Published online Sep 6, 2015. doi: 10.5527/wjn.v4.i4.455
Peer-review started: June 23, 2015
First decision: August 4, 2015
Revised: August 24, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 6, 2015
Immunoglobulin A (IgA) nephropathy is one of the most common glomerulonephritis and its frequency is probably underestimated because in most patients the disease has an indolent course and the kidney biopsy is essential for the diagnosis. In the last years its pathogenesis has been better identified even if still now several questions remain to be answered. The genetic wide association studies have allowed to identifying the relevance of genetics and several putative genes have been identified. The genetics has also allowed explaining why some ancestral groups are affected with higher frequency. To date is clear that IgA nephropathy is related to auto antibodies against immunoglobulin A1 (IgA1) with poor O-glycosylation. The role of mucosal infections is confirmed, but which are the pathogens involved and which is the role of Toll-like receptor polymorphism is less clear. Similarly to date whether the disease is due to the circulating immunocomplexes deposition on the mesangium or whether the antigen is already present on the mesangial cell as a “lanthanic” deposition remains to be clarified. Finally also the link between the mesangial and the podocyte injury and the tubulointerstitial scarring, as well as the mechanisms involved need to be better clarified.
Core tip: For few glomerular diseases a new pathogenetic pathway has been recognized in the recent years as happened for the immunoglobulin A (IgA) nephropathy. Finding in the genetics allowed identifying several loci putative for the disease progression. Spectrometry mass studies and 3 dimension studies have allowed bettering clarifying the molecules involved at glomerular level. Molecular studies of the mesangium allowed identifying new receptors responsible for the IgA immune complexes deposition and for the binding to the mesangial structure. Finally molecular and cellular studies opened new ways to understanding the link and the cross-talk between the glomeruli and the tubulointerstitial structure.
- Citation: Salvadori M, Rosso G. Update on immunoglobulin A nephropathy, Part I: Pathophysiology. World J Nephrol 2015; 4(4): 455-467
- URL: https://www.wjgnet.com/2220-6124/full/v4/i4/455.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i4.455
The immunoglobulin A (IgA) nephropathy (IgAN) is represented by a proliferation of glomerular mesangial cells jointed with the presence of IgA deposition in the mesangial area. The IgAN represents the most frequent glomerulonephritis and is represented by extremely different clinical signs and histopathologic features[1]. Because of the typical histopathological and immunological picture and the heterogeneous clinical aspects, the diagnosis of IgAN is principally based on the pathologic picture at the renal biopsy. As a consequence, the IgAN frequency is probably underestimated and the disease is the most prevalent glomerulonephritis in those countries where renal histology is more frequently used in the diagnostic algorithm. Its estimated frequency is approximately 2.5 cases/year per 100000 adults[2]. IgAN is worldwide diffused even if with different frequencies. IgAN has prevalence for male sex in the Caucasian race[3-5]. It is more frequent in some races (Asians, Hispanics, whites, American Indians) while is less frequent in other races as African and American blacks[6]. Recently new epidemiological findings, supported by the genetics, have allowed confirming that the disease incidence varies greatly according the geographical location. Indeed the IgA nephropathy is found in up to 40% of native kidney biopsies in Eastern Asia, but in less than 5% of native biopsies in central Africa[7]. In addition to the different criteria in performing renal biopsy, genetics is thought to play a significant role in explaining some geographical differences[8]. In addition, a diagnostic difficulty is represented by the huge difference in clinical presentation. Indeed, clinically, IgAN may present an asymptomatic course. Such patients are occasionally diagnosed during the work up for other diseases as hypertension or reduced glomerular filtration rate (GFR). Other patients present with macroscopic hematuria, often related to upper respiratory tract infections. In addition, some patients may present with rapidly progressive disease. Generally, the progression of the disease is related to the presence of well-known clinical risk factors that impact on the evolution of most glomerulonephritis as hypertension, proteinuria above 1 g/d, impaired renal function, smoking and obesity[9-11].
It is now clear that IgAN may be present in subjects apparently health as documented by biopsies of kidneys suitable for transplantation, or by data of autopsies not related to renal diseases[12,13]. This is further documented by the fact that most persons affected by IgAN may have a benign course or spontaneous resolution as documented by subjects followed for 10 years after diagnosis in China and in Spain[14,15]. Moreover, after transplantation of an IgAN kidney into a non IgAN recipient, disappearance of the glomerular changes has been documented, suggesting that the defect leading to IgAN is not related to the kidneys[16]. In addition, the high recurrence rates following kidney transplantation confirm that the key pathogenetic alteration in IgAN might reside outside the kidney[17].
As aforementioned, the IgAN is characterized by mesangioproliferative changes in the glomeruli with typical IgA deposits in the mesangial area. Deposits of IgG and C3 are also frequently present. The glomerular IgAs eluted from biopsies of patients affected by IgAN belong almost always to the IgA1 subclass and are principally polymeric. Interestingly, they are poorly glycosylated. In particular, these abnormal IgA1s exhibit a defect of galactose molecules that are normally linked to O-glycans in the hinge region. Defective glycosylated IgA1s exhibit higher blood levels in patients affected by IgAN than in normal subjects. However, this high circulatory level of galactose-deficient IgA1s (Gd-IgA1) per se is not able to determine the renal disease. Different steps or processes are needed for the clinical development and manifestation of the IgAN. New findings concerning these processes have been recently discovered, they are under the genetic control and genetics and immunobiology of IgAN are strictly linked[7,18].
A genome-wide association study (GWAS) performed by Gharavi et al[19] recently found five susceptibility loci for IgAN and allowed to identify the molecules responsible for the above mentioned steps.
These are represented by: (1) abnormal IgA1 glycosylation; (2) antibody production towards the abnormal IgA1; (3) binding of the anti-glycan antibodies to the abnormal IgA1 and consequent production of immune-complexes; and (4) deposition of the immune-complexes in the mesangial area and induction of the renal damage.
After the GWAS finding already mentioned[19], more recently a GWAS again has identified more candidate genes offering new views on the hits involved into the IgAN pathogenesis. The hits involved are represented by the antigen elaboration and presentation, the immunity mucosa-related, and the complement activation.
The GWAS mentioned identified three different candidate loci that might impact on this pathway. They all have been identified on chromosome 6p21, are located on major histocompatibility complex (MHC) and are called: HLA-DRB1/DQB1, HLA-DPB1/DPB2 and TAP1/PSMB9.
