk assessments. If risks are recognised as negligible at an early stage unnecessary action, overreaction and wasteful resource use can be prevented. If the risk is not negligible, a timely and effective response may contain zoonotic disease outbreaks rapidly and avert disease losses. However, only assessing the frequency of disease incursion and the magnitude of its impact with and without the system in question in comparison to either traditional approaches or a baseline of doing nothing will demonstrate if such systems are economically efficient.
3.6 One Health Activities to Prevent Zoonotic Disease Emergence and Establishment
One Health collaborations to prevent disease emergence are based on the expectation that such events can cause very large costs in terms of disease losses as well as national and international outbreak response measures. Prevention strategies are therefore adopted based on the notion that ‘prevention is better than cure’. But this is not unequivocally true and must be assessed on a case-by-case basis.
Considering the enormous number of mutations (and re-assortments) occurring in microorganisms and the vast number of animal–animal as well as animal–human contacts that occur worldwide at any time, it has to be concluded that emergence and establishment of zoonotic pathogens is a rare event. This is likely to be a consequence of each individual event being rare as such and even more so in combination, i.e. for a mutation to produce a viable and pathogenic variant, it becoming exposed to a suitable and susceptible host, which in turn occurs at densities that allow establishment of infection within that host’s local population, and which is then connected to other populations of susceptible hosts of the same or other species at the meta-population level. Any predictions in relation to occurrence of emerging infectious disease are therefore subject to high uncertainty. But based on current understanding of the relative importance of different biological, environmental and socio-economic drivers, it is likely that regions with high density domestic animal populations particularly of pigs and poultry have an important role as potential source for genetic change in pathogens as well as for amplification of new and mutated pathogens introduced from other populations, such as wild animals. The frequency and velocity (travelling time is shorter than the incubation period) of medium- to long-distance movement of animals, animal products and humans is the key parameter for spread and therefore important for successful establishment of an emerging pathogen at meta-population level. These basic principles allow the definition of risk management practices which should reduce the risk of emergence. Suitable practices include improved management of ecosystems at all levels taking into account molecular, cellular, host, species and environmental characteristics and interactions.
Because knowledge regarding disease emergence and effective prevention measures is still limited, there is no evidence available that demonstrates the economic efficiency of measures applied to animal populations aimed at preventing zoonotic disease emergence. To assess the economic efficiency, the type of zoonotic disease emerging, its epidemiology and consequences in terms of disease losses and expenditures needed to prevent or contain it must be taken into account. Data collected during past emergence events can inform economic and mathematical models to assess the economic efficiency of such initiatives in ex ante analyses. The consequences of emergence of infectious diseases have been modelled by various authors, in particular for diseases such as SARS, influenza A H5N1, H1N1 and BSE. In most cases, this referred to spread within the human population. The usage of the model predictions for policy development presented a significant challenge due to the large degree of uncertainty in relation to biological mechanisms and their quantitative parameter values (Becker et al. 2005; Ferguson et al. 2006; Ferguson and Donnelly 2003; Relman et al. 2010). Generic models focussing on the animal-human interface have also been developed (Antia et al. 2003; Lloyd-Smith et al. 2009). While disease emergence events are (still) happening, suitable data collection protocols should be developed to enhance the knowledge about such events and increase the accuracy of predictions of disease emergence.
Also, ex post economic assessments come to their limits. The reason is the simple problem that we cannot quantify things that have not occurred. Hence it is impossible to conclusively demonstrate that the emergence of a disease has been avoided.
Given the high uncertainty about such events happening, an alternative way to inform resource allocation decisions would be to ask what the frequency and/or magnitude of new zoonotic outbreaks would have to be to recover a specified amount of set-up and running costs to prevent disease emergence and to judge how likely the emergence of such an event would be. However, given the current knowledge about the process of disease emergence and establishment, it is challenging to determine what a sensible magnitude of investments would be. Sproul et al. (2012) use the ‘statistical value of a life saved’ approach to conclude that a one billion dollar annual investment in influenza risk mitigation is justified if on average 654 people are saved per year.
Additionally, decision makers should take into account the relationship between value of the non-monetary kind, meaning people’s sense of well-being, and how much resources society is prepared to devote to deterring the fears of the unknown. The monetised value of the resources committed to the avoidance of zoonotic disease emergence must be the threshold for the value society attaches to reassurance. But all the time, the actions taken to modify a practice should be reappraised and changed if evidence suggests so. Resources should be cut back if fears were unfounded and increased if risks were underestimated.
4 Discussion
We argue that efficient management of zoonotic disease risks requires interdisciplinary and intersectoral approaches, where professionals are encouraged to leave isolated institutional and intellectual silos to collaboratively design, implement and evaluate control and prevention programmes. Interdisciplinary initiatives, including One Health, have come into vogue, but robust economic evidence supporting the need for such approaches is often lacking. There is even less evidence base around the value of intersectoral approaches. This is likely to be a reason why health service providers have not systematically allocated resources towards having a cadre of people work across human and animal populations, organisations or sectors.
To justify the extra resources and effort needed to institutionalise One Health, decision makers must consider carefully the balance and trade-offs between uncertainty, risk, benefits and costs as described above. Rather than automatically favouring One Health over traditional approaches, decisions about allocating resources to One Health ideally would be based on refined economic assessments that integrate evidence from epidemiology as well as biological and social sciences. By applying comprehensive frameworks to assess the impact of zoonotic disease and the societal costs and benefits of risk mitigation measures like the one recently published by Narrod et al. (2012), resources for zoonotic disease management can be used in a more efficient way. While the framework provides a holistic approach, it also requires advanced expertise in a variety of disciplines, extensive data collection and analysis (Narrod et al. 2012). Consequently, the additional resources needed to conduct such analyses must be weighed against the potential gain in information and knowledge.
A thorough economic assessment would look carefully at political transaction costs. One Health implies adopting an interdisciplinary approach and giving up sectoral ownership of a project or programme. It also means that the credit and blame for the results of work will be shared. There are two questions that arise from this (1) How institutionalised is an interdisciplinary approach in the human and animal health sectors? (2) How entrenched are the animal and human health services in their own systems? The former is important in the acceptance of interdisciplinarity, and the latter in the ease in which intersectoral methods of working can be adopted. Often, entire structures are setup for each sector with clear mechanisms of management, budgeting, reporting, accountability and rewarding with little institutional incentive to work across sectors. Further, there may be procedures, agreements or policies that can be inhibiting because they do not allow the space or time for staff to work across sectors. Generally, the greater the entrenchment the lesser the arguments about outcomes or objectives and more about control of resources and people.
When decision makers decide to embark on One Health projects, there may be practical issues in the first phase of collaboration that may be discouraging. Lack of experience in interdisciplinary working often means that more time is needed at the beginning of One Health projects to agree on common goals and objectives, roles, responsibilities, contributions, funding and leadership. Because intersectoral work generally means giving up ownership to a certain degree, leadership vacuums or leadership struggles may result. Further, organisational and governing structures of multi-sectoral partnerships are often unclear or ambiguous and therefore bureaucracy is magnified and/or people simply do not know who does what or how to report up a hierarchy. Undervaluation of some sectors or disciplines by others in the partnership and weak methods of information sharing and communication may lead to different people having different knowledge and status.
For the human health profession, zoonotic diseases are comparably dwarfed by the burden associated with obesity, hypertension or cancer. It is only when unusual zoonoses with large economic impact, such as SARS, BSE or H1N1 occur that infectious diseases get increased attention. Likewise, for animal health professionals, whose main responsibility it is to safeguard the health/productivity of animals, zoonotic disease agents only cause a small fraction of disease burden as well. One way to harness best One Health collaborations may be to look at non-communicable diseases. Food chains process and refine food for both animals and humans and this has important implications on food intake, nutritional health and resulting diseases. These aspects are rarely treated as One Health issues and are invariably observed and worried about rather than thinking of the underlying causes. A more general systems approach rather than a disease-specific approach would be needed to understand these relationships and promote a healthy food supply.