A strength link was identified in a different HLA region that includes the HLA-DRB1-DQA1 genes[19]. The same region had been previously associated with several autoimmune diseases[20-27]. Another MHC locus has been found in the region that includes the HLA-DPA1, BPB1 and DPB2 genes.
Immunity mucosa-related: The clinical association of hematuria and infective episodes related to mucosal sites allowed to suspect that abnormalities in the IgA production might be responsible for the IgAN[28].
GWAS identified three loci involved in the mucosal pathogenesis of the IgAN. A locus is located on chromosome 17p13. This locus contains the gene TNFS 13 that codes a proliferation-inducing ligand (APRIL). APRIL might determine the proliferation of IgA-producing cells[29,30] and APRIL serum levels may be higher in subjects affected by IgAN[31].
A second locus on chromosome 22q12 affects the circulatory IgA load and the susceptibility to develop IgAN[19]. This locus includes several genes among which the genes LIF and OSM, that encode cytokines[32]. The cytokines encoded by these genes belong to the interleukin 6 (IL-6) family and influence the immunoregulation[33,34].
On the DEFA gene cluster located on the chromosome 8p23, another locus related to IgAN has been identified. It encodes the small peptides secreted by the mucosal cells with antimicrobial properties called α-defensin[35]. While α-defensin 1, 3 and 4 are secreted by neutrophil, α-defensin 5 and 6 are secreted into the gut by the Paneth cells.
On chromosome 1q32 is located the locus that contains the gene encoding complement factor H (CFH). GWAS found that the deletion of two CFH related genes (CFHR3 and CFHR1) is a protective genetic factor for IgAN[19].
Indeed, the deletion of CFHR3 and CFHR1 is associated with the lacking of their products and CFHR1 is a competitive antagonist of CFH in regulating the complement activity[36]. As a consequence, the association of elevated CFH levels with the absence or low levels of CFHR1 determines a strong inhibition of complement activation. In addition, the relationship between mesangial C3 deposits and different CFH, CFH and CFHR1 levels suggests that these proteins are related to the pathogenic IgA-IC deposition[37].
In addition to the above mentioned pathways, recently performing a GWAS in 20612 patients affected by IgAN, other relevant possible genes have been found; four in ITGAM-ITGAX, VAV3 and CARD 9 and two in the HLA-DQB1 and DEFA loci. Most loci carry genetic risk correlation with local intestinal pathogens, supporting the possibility that host pathogens might favor the IgAN in genetically predisposed patients[38]. All the candidate genes and their function are summarized in Table 1.
| Genetic locus | Genes | Function |
| 6q21 | HLA-DRB1, HLADQA1 and HLA-DQB1 | Class II major histocompatibility complex |
| PSMB8/9 and TAP/2 | Regulators for antigen processing and presentation | |
| 1q32 | CFHR1/3 | Modulators for complement activation and inflammation |
| 22q12 | HORMAD2 | Unknown |
| 17q13 | TNFSF13 | Important for B cell development and IgA isotype switching |
| 8p23 | DEFA1 | Encoding a-defensins as a type of endogenous antimicrobial mediators |
| 1p13 | VAV3 | Regulators for lymphocyte development and antigen presentation |
| 9q34 | CARD9 | Participant in antigen-induced signalosome formation (CARD9-BCL 10-MALT1) and NF-κB activation |
| 16p11 | ITGAM and ITGAX | Mediators for immune cell adhesion and phagocytosis |
Several authors[7,18] have formulated the so called “four hits” pathogenesis of the IgAN. According several studies the IgAN pathogenesis is multivariate and implies the co-existence of several factors. Indeed, after an increase in galactose-deficient circulating IgA1 (Gd-IgA1), an antibody production against these IgA1 is essential for the disease initiation. Later on IC are formed that may deposit in the kidney and activate an inflammatory response (Table 2).
| Hit | Pathogenic process | Putative environmental factors involved | Putative genetic factors involved | Potential clinical biomarkers | Potential novel therapeutic approaches |
| 1 | Hereditary increase in circulating galactose-deficient IgA1 | Potential role of mucosal exposure to infectious of dietary antigens | Strong evidence for high heritability of serum galactose-deficient IgA1 level Potential role of chromosome 22q12.2 | Serum galactose-deficient IgA1 level (HAA-based ELISA) | Suppression of synthesis of galactose-deficient IgA1 Enzymatic boost of galactose transfer to IgA1 hinge- region O-glycans Suppression of sialylation of galactose-deficient O-glycans |
| 2 | Circulating antibody directed against galactose-deficient IgA1 | Potential role of mucosal exposure to infectious or dietary antigens | Potential role of three MHC-II loci in antigen presentation and humoral response to galactose-deficient IgA1 O-glycans | Serum anti-glycan antibodies (dot blot assay) | Alteration of processing and presentation of galactose-deficient IgA1 O-glycopeptides Specific B-cell depletion therapy |
| 3 | Formation of pathogenic IgA1-containing immune complexes | Unknown | Unknown | Circulating and/or urinary immune complexes | Competitive blockade of immune complex formation by non-cross-linking anti-glycan antibodies or specific glycopeptides |
| 4 | Mesangial deposition of IgA1 containing immune complexes, cell activation and initiation of glomerular injury | Unknown | Protective effect of common deletion in CFHR1 and CFHR3 | Circulating and/or urinary complement degradation products, or novel markers of glomerular injury | Suppression of the alternative complement pathway Targeted CFHR1/3 depletion Blocking mesangial cell signaling induced by nephritogenic IgA1-containing immune complexes |
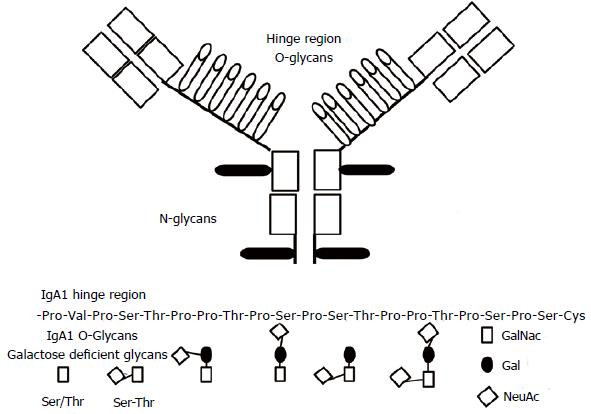
The IgA1 hinge region is located between the constant region domains CH1 and CH2 of the α 1 heavy chains. This region contains O-glycosylation sites composed of serine/threonine (Ser/Thr) and Proline residues. In normal condition only some of these sites are glycosylated[39,40]. A key role in the IgA O-glycosylation is exerted by core 1 β 1, 3 galactosyltransferase (C1β3Gal-T) and its molecular chaperone core 1-1-phosphateuridyltransferase (Gal-T)-specific chaperone (Cosmc). Patients affected by IgAN have more elevated blood levels of IgA1 with the O-glycans poorly galactosylated and having either GalNac as terminal molecule or GalNac containing the secretory J component (Figure 1)[41]. A recent study, using cell lines from a human B-lymphoma documented that the T 2 helper cytokine IL-4 may control the glycosylation of the IgA[42]. Indeed, the IL-4 stimulation decreased the messenger RNA (mRNA) levels of both core the enzyme (C1β3Gal-T) and its molecular chaperone. Studies on animals confirmed the relevance of cytokines on IgA glycosylation[43,44]. O-glycan specific lectin initially allowed identifying the defective IgA1 O-glycosylation in IgAN[45]. More recently, other techniques as the liquid chromatography and the 3D mass spectrometry have allowed us to an improved understanding of the O-glycosylation process and the molecules involved[46,47].
The origin of the poor galactosylated IgA1s is still a not resolved question. Several studies have documented a significant difference in IgA1s generated in the systemic compartment with respect to the IgA1s generated on the mucosal surface[48]. Mucosal IgAs are predominantly polymeric (pIgA), while systemic IgAs are monomeric. Interestingly, in the IgAN the pathogenic IgA immune complexes (IgA-IC) principally contain poor galactosylated pIgA with the J secretory component[49].
The high serum level of mucosal-type IgAs in the IgAN patients might suggest that mucosal sites are the origin of poor galactosylated IgA1s. In contrast, in IgAN either systemic pIgAs directed against antigens typical of the mucosa and systemic pIgA plasma cells in systemic sites have been described[50,51]. Hence the hypothesis that the overproduction in the serum of poor galactosylated IgA1 might be the result of the movement of mucosal IgA1 committed B cells from the mucosa to the systemic compartment. A mucosal B cell mis-homing to systemic sites is the most likely mechanism[52].
The abnormal activity of Toll-like receptors (TLRs) might be another factor that contributes to the increased response to mucosal antigens in IgAN. Indeed, the association of increased Toll-like receptor 4 in circulating cells and signs of renal diseases have been reported by several studies[53]. Other studies examining the single nucleotide polymorphism, found an association between the TLR-9 polymorphism and the IgAN progression. This suggests that the involvement of TLR-9/MyD88 might exert its effect over the progression of IgAN[54].
The synthesis of abnormally glycosylated IgAs is not “per se” enough to justify the mesangial lesions that characterize the IgAN. Indeed, comparing the IgA glycosylation of IgAs eluted from serum with those eluted from biopsy specimens we may observe that Gd-IgAs eluted from kidney biopsies are less glycosylated when compared to the glycosylation rate of serum IgAs from the same IgAN patients[55,56]. This fact highlights a GdIgA1 tropism for the mesangium which might contribute to explain the recurrence of IgA deposits on kidney allograft. Moreover, a study documented that in families affected by IgAN an abnormal glycosylation may be present both in IgAN patients and in asymptomatic relatives[57]. The latter observation confirms that the presence of abnormally glycosylated IgAs does not “per se” justify the mesangial lesions and that other factors should be associated.
Recent studies suggest that auto antibodies recognizing the abnormally glycosylated IgA1s are essential in the pathogenesis of the disease[58,59]. These findings document that IgAN is an autoimmune disease due to the mesangial deposition of immunocomplexes containing GdpIgA1. Other molecules such as sCD89, fibronectin, collagen and laminin are also found in IgA1 containing immune complexes, even if their role remains to be determined[60].
Circulatory auto antibodies (IgG and/or IgA) bind to Gd-IgA1s and form large pathogenetic immune complex[61]. A better understanding of these antibodies is provided from an elegant experiment that used Epstein Barr virus (EBV-immortalized) lymphocytes from subjects affected by IgAN[62]. These B cells are able to product IgG that bind Gd-IgA1 and a subsequent analysis of the cloned chains of these IgG auto antibodies identified as their unique feature the complementary-determining region (CDR) 3 of the heavy chains[61]. In particular the third portion in CD3 is typically serine in patients with IgAN. Whether bone marrow or mucosal tissues are at the origin of IgA1s in circulating immune complexes is still now a matter of controversy. The acute onset of the disease is often accompanied by a concurrent infection of the upper respiratory tract and in this site cells are present able to produce polymeric IgA1s, typical of the pathogen IgA[29]. However, in other studies, polymeric IgA1s and J chain producing cells have been found in the bone marrow of subjects affected by IgAN[63-65]. The aforementioned mis-homing with transposition of plasma cells from the mucosa to systemic sites might explain this finding[52].
Recently Barratt et al[66,67] postulated the so called hypothesis of the “right antibodies in the wrong place at the wrong time”. According this hypothesis, the “right” antigen represented by the mucosal derived Gd-IgA is in the systemic compartment that is the wrong place. Later on, when a large quantity of Gd-IgA1 is in circulation, a large quantity of the right antibody anti Gd-IgA1 is secreted at the wrong time.
The stimuli leading to the production of these auto antibodies remains to be explained.
A hypothesis might be that these antibodies are secreted against pathogen cell surface GalNac-containing glycoconiugates cross-reacting with GdIgA1, realizing a molecular mimicry[68]. A prevalence of IgA1 autoantibody response[61] anti GdIgA1 may justify the fact that some patients have only IgA1 antibody in the glomeruli[69].
In conclusion, a portion of the IgA1 molecules secreted by the plasma cells in patients affected by IgAN is Gal-deficient and is identified by the anti-glycan IgG (or IgA1) antibodies[70]. The formed IC, due to their size, cannot reach and bind the asialoglycoprotein receptor (ASGP-R) in the liver, and be metabolized. Moreover, the terminal GalNac residues, which might interact with the ASGP-R, are covered by specific antibodies that prevent such interaction[71]. As a consequence, a larger fraction of the IgA-IC may reach the glomerular capillaries overlying the mesangium.