We discuss available economic evidence of the One Health paradigm based on the concept of alternative approaches for disease risk mitigation. Thus, one approach is considered to be more efficient than its alternatives, if the same mitigation outcome can be achieved at lower cost or if the same ‘expenditure’ for risk mitigation results in lower overall risk. In order to make the subject more tractable, we present envisaged outcomes for four types of intersectoral collaboration with increasing degrees of integration.
A low degree of intersectoral integration is sufficient to share and save operational costs. This is for example the case in settings where cost reductions for health service delivery are achieved through economies of scale. The same level of disease risk can thus be obtained at lower cost and the resources saved can be used for other purposes. Areas of low human population densities coupled with high livestock numbers, i.e. pastoral settings, are one circumstance in which neither basic human nor animal health services can be provided at an affordable price due to high transportation costs, poor infrastructure and low aggregate demand, unless subsidised. Another example of potential savings by sharing operational costs would be joint funding of high cost research infrastructure such as high-security laboratories used for diagnostics and research on dangerous exotic pathogens.
A medium degree of intersectoral integration is required for control programmes for known zoonotic diseases in which interventions carried out by animal health services provide benefits to the human health sector. Although this form of intervention is ‘standard’ in veterinary public health with a long history of control programmes against diseases such as tuberculosis, brucellosis and rabies, the economic efficiency of such programmes has rarely been assessed from a One Health perspective. Rough estimates of the value of human health benefits of zoonotic disease control in animals often show orders of magnitude higher than the resulting benefits to the livestock sector, as for example reported for brucellosis in Mongolia (Roth et al. 2003) and tuberculosis in the USA (Olmstead and Rhode 2012). The current institutional architecture in which public funds are allocated to specific ministries does not favour development of joint public health programmes and thus is likely to result in inefficiencies of resource use as each ministry carries out its partial economic assessment.
A high degree of intersectoral integration moves beyond management of known disease risks and is concerned with early detection of emerging/exotic zoonotic pathogens through integrated surveillance mechanisms. While economic evaluations can be carried out for the first two types of One Health collaboration, economic assessment of the efficiency of integrated surveillance systems is severely complicated by the uncertainty surrounding disease emergence or introduction and subsequent disease spread. The available literature is largely theoretical and focuses on the balance between the marginal cost of the additional surveillance effort and the marginal reduction of expected damage. The latter will not only depend on the timeliness of disease detection but also on the effectiveness of the outbreak response. In a large number of countries, outbreak response mechanisms are weak and as a result the benefits of early detection may be minimal. Enhancing surveillance through intersectoral integration therefore only provides the expected efficiency gains if response capacity of the animal and human health sectors is sufficiently developed or if surveillance investments are accompanied by concurrent investments in disease response capabilities.
Identification and implementation of measures that reduce the likelihood of zoonotic disease emergence and establishment in the first place represent the highest degree of intersectoral integration. Economic assessment of the potential benefits of such measures is not only complicated by the uncertainty of associated outcomes (as is the case with early warning surveillance) but also by the wide-ranging externalities of potential measures and impacts.