Summarizing the two steps above described in the IgA pathogenesis, in the first time a high quantity of under-galactosylated IgA1 is present in the blood. At this regard several points remain to be clarified. The plasma cell defect is inherited or acquired? In addition, to justify the systemic presence of GdIgA1 high quantity, a plasma cell mis-homing from mucosa to systemic sites is needed or not?
In the second step we have the IgG auto antibodies production anti the GdIgA1. At this regard several questions remain to be answered.
Are these antibodies the result of a molecular mimicry triggered by infections?
In addition, are these antibodies the result of a Toll-like receptor polymorphism?
Finally, is there a genetically determination induced by somatic mutations in the IgG heavy chains?
Circulating anti-glycan auto antibodies recognize Gd-IgA1 and pathogenic immune complexes are formed as a consequence. IgA1s must be within an immune complex to activate the mesangial cell proliferation; indeed not complexed GdIgA1s do not stimulate the proliferation of mesangial cells[72-74]. Additional components from serum need to be present to form stimulatory complex[72].
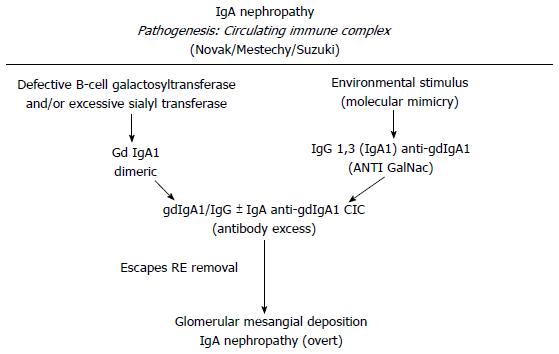
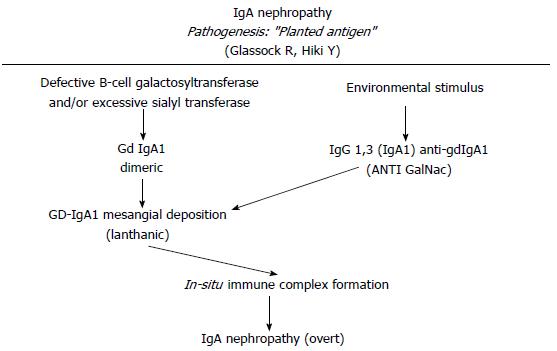
Several models have been proposed to explain the IgA1 immune complex depositions on the mesangial cells. Immune complexes containing IgA1/IgG or IgA could directly deposit on mesangial cell[61,62,75]. Another hypothesis is that poor glycosylated IgA1 might be present in the mesangial area as lanthanic deposits and later newly generated anti-glycan antibodies might bind and realize immune complexes in situ capable to activate mesangial cells[3] (Figures 2 and 3). In addition, a further theory is that self-aggregated Gd-IgA1s might deposit or bind to specific receptors in the mesangial area realizing “planted” depositions that are not pathogenic “per se”. When an exposure to similar environmentally derived antigens occurs, an auto-antibody production and the involvement of several mediators cascade lead to the disease[76,77]. Further studies led to identify the relevance of IgA receptors (IgA-R) in the deposits. Several IgA-Rs have been recognized[78-80]. In the IgAN pathogenesis, two IgA-R expressing cells have been principally involved: (1) mesangial cells which have been documented to be implicated in kidney injury; and (2) myeloid cells (essentially kidney infiltrating macrophages) which have been documented to modulate the extent of the inflammatory response. Studies from several groups have ruled out that the mesangial expression of receptors as ASGP-R, CD 89 and pIgAR might have a role[81]. In addition, although on the mesangial cells is located the Fc alpha mu receptor (Fcα/µR), neither IgM nor the recombinant Fcα/µR inhibit the IgA1 binding to mesangium[82]. The transferrin receptor (TfR1 or CD71) has been identified as the mesangial IgA1 receptor implicated and the pIgA1 binding to TfR1 induces mesangial cells activation[83,84]. Moreover, TfR1 co-localizes with deposited IgA in the IgAN biopsies[85]. The same group documented that both glycosylation and size of IgA1 are relevant factors for the TfR-IgA1 interaction. This probably is the first step of the IgAN injury[86]. Finally, as an alternative hypothesis, has been proposed that the soluble form of the Fcα receptor (sCD89) might “per se” generate complexes with Gd-IgA1[87]. The formation of circulating Gal deficient pIgA1 immune complexes (IgA1-CIC) induces an alteration in the interaction between IgA and CD89 that are found in the mesangial deposits and is implicated in the diseases exacerbation through the activation of pro-inflammatory cytokines and the secretion of chemokines[88,89].
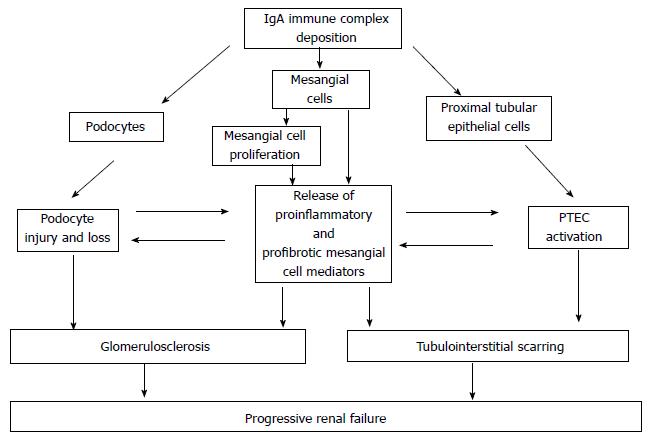
The renal damage after mesangial cell deposition of immune complexes may be distinguished into three phases: (1) mesangial cell activation; (2) podocyte injury; and (3) tubulo-interstitial scarring. All these kinds of renal injuries may be mediated by different pathways as the complement activation, the innate immunity activation, and the non-complement mediators of IgA nephropathy (Figure 4).
Mesangial cells are activated by IgAs and may transform into inflammatory and fibrotic cells after the exposure to IgA-IC. Indeed, the mesangial cells binding to IgA-IC containing poorly galactosylated IgA1 triggers the proliferation and the programmed death of the mesangial cells. In addition a reduced synthesis of vascular endothelial growth factor (VEGF), an abnormal integrin production and an abnormal production of extracellular matrix increase the renal damage[86,90-92].
The role of complement in the activation of the mesangial cells in IgAN has been recently reviewed by Maillard et al[93]. In IgAN, polymeric or aggregated IgA1s, principally Gd-IgA1s, may stimulate the alternative complement pathway, determine the C3 deposition and the production of the complement terminal complex (C5b-C9)[94-97]. Similarly the same pathways might be locally stimulated on the mesangial cells by polymeric Gd-IgA1s as well as by the secretory IgAs (SIgA)[97]. In addition, the complement involvement by the alternative and the mannan binding lectin (MBL) pathways may be activated by the polymeric IgA1, thus participating to the pathogenesis of the IgAN and characterizing a more severe disease.
Components of the innate immunity are similarly involved in the pathogenesis of the IgAN. Some studies have documented that the TLRs are able to induce the IgA production by the B cells[98]. It has also been documented a link between the TLRs stimulation, the overproduction of Gd-IgA1s, the more aggressive aspects of IgAN and the activation of the enzyme and molecules involved in the IgA glycosylation[54,99,100].
Mesangial injury: IgA1-ICs containing secretory IgA with a high sialic acid content and anionic IgA-IC stimulate mesangial cells, may stimulate the p42/p44 mitogen activated protein kinase, activator protein 1, and NF-kB signal transduction. Similarly other chemokines and cytokines are up-regulated, among which the IL-6, the transforming growth factor β (TGFβ), the tumor necrosis factor α (TNFα) and the monocyte chemo attractant protein (MCP-1)[101-103].
The platelet derived growth factors (PDGFs) are among the growth factors most involved in the mesangial cell proliferation[104]. The PDGFs are five potent mitogens and chemoattractants that play important roles in the mesangio-proliferative diseases, principally in the IgA nephropathy. The PDGF-BB and PDGF receptors (PDGFR-β) are over expressed in the experimental mesangio-proliferative nephritis and in the human IgAN[104].
The pathogen IgA1s are also able to activate the renin-angiotensin-aldosterone system (RAAS) intrarenally[105]. This system too is involved in the IgAN injury.
Finally a protective affect against mesangial cell activation by IgA-IC is exerted by bone morphogenetic protein 7 (BMP-7)[106]. BMP-7 suppresses TNFα induced synthesis of proinflammatory cytokines, and has an anti-fibrotic effect through antagonism of the cellular actions of TGF β[107,108].
Podocyte injury: Podocyte necrosis and detachment from the glomerular basal membrane has been reported in the IgAN and the degree of podocytopenia is closely related to the increasing severity of glomerular lesions[109]. Nephrin is a key component of the podocyte slit diaphragm and is essential for the maintenance of an intact glomerular filtration barrier. Interestingly, nephrin mRNA and extracellular nephrin expression are reduced in the IgAN[110,111]. In addition, evidence from in vitro studies suggests that the podocyte injury in the IgAN is likely to be mediated by both the mesangial cell derived soluble mediators and by the direct contact with filtered IgAs[112,113].
It has also been documented that IgA1-IC in IgAN patients might up regulate the production of CXCL1 and TGF beta from the mesangial cells. CXCL1 and TGF beta might exert a synergistic effect upon the podocytes, inducing podocyte dysfunction and podocyte death[114].
Tubulointerstitial scarring: It has until recently been thought that the mechanisms of the subsequent tubulointerstitial injury were generic and common to all forms of chronic kidney diseases. Recent studies have documented that specific mechanisms are operating in the IgAN.
Among the factors that are up regulated after stimulation of mesangial cells, the TGFβ is the most involved in generating fibrotic tissue related to the cell damage. It is generated by growth factors involved in the connective tissue generation[115,116]. In addition to producing fibrotic tissue, TGFβ also acts favoring the transformation of the tubular cells into a fibrotic phenotype.
With an increasing damage to the perm selective barrier, increasing amounts of high-molecular weight IgA-ICs enter the urine. In IgAN these IgA-ICs are enriched by GdIgA1s that reflects their localization within the mesangium[117]. Therefore the proximal tubule epithelial cells (PTEC) are constantly exposed to filtered IgA-ICs because of the impairment of the glomerular size barrier.
Recent studies suggest that there may be a direct and specific interaction between filtered IgA-ICs, mesangial cell-derived mediators, and PTEC. Indeed, when the mesangial cells are activated by the IgAs, they release mediators, which subsequently lead to up-regulation of angiotensin II production, inflammatory changes and apoptosis of PTECs[118]. Similarly, the mesangial cell derived TNFα is known to activate the tubular cells inducing pro-inflammatory mediators, thus establishing an IgAs specific glomerular-tubular cross-talk[119]. There is also convincing in vitro evidence that the filtered mesangial cell-derived mediators also may determine a proinflammatory and profibrotic transformation of PTEC[118-121]. As a consequence, an IgA specific pathogenetic effects might exist, which further accelerate the progressive lost of renal function. In addition, a recent study documents that[122] IgAN might be associated to the Epithelial-mesenchimal transition and the apoptosis of renal tubular epithelial cells favoring the renal scarring.
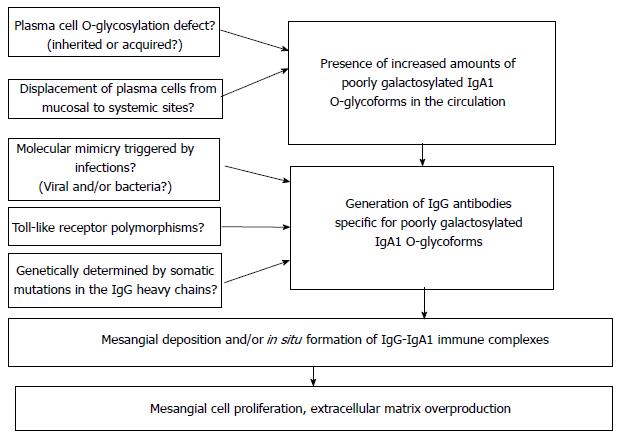
Figure 5 summarizes the four steps along which the IgAN pathogenesis develops. Every step is far to be completely understood. From one side several questions remain to be answered, from the other side new disease specific therapeutic approaches might be opened.