One Health disease management measures should not only aim to reduce the likelihood of emergence of highly virulent pathogens, such as influenza viruses, but also take into account ‘low profile’ pathogens such as Campylobacter jejuni. This pathogen has become one of the most costly human health hazards associated with the livestock industries in developed countries, responsible for more than 10,000 hospitalisations per year in the USA alone (Mead et al. 1999). Another phenomenon warranting One Health attention is the increased prevalence of antimicrobial resistance genes in pathogens and commensals of animals as these can be transferred to microbes of humans through horizontal gene transfer (Smillie et al. 2011; Witte 2000). In the USA infection with resistant microbes has been estimated to be associated with an 11-day increase of hospitalization, increasing medical costs per patient by around US $20,000, while societal costs were estimated to amount to around US $60,000 per patient (Roberts et al. 2009). The usage of antimicrobials in animals and humans is regulated fairly effectively in developed countries. In contrast, in the parts of the world currently experiencing the highest levels of growth in animal production, particularly in Asia, drugs are commonly traded illegally, used inappropriately or may be tainted, all of which will increase the risk of antibiotic resistance emerging. While the magnitude of these impacts suggest that closer cooperation between human and animal health sectors to mitigate risks may be beneficial, only systematic economic appraisal will demonstrate its economic efficiency and guide the allocation of resources across sectors.
5 Conclusions
Zoonotic diseases create negative impacts to society either directly or indirectly. If price mechanisms of the markets do not take into account the full social costs and benefits of such externalities, they may lead to market failure and undersupply of prevention and control methods for such diseases by the livestock industry (and even by individual country governments) unless social planners intervene.
Given that externalities of disease risk extend beyond national and regional boundaries, international bodies have an important role in providing normative guidance to countries and regions on One Health implementation. At the institutional level it is clear that the broadening of health management and the creation of safer, more disease resilient agricultural landscapes goes beyond the veterinary and human medical services. Extending the efforts towards sustainable agriculture and rural development, environment protection and socio-economic development entails involvement of many institutional stakeholders, requiring a major challenge in terms of fostering partnerships and communication. Moving One Health forward may not require new organisations, but it does require new institutional rules of organisation. It may also not require major additional funding, but it will require different means in how funding is distributed and managed. Such changes are not cost free, and these costs need to be estimated and compared with the benefits gained in terms of better disease management and prevention.
Notes
- 1.
A recent meeting on human health services in low and middle income countries made no reference to One Health.
- 2.
Mitigation of zoonotic disease risk refers to the elimination or reduction of the frequency, magnitude or severity of exposure to zoonotic disease, or minimisation of the potential impact of zoonotic disease.
- 3.
Investments are taken to be the physical infrastructure, the training of people and the changes in logistic and administration.
- 4.
This is an argument not about scarcity of resource but the sheer scale of resource required limiting it to large, rich countries or countries that band together in Unions.
- 5.
To the authors’ knowledge all countries continue to have separate Ministries of Health and Agriculture, the latter includes animal health. Many OECD countries have consumer protection agencies, but these tend not to work closely across sectors. At international level the UN continues to have WHO and FAO supported by OIE. While a tripartite agreement for One Health exists between these international organisations there are few programmes or projects with significant budget and actions that are truly One Health.
- 6.
Compare this to the problems faced in developed countries that struggle to maintain a veterinary presence in remote rural areas and have adopted policies to encourage veterinarians to continue to work in such areas.
- 7.
With exotic we describe a previously defined (known) disease that crosses political boundaries to occur in a country or region in which it is not currently recorded as present. We distinguish it from emerging disease, because the surveillance and response strategies for known diseases are expected to be different from those for new diseases.