The first step is the presence in circulation of elevated levels of Gd-IgA1. This step is under the control of several putative genetic factors. Still opened questions are whether the IgA under-glycosylation defect is genetically or environmentally generated and whether there is an abnormal plasma cell mis-homing from mucosal to systemic areas.
Second step is represented by the production of auto antibodies against Gd-IgA1. GWAS has identified the possible role of MHC-II loci involved either in the process of antigen elaboration or in the antibody response. Open questions at this regard are whether a molecular mimicry is triggered by infections and which is the role of Toll-like receptors and their polymorphisms. In addition, is not clear whether a somatic mutation genetically determined for the IgG heavy chains structure does exist.
The third step is the production of pathogenic immune complexes containing IgA and their following localization on the mesangium. Open questions are the exact composition of the immune complexes and whether there is a circulating immune complexes deposition or the Gd-IgA1 are already present in the mesangium as a lanthanic deposition followed by binding of auto antibodies.
The fourth step is represented by the renal injury IgA induced and caused by the mesangial IC deposition. The role of podocyte injury in determining the renal lesions is far to be clarified and similarly the tubulointerstitial scarring pathogenesis seems to be peculiar of IgAN, but still not completely clarified.
P- Reviewer: Roberto S, Tanaka H, Watanabe T S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402-2414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 797] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 2. | Floege J, Moura IC, Daha MR. New insights into the pathogenesis of IgA nephropathy. Semin Immunopathol. 2014;36:431-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Glassock RJ. The pathogenesis of IgA nephropathy. Curr Opin Nephrol Hypertens. 2011;20:153-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, Sinclair R, McNeil JJ, Atkins RC. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16:1364-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 6. | Julian BA, Wyatt RJ, Waldo FB, Koopman WJ, Jackson S, Schrohenloher RE, Galla JH, Mestecky J, Czerkinsky C. Immunological studies of IgA nephropathy: familial and racial aspects. Adv Exp Med Biol. 1987;216B:1489-1498. [PubMed] [Cited in This Article: ] |
| 7. | Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124:2325-2332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Novak J, Rizk D, Takahashi K, Zhang X, Bian C, Ueda H, Ueda Y, Reily C, Lai L, Hao . New insights into the pathogenesis of IgA nephropathy. Kidney Dis. 2015;1:8-18. [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Bonnet F, Deprele C, Sassolas A, Moulin P, Alamartine E, Berthezène F, Berthoux F. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Orth SR, Stöckmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, Rambausek M, Roccatello D, Schäfer K. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Waldherr R, Rambausek M, Duncker WD, Ritz E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant. 1989;4:943-946. [PubMed] [Cited in This Article: ] |
| 13. | Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286-2294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Szeto CC, Lai FM, To KF, Wong TY, Chow KM, Choi PC, Lui SF, Li PK. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med. 2001;110:434-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, Olea T, Martínez-Ara J, Segarra A, Bernis C. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol. 2012;23:1753-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Floege J. Recurrent IgA nephropathy after renal transplantation. Semin Nephrol. 2004;24:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Berger J, Yaneva H, Nabarra B, Barbanel C. Recurrence of mesangial deposition of IgA after renal transplantation. Kidney Int. 1975;7:232-241. [PubMed] [Cited in This Article: ] |
| 18. | Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 19. | Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 458] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 20. | Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7131] [Cited by in F6Publishing: 6966] [Article Influence: 409.8] [Reference Citation Analysis (0)] |
| 21. | Radstake TR, Gorlova O, Rueda B, Martin JE, Alizadeh BZ, Palomino-Morales R, Coenen MJ, Vonk MC, Voskuyl AE, Schuerwegh AJ. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet. 2010;42:426-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 22. | Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 23. | Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, Yuan GY, Li CG, Xue LQ, Shen M. A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet. 2011;43:897-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Ferreira RC, Pan-Hammarström Q, Graham RR, Gateva V, Fontán G, Lee AT, Ortmann W, Urcelay E, Fernández-Arquero M, Núñez C. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency. Nat Genet. 2010;42:777-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Yang W, Tang H, Zhang Y, Tang X, Zhang J, Sun L, Yang J, Cui Y, Zhang L, Hirankarn N. Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1, and ARID5B as associated with systemic lupus erythematosus in Asians. Am J Hum Genet. 2013;92:41-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1265] [Cited by in F6Publishing: 1203] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 27. | Okada Y, Yamazaki K, Umeno J, Takahashi A, Kumasaka N, Ashikawa K, Aoi T, Takazoe M, Matsui T, Hirano A. HLA-Cw*1202-B*5201-DRB1*1502 haplotype increases risk for ulcerative colitis but reduces risk for Crohn’s disease. Gastroenterology. 2011;141:864-871.e1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Béné MC, Faure GC. Mesangial IgA in IgA nephropathy arises from the mucosa. Am J Kidney Dis. 1988;12:406-409. [PubMed] [Cited in This Article: ] |
| 29. | Stein JV, López-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodríguez D, Gómez-Caro R, De Jong J, Martínez-A C, Medema JP, Hahne M. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812-826. [PubMed] [Cited in This Article: ] |
| 31. | McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121:3991-4002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791-1797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 33. | Rockman SP, Demmler K, Roczo N, Cosgriff A, Phillips WA, Thomas RJ, Whitehead RH. Expression of interleukin-6, leukemia inhibitory factor and their receptors by colonic epithelium and pericryptal fibroblasts. J Gastroenterol Hepatol. 2001;16:991-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Guimbaud R, Abitbol V, Bertrand V, Quartier G, Chauvelot-Moachon L, Giroud J, Couturier D, Chaussade DC. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur Cytokine Netw. 1998;9:607-612. [PubMed] [Cited in This Article: ] |