Abbreviations
- BSE:
-
Bovine spongiform encephalopathy
- CHORDS:
-
Connecting Health Organizations for Regional Disease Surveillance
- CSCHAH:
-
Canadian Science Centre for Human and Animal Health
- FAO:
-
Food and Agricultural Organization of the United Nations
- GLEWS:
-
Global early warning system for major animal diseases including zoonoses
- HAIRS:
-
Human animal infections and risk surveillance
- HPAI:
-
Highly pathogenic avian influenza
- NGO:
-
Non-government organisation
- OIE:
-
World Organisation for Animal Health
- SARS:
-
Severe acute respiratory syndrome
- WHO:
-
World Health Organization
References
Altizer S, Harvell D, Friedle E (2003) Rapid evolutionary dynamics and disease threats to biodiversity. Trends in Ecology & Evol 18:589–596. doi: 10.1016/j.tree.2003.08.013
Anonymous (2012) The evolving threat of antimicrobial resistance: Options for action. World Health 119
Antia R, Regoes RR, Koella JC, Bergstrom CT (2003) The role of evolution in the emergence of infectious diseases. Nature 426:8–11. doi: 10.1038/nature02177.1
Becker NG, Glass K, Li Z, Aldis GK (2005) Controlling emerging infectious diseases like SARS. Math Biosci 193:205–221. doi: 10.1016/j.mbs.2004.07.006
Bennett M (2006) Bats and human emerging diseases. Epidemiol Infect 134:905–7. doi: 10.1017/S0950268806006674
Breed AC, Field HE, Epstein JH, Daszak P (2006) Emerging henipaviruses and flying foxes—conservation and management perspectives. Biol Conserv 131:211–220. doi: 10.1016/j.biocon.2006.04.007
Calisher CH, Childs JE, Field HE et al. (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545. doi: 10.1128/CMR.00017-06
Chen M, Feng Z (1999) Schistosomiasis control in China. Parasitol Int 48:11–19
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science 287:443–449
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mole Biol Rev: MMBR 74:417–433. doi: 10.1128/MMBR.00016-10
De Haan C, Umali DL (1992) Public and private sector roles in the supply of veterinary services. 12th Agricultural Sector Symposium. The World Bank, Washington pp 125–137
Dobson AP, Carper ER (1996) Infectious diseases and human population history effect of the growth of civilization. BioScience 46:115–126
Drew TW (2011) The emergence and evolution of swine viral diseases: to what extent have husbandry systems and global trade contributed to their distribution and diversity? Rev—Off Int Epizoot 30:95–106
Drexler JF, Corman VM, Müller MA et al. (2012) Bats host major mammalian paramyxoviruses. Nat Commun 3:796. doi: 10.1038/ncomms1796
Elbasha EH, Fitzsimmons TD, Meltzer MI (2000) Surveillance system for identifying Escherichia coli O157: H7 outbreaks. Emerg Infect Dis 6:7–11
Ferguson NM, Cummings D a T, Fraser C et al. (2006) Strategies for mitigating an influenza pandemic. Nature 442:448–452. doi: 10.1038/nature04795
Ferguson NM, Donnelly C a (2003) Assessment of the risk posed by bovine spongiform encephalopathy in cattle in Great Britain and the impact of potential changes to current control measures. Proc R Soc Lond B 270:1579–1584. doi: 10.1098/rspb.2003.2484
Field HE (2009) Bats and emerging zoonoses: henipaviruses and SARS. Zoonoses Public Hlth 56:278–284. doi: 10.1111/j.1863-2378.2008.01218.x
Graham JP, Leibler JH, Price LB et al. (2008) The animal-human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep 123:282–299
Greger M (2007) The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol 33:243–299. doi: 10.1080/10408410701647594
Harper K, Armelagos G (2010) The changing disease-scape in the third epidemiological transition. Int J Environ Res Publ Health 7:675–697. doi: 10.3390/ijerph7020675
Hoinville L, Ronello A, Alban L et al. (2011) Animal health surveillance terminology final report from Pre-ICAHS workshop. Health 2011:1–26
Howe, KS, Häsler, B, Stärk, KD, 2012. Economic principles for resource allocation decisions at national level to mitigate the effects of disease in farm animal populations. Epidemiol. Infect., doi:10.1017/S095026881200060X