| 35. | Lehrer RI, Lu W. α-Defensins in human innate immunity. Immunol Rev. 2012;245:84-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 36. | Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci USA. 2013;110:4685-4690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 37. | Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J Am Soc Nephrol. 2015;26:1195-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 402] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 39. | Renfrow MB, Mackay CL, Chalmers MJ, Julian BA, Mestecky J, Kilian M, Poulsen K, Emmett MR, Marshall AG, Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem. 2007;389:1397-1407. [PubMed] [Cited in This Article: ] |
| 40. | Takahashi K, Smith AD, Poulsen K, Kilian M, Julian BA, Mestecky J, Novak J, Renfrow MB. Naturally occurring structural isomers in serum IgA1 o-glycosylation. J Proteome Res. 2012;11:692-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | de Wolff JF, Dickinson SJ, Smith AC, Molyneux K, Feehally J, Simon A, Barratt J. Abnormal IgD and IgA1 O-glycosylation in hyperimmunoglobulinaemia D and periodic fever syndrome. Clin Exp Med. 2009;9:291-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Yamada K, Kobayashi N, Ikeda T, Suzuki Y, Tsuge T, Horikoshi S, Emancipator SN, Tomino Y. Down-regulation of core 1 beta1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol Dial Transplant. 2010;25:3890-3897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Kobayashi I, Nogaki F, Kusano H, Ono T, Miyawaki S, Yoshida H, Muso E. Interleukin-12 alters the physicochemical characteristics of serum and glomerular IgA and modifies glycosylation in a ddY mouse strain having high IgA levels. Nephrol Dial Transplant. 2002;17:2108-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327-2333. [PubMed] [Cited in This Article: ] |
| 45. | Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598-2604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | Smith AC, Molyneux K, Feehally J, Barratt J. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol. 2006;17:3520-3528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28:78-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | van den Wall Bake AW, Daha MR, Haaijman JJ, Radl J, van der Ark A, van Es LA. Elevated production of polymeric and monomeric IgA1 by the bone marrow in IgA nephropathy. Kidney Int. 1989;35:1400-1404. [PubMed] [Cited in This Article: ] |
| 51. | Barratt J, Bailey EM, Buck KS, Mailley J, Moayyedi P, Feehally J, Turney JH, Crabtree JE, Allen AC. Exaggerated systemic antibody response to mucosal Helicobacter pylori infection in IgA nephropathy. Am J Kidney Dis. 1999;33:1049-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Barratt J, Feehally J. Primary IgA nephropathy: new insights into pathogenesis. Semin Nephrol. 2011;31:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, Loiacono E, Vatrano S, Rollino C, Sepe V, Rampino T. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol. 2010;159:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 54. | Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, Kanou T, Tsukaguchi H, Novak J, Horikoshi S. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 56. | Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 57. | Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 59. | Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23:1579-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 60. | Monteiro RC, Moura IC, Launay P, Tsuge T, Haddad E, Benhamou M, Cooper MD, Arcos-Fajardo M. Pathogenic significance of IgA receptor interactions in IgA nephropathy. Trends Mol Med. 2002;8:464-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73-81. [PubMed] [Cited in This Article: ] |
| 62. | Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 63. | Harper SJ, Allen AC, Layward L, Hattersley J, Veitch PS, Feehally J. Increased immunoglobulin A and immunoglobulin A1 cells in bone marrow trephine biopsy specimens in immunoglobulin A nephropathy. Am J Kidney Dis. 1994;24:888-892. [PubMed] [Cited in This Article: ] |
| 64. | Harper SJ, Feehally J. The pathogenic role of immunoglobulin A polymers in immunoglobulin A nephropathy. Nephron. 1993;65:337-345. [PubMed] [Cited in This Article: ] |
| 65. | Harper SJ, Pringle JH, Gillies A, Allen AC, Layward L, Feehally J, Lauder I. Simultaneous in situ hybridisation of native mRNA and immunoglobulin detection by conventional immunofluorescence in paraffin wax embedded sections. J Clin Pathol. 1992;45:114-119. [PubMed] [Cited in This Article: ] |
| 66. | Barratt J, Eitner F, Feehally J, Floege J. Immune complex formation in IgA nephropathy: a case of the ‘right’ antibodies in the ‘wrong’ place at the ‘wrong’ time? Nephrol Dial Transplant. 2009;24:3620-3623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Barratt J, Smith AC, Molyneux K, Feehally J. Immunopathogenesis of IgAN. Semin Immunopathol. 2007;29:427-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Agmon-Levin N, Blank M, Paz Z, Shoenfeld Y. Molecular mimicry in systemic lupus erythematosus. Lupus. 2009;18:1181-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Bellur SS, Troyanov S, Cook HT, Roberts IS. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533-2536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Mestecky J, Novak J, Julian BA, Tomana M Pathogenetic potential of galactose-deficient IgA1 in IgA nephropathy. Nephrology. 2002;7:S92-S99. [Cited in This Article: ] |
| 71. | Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J. Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res. 2008;31:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Yanagihara T, Brown R, Hall S, Moldoveanu Z, Goepfert A, Tomana M, Julian BA, Mestecky J, Novak J. In vitro-generated immune complexes containing galactose-deficient IgA1 stimulate proliferation of mesangial cells. Results Immunol. 2012;2:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant. 2011;26:3451-3457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171-179. [PubMed] [Cited in This Article: ] |
| 76. | Glassock RJ. Future prospects for IgA nephropathy. Recent advances in IgA nephropathy. Singapore: World Scientific 2009; 403-412. [Cited in This Article: ] |
| 77. | Glassock RJ. Analyzing antibody activity in IgA nephropathy. J Clin Invest. 2009;119:1450-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 376] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 79. | Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Diana J, Moura IC, Vaugier C, Gestin A, Tissandie E, Beaudoin L, Corthésy B, Hocini H, Lehuen A, Monteiro RC. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol. 2013;191:2335-2343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Leung JC, Tsang AW, Chan DT, Lai KN. Absence of CD89, polymeric immunoglobulin receptor, and asialoglycoprotein receptor on human mesangial cells. J Am Soc Nephrol. 2000;11:241-249. [PubMed] [Cited in This Article: ] |