Jiménez S, Pérez A, Gil H et al. (2002) Progress in control of cystic echinococcosis in La Rioja, Spain: decline in infection prevalences in human and animal hosts and economic costs and benefits. Acta Tropica 83:213–221
Jones KE, Patel NG, Levy MA et al. (2008) Global trends in emerging infectious diseases. Nature 451:990–993. doi: 10.1038/nature06536
Keesing F, Belden LK, Daszak P et al. (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652. doi: 10.1038/nature09575
King LJ, Marano N, Hughes JM (2004) New partnerships between animal health services and public health agencies. Rev Sci Tech Off Int Epiz 23:717–726
Kurowski C, Wyss K, Abdulla S, Mills A (2007) Scaling up priority health interventions in Tanzania: the human resources challenge. Health Policy Plann 22:113–127. doi: 10.1093/heapol/czm012
Lehmann U, Sanders D (2007) Community health workers: what do we know about them? World Health Organization, Geneva, p 34
Leonard DK (2010) The new institutional economics and the restructuring of animal health services in Africa. In: Leonard DK (ed) Africa’s changing markets for health and veterinary services. The new institutional issues. Macmillan Press Ltd, London, pp 1–40
Leyland T, Catley A (2002) Community-based animal health delivery systems: improving the quality of veterinary service. OIE seminar Organisation of Veterinary Services and Food Safety. World Veterinary Congress, Tunis, pp 1–15
Lloyd-Smith JO, George D, Pepin KM et al. (2009) Epidemic dynamics at the human-animal interface. Science 326:1362–1367. doi: 10.1126/science.1177345
Ma W, Kahn RE, Richt JA (2009) The pig as a mixing vessel for influenza viruses: human and veterinary implications. J Mole Gene Med 3:158–166
McDermott JJ, Randolph TF, Staal SJ (1999) The economics of optimal health and productivity in smallholder livestock systems in developing countries. Rev—Off Int Epizoot 18:399–424
McMichael AJ (2004) Environmental and social influences on emerging infectious diseases: past, present and future. Philos Trans R Soc Lond B Biol Sci 359:1049–1058. doi: 10.1098/rstb.2004.1480
Mead PS, Slutsker L, Dietz V et al. (1999) Food-related illness and death in the United States. Emerg Infect Dis 5:840–842
Morgan D, Kirkbride H, Hewitt K et al. (2009) Assessing the risk from emerging infections. Epidemiol Infect 137:1521–1530. doi: 10.1017/S0950268809990227
Morse SS (2004) Factors and determinants of disease emergence. Rev—Off.Int Epizoot 23:443–451
Morse SS (1995) Factors in the emergence of infectious diseases. Emerg Infect Dis 1:7–15
Narrod C, Zinsstag J, Tiongco M (2012) A one health framework for estimating the economic costs of zoonotic diseases on society. EcoHealth. doi: 10.1007/s10393-012-0747-9
Newcomb J, Harrington T, Aldrich S (2011) The economic impact of selected infectious disease outbreaks. 1–25
Olmstead AL, Rhode PW (2012) The eradication of bovine tuberculosis in the united states in a comparative perspective. In: Zilberman D, Otte J, Roland-Holst D, Pfeiffer D (eds) Health and animal agriculture in developing countries. Springer, New York, pp 7–30
Olsen L, Choffnes ER, Relman DA, Pray L (2011) Fungal diseases: an emerging threat to human, animal and plant health. National Academies Press, New York, pp 1–99
Palumbi SR (2001) Humans as the world’s greatest evolutionary force. Science 293:1786–1790
Pearce-Duvet JMC (2006) The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc 81:369–382. doi: 10.1017/S1464793106007020
Peeling D, Holden S (2004) The effectiveness of community-based animal health workers, for the poor, for communities and for public safety. Rev—Off Int Epizoot 23:253–276; discussion 391–401
Randolph TF, Schelling E, Grace D et al. (2007) Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. J Anim Sci 85:2788–2800. doi: 10.2527/jas.2007-0467
Relman DA, Choffnes ER, Mack A (2010) The domestic and international impacts of the 2009-H1N1 influenza A pandemic: global challenges, global solutions: Workshop summary. 440
Roberts RR, Hota B, Ahmad I et al. (2009) Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 49:1175–1184. doi: 10.1086/605630
Roth F, Zinsstag J, Orkhon D et al. (2003) Human health benefits from livestock vaccination for brucellosis: case study. B World Health Organ 81:867–876
Rushton J, Leonard DK (2009) The new institutional economics and the assessment of animal disease control. In: Rushton J (ed) The economics of animal health and production. CABI, Wallingford, pp 144–149
Schelling E, Bechir M, Ahmed MA, et al. (2007) Human and animal vaccination delivery to remote nomadic. Emerg Infect Dis 13:373–379
Schelling E, Wyss K, Béchir M, et al. (2005) Synergy between public health and veterinary services to deliver human and animal health interventions in rural low income settings. BMJ (Clinical research ed) 331:1264–1267. doi: 10.1136/bmj.331.7527.1264
Schreuder BEC, Moll HAJ, Noorman N, et al. (1996) A benefit-cost analysis of veterinary interventions in Afghanistan based on a livestock mortality study. Prev Vet Med 26:303–314. doi: 10.1016/0167-5877(95)00542-0
Smillie CS, Smith MB, Friedman J, et al. (2011) Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480:241–244. doi: 10.1038/nature10571
Smith JP (1999) Healthy bodies and thick wallets: the dual relation between health and economic status. J Econ Perspect: J Am Eco Assoc 13:144–166
Smith TC, Harper AL, Nair R et al. (2011) Emerging swine zoonoses. Vector Borne Zoonot Dis 11:1225–1234. doi: 10.1089/vbz.2010.0182
Smolinski MS, Hamburg MA, Lederberg J (2003) Microbial threats to health: emergence, detection and response. Health, San Francisco 398
Sproul TW, Zilberman D, Roland-Holst D, Otte J (2012) The cost of saving a statistical life: a case for influenza prevention and control. In: Zilberman D, Otte JM, Roland-Holst D, Pfeiffer DU (eds) Health and animal a1griculture in developing countries. Springer Science + Business Media, New York, pp 135–141
Square D (1999) The strange world inside Canada’s only level-4 containment laboratory. Health 161:1171–1172
Taylor LH, Latham SM, Woolhouse MEJ (2001) Risk factors for human disease emergence. Philos Trans Royal Society Lond B, Biol Sci 356:983–989. doi: 10.1098/rstb.2001.0888
Walsh AL, Morgan D (2005) Identifying hazards, assessing the risks. Vet Rec 157:684–687
Ward DE, Ruppanner R, Marchot PJ, Hansen JW (1993) One medicine—practical application for non-sedentary pastoral populations. 10
zur Wiesch PA, Kouyos R, Engelstädter J et al. (2011) Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infect Dis 11:236–247. doi: 10.1016/S1473-3099(10)70264-4
Witte W (2000) Selective pressure by antibiotic use in livestock. Int J Antimicrobial Agents 16 Suppl 1:S19–24
Wolfe ND, Dunavan CP, Diamond J (2007) Origins of major human infectious diseases. Nature 447:279–283. doi: 10.1038/nature05775
Woolhouse M, Gaunt E (2007) Ecological origins of novel human pathogens. Critic Rev Microbiol 33:231–242. doi: 10.1080/10408410701647560
Wyss K, Doumagoum Moto D, Callewaert B (2003) Constraints to scaling-up health related interventions: the case of Chad, Central Africa. J Int Dev 15:87–100. doi: 10.1002/jid.967
Yahya SRR (1990) Indonesia: implementation of the health-for-all strategy. Achieving health for all by the year 2000.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Häsler, B., Gilbert, W., Jones, B.A., Pfeiffer, D.U., Rushton, J., Otte, M.J. (2012). The Economic Value of One Health in Relation to the Mitigation of Zoonotic Disease Risks. In: Mackenzie, J., Jeggo, M., Daszak, P., Richt, J. (eds) One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases. Current Topics in Microbiology and Immunology, vol 365. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2012_239
Download citation
DOI: https://doi.org/10.1007/82_2012_239
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36888-2
Online ISBN: 978-3-642-36889-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)