| 82. | McDonald KJ, Cameron AJ, Allen JM, Jardine AG. Expression of Fc alpha/mu receptor by human mesangial cells: a candidate receptor for immune complex deposition in IgA nephropathy. Biochem Biophys Res Commun. 2002;290:438-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Moura IC, Benhamou M, Launay P, Vrtovsnik F, Blank U, Monteiro RC. The glomerular response to IgA deposition in IgA nephropathy. Semin Nephrol. 2008;28:88-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, Monteiro RC. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194:417-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Haddad E, Moura IC, Arcos-Fajardo M, Macher MA, Baudouin V, Alberti C, Loirat C, Monteiro RC, Peuchmaur M. Enhanced expression of the CD71 mesangial IgA1 receptor in Berger disease and Henoch-Schönlein nephritis: association between CD71 expression and IgA deposits. J Am Soc Nephrol. 2003;14:327-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol. 2004;15:622-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Monteiro RC. Pathogenic role of IgA receptors in IgA nephropathy. Contrib Nephrol. 2007;157:64-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 88. | Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 89. | Monteiro RC. The role of IgA and IgA Fc receptors as anti-inflammatory agents. J Clin Immunol. 2010;30 Suppl 1:S61-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Amore A, Cirina P, Conti G, Brusa P, Peruzzi L, Coppo R. Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol. 2001;12:1862-1871. [PubMed] [Cited in This Article: ] |
| 91. | Amore A, Conti G, Cirina P, Peruzzi L, Alpa M, Bussolino F, Coppo R. Aberrantly glycosylated IgA molecules downregulate the synthesis and secretion of vascular endothelial growth factor in human mesangial cells. Am J Kidney Dis. 2000;36:1242-1252. [PubMed] [Cited in This Article: ] |
| 92. | Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M. Interactions of human mesangial cells with IgA and IgA-containing immune complexes. Kidney Int. 2002;62:465-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 93. | Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26:1503-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 94. | Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276-J286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 95. | Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 303] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 96. | Oortwijn BD, Eijgenraam JW, Rastaldi MP, Roos A, Daha MR, van Kooten C. The role of secretory IgA and complement in IgA nephropathy. Semin Nephrol. 2008;28:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 97. | Stangou M, Alexopoulos E, Pantzaki A, Leonstini M, Memmos D. C5b-9 glomerular deposition and tubular alpha3beta1-integrin expression are implicated in the development of chronic lesions and predict renal function outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol. 2008;42:373-380. [PubMed] [Cited in This Article: ] |
| 98. | Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R. Innate immunity and IgA nephropathy. J Nephrol. 2010;23:626-632. [PubMed] [Cited in This Article: ] |
| 99. | Qin W, Zhong X, Fan JM, Zhang YJ, Liu XR, Ma XY. External suppression causes the low expression of the Cosmc gene in IgA nephropathy. Nephrol Dial Transplant. 2008;23:1608-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 100. | Eijgenraam JW, Woltman AM, Kamerling SW, Briere F, de Fijter JW, Daha MR, van Kooten C. Dendritic cells of IgA nephropathy patients have an impaired capacity to induce IgA production in naïve B cells. Kidney Int. 2005;68:1604-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 101. | Oortwijn BD, van der Boog PJ, Roos A, van der Geest RN, de Fijter JW, Daha MR, van Kooten C. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 2006;69:1131-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Leung JC, Tang SC, Chan LY, Tsang AW, Lan HY, Lai KN. Polymeric IgA increases the synthesis of macrophage migration inhibitory factor by human mesangial cells in IgA nephropathy. Nephrol Dial Transplant. 2003;18:36-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Lai KN, Tang SC, Guh JY, Chuang TD, Lam MF, Chan LY, Tsang AW, Leung JC. Polymeric IgA1 from patients with IgA nephropathy upregulates transforming growth factor-beta synthesis and signal transduction in human mesangial cells via the renin-angiotensin system. J Am Soc Nephrol. 2003;14:3127-3137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant. 2014;29 Suppl 1:i45-i54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Lai KN, Leung JCK. Renin-angiotensin system. Recent advances in IgA nephropathy. Singapore: World Scientific 2009; 289-308. [Cited in This Article: ] |
| 106. | Chan WL, Leung JC, Chan LY, Tam KY, Tang SC, Lai KN. BMP-7 protects mesangial cells from injury by polymeric IgA. Kidney Int. 2008;74:1026-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 108. | Wang S, Hirschberg R. BMP7 antagonizes TGF-beta -dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol. 2003;284:F1006-F1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 109. | Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475-1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 110. | Gagliardini E, Benigni A, Tomasoni S, Abbate M, Kalluri R, Remuzzi G. Targeted downregulation of extracellular nephrin in human IgA nephropathy. Am J Nephrol. 2003;23:277-286. [PubMed] [Cited in This Article: ] |
| 111. | Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 112. | Lai KN, Leung JC, Chan LY, Saleem MA, Mathieson PW, Lai FM, Tang SC. Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol. 2008;294:F945-F955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Wang C, Ye Z, Peng H, Tang H, Liu X, Chen Z, Yu X, Lou T. Effect of aggregated immunoglobulin A1 from immunoglobulin A nephropathy patients on nephrin expression in podocytes. Nephrology (Carlton). 2009;14:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Zhu L, Zhang Q, Shi S, Liu L, Lv J, Zhang H. Synergistic effect of mesangial cell-induced CXCL1 and TGF-β1 in promoting podocyte loss in IgA nephropathy. PLoS One. 2013;8:e73425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Floege J, van Roeyen C, Boor P, Ostendorf T. The role of PDGF-D in mesangioproliferative glomerulonephritis. Contrib Nephrol. 2007;157:153-158. [PubMed] [Cited in This Article: ] |
| 116. | Floege J, Ostendorf T. Cytokines and growth factors. Recent advances in IgA nephropathy. Singapore: World Scientific 2009; 289-308. [Cited in This Article: ] |
| 117. | Matousovic K, Novak J, Yanagihara T, Tomana M, Moldoveanu Z, Kulhavy R, Julian BA, Konecny K, Mestecky J. IgA-containing immune complexes in the urine of IgA nephropathy patients. Nephrol Dial Transplant. 2006;21:2478-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J Am Soc Nephrol. 2005;16:2306-2317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Chan LY, Leung JC, Tsang AW, Tang SC, Lai KN. Activation of tubular epithelial cells by mesangial-derived TNF-alpha: glomerulotubular communication in IgA nephropathy. Kidney Int. 2005;67:602-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 120. | Xiao J, Leung JC, Chan LY, Guo H, Lai KN. Protective effect of peroxisome proliferator-activated receptor-gamma agonists on activated renal proximal tubular epithelial cells in IgA nephropathy. Nephrol Dial Transplant. 2009;24:2067-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Xiao J, Leung JC, Chan LY, Tang SC, Lai KN. Crosstalk between peroxisome proliferator-activated receptor-gamma and angiotensin II in renal tubular epithelial cells in IgA nephropathy. Clin Immunol. 2009;132:266-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Yao J, Ke Z, Wang X, Peng F, Li B, Wu R. Epithelial-mesenchymal transition and apoptosis of renal tubular epithelial cells are associated with disease progression in patients with IgA nephropathy. Mol Med Rep. 2014;10:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |