Published online Nov 27, 2013. doi: 10.5496/wjmg.v3.i4.22
Revised: October 28, 2013
Accepted: November 7, 2013
Published online: November 27, 2013
Head and neck squamous cell carcinoma is the sixth most common cancer in the world with approximately 650000 new cases diagnosed annually. Next-generation molecular techniques and results from phase 2 of the Cancer Genome Atlas becoming available have drastically improved our current knowledge on the genetics basis of head and neck squamous cell carcinoma. New insights and new perspectives on the mutational landscape implicated in head and neck squamous cell carcinoma provide improved tools for prognostication. More importantly, depend on the patient’s tumor subtypes and prognosis, deescalated or more aggressive therapy maybe chosen to achieve greater potency while minimizing the toxicity of therapy. This paper aims to review our current knowledge on the genetic mutations and altered molecular pathways in head and neck squamous cell carcinoma. Some of the most common mutations in head and neck squamous cell carcinoma reported by the cancer genome atlas including TP53, NOTCH1, Rb, CDKN2A, Ras, PIK3CA and EGFR are described here. Additionally, the emerging role of epigenetics and the role of human papilloma virus in head and neck squamous cell carcinoma are also discussed in this review. The molecular pathways, clinical applications, actionable molecular targets and potential therapeutic strategies are highlighted and discussed in details.
Core tip: Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world with approximately 650000 new cases diagnosed annually. Understanding the molecular pathways that are implicated in the pathogenesis of HNSCC enable clinicians to be able to classify and to prognosticate the disease based on subtypes, such as human papilloma virus (HPV)-positive vs HPV-negative HNSCC. More importantly, patients may be placed on de escalated or more aggressive therapies depend on their tumor subtypes and prognosis. This paper aims to review our current knowledge of the most common genetic alterations in HNSCC.
- Citation: Nguyen TK, Iyer NG. Genetic alterations in head and neck squamous cell carcinoma: The next-gen sequencing era. World J Med Genet 2013; 3(4): 22-33
- URL: https://www.wjgnet.com/2220-3184/full/v3/i4/22.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v3.i4.22
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world with approximately 650000 new cases diagnosed annually[1]. Lesions in the head and neck area impair both forms and functions significantly. Surgery, one of the three pillars of treatments, is not only technically demanding but surgery also presents difficult challenges during rehabilitation in the postoperative period. On the other hand, radiotherapy is associated with significant complications and side effects that render the treatments intolerable for many patients. Thus, understanding the molecular pathways that are implicated in the pathogenesis of HNSCC enables clinicians to be able to classify the disease based on subtypes, such as human papilloma virus (HPV)-positive vs HPV-negative HNSCC and to prognosticate better. More importantly, patients may be placed on deescalated or more aggressive therapies depend on their tumor subtypes and prognosis. Moreover, chemotherapy has been useful to prevent recurrence in other cancers such as breast and may become relevant in HNSCC when an actionable target is discovered. The aim of this paper is to review our current knowledge of the molecular pathogenesis of HNSCC- knowledge made available by advanced, next-generation molecular techniques. We also discuss how this growing body of evidence currently shapes research interests and research directions in the quest of finding better treatments for HNSCC.
TP53 encodes for the p53 protein and is widely touted as “the guardian of the genome” due to its master regulatory role in monitoring DNA damage, promoting senescence, inducing cell cycle arrest and apoptosis. Early studies revealed that somatic mutations in TP53 are found in 47% to 70% of HNSCC making TP53 mutations the most commonly mutated genes implicated in HNSCC[2-5]. Smoking and alcohol, two well-known causes of TP53 mutations are the leading risk factors in HNSCC[6,7]. Furthermore, evidence suggests that these mutations occur relatively early in the course of HNSCC development. Premalignant dysplastic lesions for HNSCC such as leukoplakia contain TP53 mutations in as high as 27% of cases[8]. Additionally, the presence of p53 mutations in these premalignant lesions also increases the risk of progression to invasive carcinoma[8,9]. It is important to point out that such precursor lesions indicate a field defects and that both clonal and non-clonal TP53 mutations can be found in macroscopically normal epithelium[5]. In as high as 35% of oral and oropharyngeal carcinomas, the primary tumor is surrounded by mucosal epithelium that contains TP53 mutations[10].
TP53 is not only a significant determining factor in the carcinogenesis but patients with TP53 mutations also have worse prognoses. In a study by Poeta et al[11] HNSCC patients with disruptive TP53 mutations have a decreased survival rate of more than 1.5 fold when compared with TP53 wild-type HNSCC. More specifically, a truncating TP53 mutation is associated with a worse overall survival and progression-free survival[12]. These could be due to several factors. Firstly, disruptive mutations of TP53 can lead to a complete shutdown of intracellular restorative processes. TP53 mutants also disrupt tissue architecture[13], upregulate angiongenesis[14], as well as participate in migration, invasion and metastasis[15,16]. All these factors contribute to a much more aggressive tumor biology. Secondly, TP53 mutants are extremely resistant to treatments. Several prospective trials have shown that patients with TP53 mutations respond poorly to cisplatin and fluorouracil[17,18]. Additionally, field defects with TP53 mutations are found to often present in surgical margins during tumor resection[19]. Retrospective studies have shown that both local recurrence and metachronous primary can arise from within the field cancerization[20,21]. Thus, because of TP53 mutants within the field cancerization, an R0 surgical margin may not result in improved survival. Both primary and adjuvant radiotherapy are found to be less effective in HNSCC patients with TP53 mutations and have a much higher rate of locoregional recurrence and failure rate, respectively[22,23].
Given the significant role of TP53 mutations in HNSCC and associated clinical implications discussed above, considerable efforts have been devoted to explore treatment strategies. This has proven to be challenging due to the wide spectrums of mutation patterns in TP53. Missense mutations in the DNA binding domain of the p53 protein are the most common type of TP53 mutations and account for 50%-70% of mutations[2,3,24]. Other patterns of TP53 mutations have also been described, for example, 16% nonsense, 16% insertion or deletion and 8% splice site mutation in one series[1]. Although most of these mutations are loss-of-function mutations, gain-of-function oncogenic activities associated with TP53 mutations have also been documented. In fact, for almost an entire decade after it was first discovered, TP53 was considered a proto-oncogene[25]. These gain-of-function mechanisms remain poorly understood. Loyo et al[26] suggest that such gain-of-function activities arise from the interactions of defect p53 with other regulatory proteins. Examples of p53 gain-of-function activities are the interactions of p53 with p63/p73[25], ability of TP53 mutants to escape growth arrest induced by v-Ki-ras2 Kirsten rat sarcoma viral oncogene[27], and the ability to promote invasion and metastasis via integrin and EGFR upregulations[15].
Thus, given such complex and paradoxical activities of p53 mutants, several therapeutic strategies have been proposed and tested in clinical trials. One strategy aims to restore wild-type p53 functions in tumor cell. This stems from an important proof-of-principle in several mouse models in which reactivating wild-type p53 functions results in tumor regression[28]. For example, adenovirus gene therapy can reactivate wild-type p53 functions. Clinical trials studying adenoviral based treatment such as Advexin (Introgen Therapeutics Inc., Austin, TX) and ONYX-015 (Onyx Pharmaceuticals Inc., San Francisco, CA) have yielded some positive results in phase I, IIand several pending results in phase III[29,30]. In a trial that assessed response to the treatment of ONYX-015 in combination with cisplatin and 5-fluorouracil, the complete and partial response rates were 27% and 36% respectively. Patients with ONYX-015 injection also have longer time to tumor progression[30]. This trial also yielded some other important findings: (1) patients with documented chemoresistance have objective tumor regression after injection with ONYX-015; and (2) side effects include flu-like symptoms, injection site pain and mucous membrane disease. ONYX-015 has recently been approved for treatment of HNSCC in China[31]. Furthermore, there are other innovative approaches to reactivate wild-type p53. These approaches revolve around our progressively expanding knowledge of the biomechanism within the p53 pathway: by targeting the MDMX-p53 ubiquitination pathway[28], by using proteasome inhibitor bortezomib[32], or by using p53 reactivating molecules such as PRIMA-1[33,34]. Another avenue of approach for therapy in HNSCC patients with TP53 mutations is to target p53 mutants directly. As discussed above, high levels of mutant p53 is critical in tumorigenesis. Thus, molecules that can destabilize or reduce mutant p53 turnover may be beneficial for HNSCC patients with TP53 mutations. Li et al[35,36] have shown that p53 mutants level can be reduced by inhibiting either Hsp90 or HDAC6. This approach maybe quickly translated into clinical use with the availability of Vorinostat (Merck & Co., White House Station, New Jersey), an FDA-approved HDAC6 inhibitor that is widely used for lymphoma and other solid tumors[35].
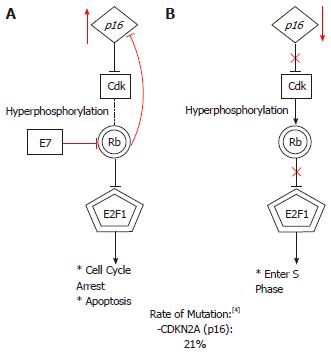
The Retinoblastoma (Rb) protein, located on chromosome 13, is a critical regulatory protein of the G1 checkpoint. The Rb pathway is described schematically in Figure 1. In healthy cells, hypophosphorylated Rb protein forms a complex with the transcription factor E2F to promote G1 arrest. Cells progress to S-phase after hyperphosphorylation of Rb occurs. Thus, epithelial carcinogenesis in HNSCC is thought to arise from excessive hyperphosphorylation of Rb proteins. This event can occur with mutations in several different loci. Rb mutations can happen due to loss of heterozygosity (LOH) or microsatellite instability (MI)[37]. LOH has been implicated in many malignancies, including HNSCC[38,39]. The rate of LOH in Rb gene is as high as 60% in laryngeal SCC in some series whereas MI is reported at 34% in a series of stage 2 laryngeal SCC patients[39]. Although the inactivation of Rb gene product (pRb) due to viral oncogenes in HPV-positive HNSCC is well established, the inactivation of pRb as a result of LOH/MI in HPV-negative HNSCC is much more contentious. Recent evidence suggests that a mutation at one Rb locus is not enough to stop Rb expression and that loss of pRb expression only arises when there are mutations of both Rb alleles[37]. This study also suggests that despite the high frequency of LOH or MI in this series, such presence does not offer additional information to tumor biology or patient prognosis[37]. Rather, a multi-step tumorigenesis in which Rb is an intermediary is a more likely process in vivo.
Cyclin-dependent kinase inhibitor 2A (CDKN2A) at 9p21 locus controls the phosphorylation of Rb. In the presence of CDKN2A, cyclin-dependent kinases CDK4 and CDK6 are prevented from phosphorylating Rb. Without this regulation, Rb-E2F complexes become destabilized due to hyperphosphorylation and progression to S phase proceeds unchecked[40]. The Cancer Genome Atlas (TCGA) reported a mutation rate of 21% in the CDKN2A locus in HNSCC[4]. LOH at the CDKN2A locus is common in premalignant oral lesions such as leukoplakia and can be found in as high as 80% of HNSCC tumors[41-43]. CDKN2A encodes for two tumor suppressors: p16 and p14. In HPV-negative hypopharyngeal and oropharyngeal SCC, p16 downregulation and concurrent cyclin D1 overexpression have been linked with poorer outcome[44,45]. Furthermore, an alternate reading frame p14 has been shown to be associated with a slightly higher risk of developing a second primary malignancy after an index HNSCC[46]. Such recent evidence, together with the feasibility of detecting p14 and p16, have led to increased interest in detecting p14 and p16 as surrogate markers and as prognostication tool in HNSCC[45,46].
Evidence of tumor arrest after transfection of p16 in negative p16 squamous carcinoma cell lines has offered some therapeutic directions[47,48]. The demethylating agent 5-aza-2’-deoxycytidine has been shown to recover p16 expression[49]. Moreover, 5-aza has also been shown to increase the radiosensitivity of HNSCC tumors[50].
The discovery of NOTCH1 as a commonly mutated gene in HNSCC owes much to the availability of next-generation sequencing. NOTCH 1, a large gene of 34 exons, was first shown to be involved in tumorigenesis while studying T-cell leukemias[51]. Further evidence emerged and different patterns of NOTCH1 mutations have been found to be associated with lung cancer, various forms of leukemia and HNSCC[2,52,53]. TCGA reports a mutation rate of 19% for NOTCH1 in HNSCC[4]. Function of NOTCH1 is highly contextual in normal biology as well as in pathology. In normal biology, activation of NOTCH1 causes terminal differentiation in some tissues while performs stem cell maintenance in other tissues[54]. From studies of T-cell lymphoblastic and chronic lymphocytic leukemia, the NOTCH1 pathway is found to be upregulated and thus becomes oncogenic. However, reduced NOTCH1 signaling has been found in HNSCC, suggesting tumor suppressing activities of NOTCH1 in these cell lines[2,3]. Several animal models have also been shown to support this paradoxical biological duality of NOTCH1 in tumorigenesis[55-57]. Unsurprisingly, NOTCH1 mutations in HNSCC are fundamentally distinctive from oncogenic mutations found in other types of cancers. The majority of NOTCH1 mutations in HNSCC were found in N-terminal of the transmembrane region or in the N-terminal EGF-like ligand binding domain; in contrast, oncogenic mutations of NOTCH1 clustered in the heterodimerization domain and the PEST C-terminal domain[2,26]. More importantly, the inactivating mutations of NOTCH1 in HNSCC are strongly implicated as the main driver of tumorigenesis, rather than being simply passenger mutations[26].
The role of TP63 in regulating NOTCH1 adds to the complexity of NOTCH1 expression. TP63 is a p53-related transcription factor that is expressed in keratinocytes of the basal layer and participate in epidermal differentiation and proliferation[58]. In mature epithelium, p63 inhibits NOTCH1 expression[59]. Dysplastic mucosa in the head and neck as well as HNSCC tumors have been shown to harbor cells that overexpress TP63[59,60]. Additionally, besides contributing to tumorigenesis via NOTCH1 suppression, evidence suggests that p63 also plays an intricate role in the interactions with other cell cycle regulators such as p73, p16, and EGFR in solid tumors[60-62].
Compared to other mutations in HNSCC, mutations in NOTCH1 came under investigation only recently. Furthermore, any treatment strategy has to negotiate the complexity of NOTCH1 expression being both oncogene and tumor suppressor. In fact, a recent trial investigating γ-secretase inhibitors (GSI), an agent that can shut down constitutively active NOTCH1 pathway, has to be halted due to serious adverse events of patients developing skin cancer[63]. On the other hand, combining a popular histone deacetylase inhibitors SAHA (suberoylanilide hydroxamic acid) with gene therapy of p63, a potent regulator in the NOTCH1 pathway as discussed above, has shown promising anticancer effect in HNSCC[64]. Thus, whether or not NOTCH1 can be targeted as an actionable target in treating HNSCC needs better understanding of its functional pathway.
In the wide spectrum of mutations found in HNSCC cell line, EGFR has an interesting role. EGFR expression is found to be upregulated in 90% of HNSCC and is associated with a poorer disease presentation: higher stage, increased relapse rate and lower overall survival[65-67]. More importantly, the significance of EGFR biological function in HNSCC is further underlined by the success of cetuximab, the first ever targeted therapy developed for HNSCC[68]. Cetuximab is effective in locally advanced disease when combined with radiotherapy and in recurrent or highly staged disease when combined with cisplatin and 5-fluorouracil[68,69]. Despite the high incidence of EGFR overexpression in HNSCC, it is very rarely mutated[24,26]. In fact, Loeffer-Ragg et al[70] report only one incidence of somatic mutation of the EGFR domain in a series of 100 Caucasian patients. Thus, unlike other types of cancer such as lung cancer, EGFR mutations are not sensitizing mutations for EGFR inhibition.
One current research focus in EGFR targeting therapy is to study its mechanism of resistance to cetuximab. Patients who initially showed responses to cetuximab eventually become refractory to treatment[71,72]. Early evidence suggested that cross-activations of other receptor tyrosine kinase (RTK) pathways such as c-MET, IGFR1 and the Her family members confer to resistance[71,73,74]. This suggests that EGFR inhibition by itself is inadequate. An irreversible, combined EGFR and HER-2 inhibitor, afatinib, has been shown to reverse tumor development in a xenograft SCC model[71]. Thus, besides tyrosine kinase inhibitors (TKIs) that inhibit both EGFR and HER2 such as the aforementioned afatinib, dacomitinib, and lapatinib that are currently used in Her2 positive breast cancers, other Her family receptors inhibitors such as Herceptin may be useful when used together with cetuximab[75]. Dacomitinib, lapatinib and afatinib are currently in phase 1, 2, and 3 trial respectively[76-78]. c-MET is also an attractive target to reduce resistance to EGFR therapy with the recent approval of crizotinib for use in the treatment of lung cancer[79]. Another possible mechanism of resistance to cetuximab is via the expression of EGFR variant III (EGFRvIII) as cetuximab binds with much less affinity to EGFRvIII. This variant presents in approximately 42% of HNSCC and arises due to exon 2-7 iframe deletion that makes it resistant to ubiquination[80]. Investigators have been hopeful that EGFRvIII activation can be blocked by either TKIs or by a newer generation of EGFR mAbs. So far, clinical trials results investigating TKIs such as erlotinib have been perplexing. A retrospective review of four clinical trials failed to identify any benefits from using erlotinib in HNSCC treatment. Interestingly, it led to a paradoxical discovery that EGFRvIII was a surprised biomarker of improved disease control with a caveat that the sample size was small[81]. Successful phase I trials for ABT-806, a next generation of EGFR mAbs, have paved way for some recent phase II trials with pending results[82-84].
Understanding the mechanisms of EGFR resistance in both treatment-naïve and treatment-experienced settings holds the key to unlock many translational opportunities. A phenomenon observed to be highly correlated with cetuximab resistance and worse disease progression is the epithelial-to-mesenchymal transition (EMT)[85]. Interestingly, early evidence suggests that reversing EMT will re-sensitize resistant HNSCC cells to cetuximab and TKIs such as gefitinib[86,87]. Moreover, agents that specifically kill EMT transformed cells such as salinomycin, may be synergistic with cetuximab[88]. Thus, the EGFR pathway remains to be a very promising domain to investigate for the next generation targeted treatment for HNSCC.
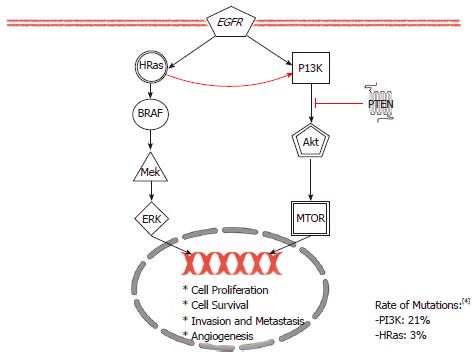
Conflicting early evidence reported a rate of mutations of HRAS gene in 35% of oral cancers from in India but none in the United States[89,90]. Recently, with the availability of deep sequencing technique, HRAS mutations have been shown to be among the most common mutations in HNSCC in the United States with the incidence of 3% to 5%[2-4]. HRAS is the only one of the three Ras genes found to be implicated in HNSCC[91]. These three isoforms of Ras proteins exhibit tissue-specific functions due to differences in the C-termini that determine their lineage-specific roles[92]. Because attempts to directly inhibit the Ras signaling in clinical trials have been disappointing, this review will focus on newer research direction in investigating a major downstream effectors of Ras: the Phosphoinositide-3 kinase (PI3K) pathways[93,94]. As shown in Figure 2, the PI3K pathway is downstream of Ras and is important for cell growth and survival[95].
The PI3K pathway can become over activated by PIK3CA mutations or as shown in Figure 2, by a loss of inhibition from phosphatase and tensin homolog (PTEN), a negative regulator. Two “hot spot” domains in the PIK3CA gene contain activating mutations in 6%-11% of HNSCC[96,97]. On the other hand, LOH of PTEN is as frequent as 40% of HNSCC[98]. Evidence suggests that LOH alone is adequate to drive tumorigenesis[99]. LOH mechanisms can arise from either epigenetic or silencing somatic mutations[100]. Regardless of the how, when the PI3K pathway becomes over activated, many broad downstream effects occur: angiogenesis, increased metabolism, enhanced proliferation and apoptosis inhibition. Akt and its downstream agent mTOR have been implicated in these downstream effects[101-103].
Targeting the PI3K/AKT/mTOR axis has been shown to show some positive responses of tumors to treatments[104]. This pathway can be targeted at multiple different targets for therapy. Currently, a pilot trial investigating the neoadjuvant use of Rapamycin, a known mTOR inhibitor, to treat advanced HNSCC patients is being conducted[105]. Other mTOR inhibitors such as everolimus or temsirolimus are also available for testing. On the other hand, MK2206, an Akt inhibitor, is also being tested in recurrent or metastatic HNSCC patients[106]. Existing PI3K inhibitors such as PX-866 or BKM120 are also being tested alone, or in combination with paclitaxel, docetaxel or cetuximab in recurrent and metastatic HNSCC[107-111]. The basis for trials that investigate PI3K inhibitors together with cetuximab stems from an observation that PI3K amplification may have causes resistance to EGFR inhibition[101].
Deep exome sequencing studies of HNSCC cell lines have found, at considerable frequencies, mutations in several genes that act at an epigenetic level. These genes are MLL2, NSD1 and SYNE1[2,3]. According to TCGA, the rates of mutation for MLL2 and NSD1 in HNSCC are 18% and 11% respectively[4]. Both MLL2 and NSD1 code for histone methyltransferases. Studies that specifically investigate MLL2 and NSD1 in HNSCC are currently lacking. However, MLL2 have been reported to be a major tumor suppressor gene in non-Hodgkin lymphoma and inactivating somatic mutation of MLL2 is indicated as a driver mutation for this malignancy[112]. Other histone modifications enzymes have also been found to be associated with solid human cancers such as renal cell carcinoma (RCC), breast, gastric and colorectal carcinomas[113-115]. Histones acetyltransferases p300/CBP mutations are found in solid and hematological tumors, also suggesting their involvements in critical tumorigenic pathways[116]. A study by Yang et al[117] from China reported histone modification as a major step in the pathogenesis of laryngeal carcinoma. Histone modification enzymes in HNSCC remain to be a new frontier for research.
Besides histone modifications, DNA methylation is another form of epigenetic regulation. DNA methylation blocks transcription factors from binding to initiate transcription complex formation. Furthermore, methylated DNA sequences also have a higher affinity for histone modification enzymes and recruitments of these enzymes induce genes silencing[118,119]. Body of evidence that links abnormal DNA methylation to HNSCC is well established. Many genes that are involved in cell cycle, cell-cell adhesion, migration, angiogenesis and metastasis in HNSCC cell lines have been found to be associated with DNA methylation. Examples of these genes are p15, p16, cyclin A1, RAR-B2, CDKN2A, E-cadherin, DAPK and others[120-124]. Interestingly, DNA hypermethylation is only one side of the story: DNA hypomethylation has also been implicated in laryngeal carcinoma from investigating S100A4. The gene S100A4 has been reported as an important mediator of EMT and metastasis[125-127]. Recent evidence has suggested that S100A4 is also important in the maintenance and development of head and neck cancer-initiating cells (CIC). In this CICs population, S100A4 promoter is hypomethylated[127]. Other supporting evidence for hypomethylation of S100A4 exists as well: demethylating agents induce both S100A4 RNAs and proteins expressions[128]. A possible explanation for this methylation paradox is that when DNA methylation becomes aberrant and an imbalance occurs, global hypomethylation could lead to activation of oncogenes whereas focal hypermethylation can silence tumor suppressor gene.
In the genetic landscape of HNSCC, activating of oncogenes is an exception rather than the norm. In principle, the majority of HNSCC harbored inactivating mutations in tumor suppressor genes. Results of HNSCC treated with agents that modulate epigenetic regulations such as histone deacetylase (HDAC) inhibitors and demethylation agents are encouraging and lend support to this observation. Demethylation treatments have been shown to restore tumor suppressor gene functions, arrest tumor growths, and increase readiosensitivity of HNSCC cells[49,50,129]. Additionally, HDAC inhibitors have also yielded some promising results. Valproic acid (VPA), a relatively weak HDAC inhibitors, have been shown to inhibit both acute and chronic growth of HNSCC cells[130]. VPA has also been shown to improve tumor arrest when used together with a recombinant adenovirus in an HNSCC xenograft mouse model[131]. A phase 2 trial currently evaluates the addition of VPA to standard platinum-based chemoradiation[132]. Another HDAC inhibitor, Vorinostat, is currently in phase 1 for stage III and IV SCC of the oropharynx and in phase 2 for combination with capecitabine in recurrent and metastatic HNSCC[133,134].
Both from a genetic and a clinical perspective, HPV-positive HNSCC is a distinct entity from HPV-negative HNSCC. Most importantly, the overall prognosis of HPV-positive HNSCC is much more favorable than HPV-negative HNSCC[135,136]. This presents an opportunity to carry out de escalated therapies to minimize treatment related toxicities with ongoing trials investigating this strategy[137]. The pathogenesis of HPV is due to viral oncoproteins E6 and E7 inactivating tumor suppressors p53 and Rb. E6 targets p53 and E7 targets Rb, as shown in Figure 1 and cause ubiquitin-dependent protein degradation[138]. Understanding the mechanism of E6 and E7 has led to some important applications. Because E7 degrades Rb, it also leads to an upregulation of p16, an upstream regulator of Rb. Thus, p16 has been used as a biomarker to diagnose HPV-positive HNSCC[138,139]. Moreover, E6 and E7 are appealing molecular targets for therapy. In HPV-positive HNSCC cells lines, short hairpin RNAs that target and suppress E6 and E7 have been shown to restore the level of p53 and Rb[140]. Researches investigating how E6 and E7 can be inhibited in vitro are at early stages. Two strategies currently exist: disruption of E6/E7 binding with its ubiquitin ligase enzyme or blocking the activation of downstream ubiquitin/proteasome systems (UPS)[141,142]. So far, two trials have shown that Bortezomib, an UPS inhibitor, has a very poor response rate in locally recurrent or advanced HNSCC. However, it must be pointed out that the rate of HPV-positive was low in one trial (1 out of 20 tumors) and was not reported in the other trial[143,144]. Thus, further studies and trials are indeed necessary in this area. Immunotherapy for HPV-positive patients with HNSCC is another area of active research. Proof of principle studies, mainly in mouse models, have demonstrated that engaging CD8+ T cell response to target E6/E7-specific antigens have led to tumor eradication[145,146]. In HPV-16-positive oropharyngeal cancers, improved adaptive immunity as measured by CD8 cell counts is associated with a better prognosis[147]. Besides immunotherapy, vaccination is also gaining traction when HPV vaccines are now being recommended by the Center for Disease Control and Prevention for the prevention of anogenital and oropharyngeal cancers in male. Thus, the role of immunotherapy is becoming more and more relevant in HPV-positive HNSCC.
Next-gen sequencing has allowed us to accumulate an unprecedented amount of knowledge about mutations found in HNSCC. In summary, we can make the following observations. Firstly, inactivation of tumor suppressor genes is much more common in HNSCC cells than activation of oncogenes. Secondly, it is unlikely that a single target therapeutic approach will work and patients will benefit more from agents that can target more than one receptor or from combination therapy. Thirdly, mutations in HNSCC are heterogenous with complex interplay between many different molecular pathways at both the genetic and epigenetic levels. In our humble opinion, this heterogeneity should be seen as opportunity rather than obstacle. It seems inevitable that as our knowledge continues to expand and becomes more refined, we will be able to classify HNSCC into subtypes based on the pattern of mutations. By classifying into subtypes, we will be able to improve our ability to diagnosis, stage, and prognosticate. More importantly, we will be able to give therapy with greater potency and less toxicity. Certainly, this is already happening to an extent with HPV-positive and HPV-negative HNSCC. As we identify more biomarkers and invent new therapies to target these biomarkers, the trend in management of HNSCC continues its shift towards a more personalized therapeutic approach.
P- Reviewer: Mandic R S- Editor: Song XX L- Editor: A E- Editor: Liu XM
| 1. | Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1418] [Cited by in F6Publishing: 1318] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 3. | Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1986] [Cited by in F6Publishing: 1886] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 4. | The Cancer Genome Atlas. NCI and the NHGRI. Retrieved 2013-06-01. Available from: http://www.cancergenome.nih.gov/ Retrieved 2013-06-01. [Cited in This Article: ] |
| 5. | Leemans CR, Brakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1786] [Cited by in F6Publishing: 1799] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 6. | Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1341] [Cited by in F6Publishing: 1423] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 7. | Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712-717. [PubMed] [Cited in This Article: ] |
| 8. | Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM, Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53:4477-4480. [PubMed] [Cited in This Article: ] |
| 9. | Ebrahimi M, Boldrup L, Coates PJ, Wahlin YB, Bourdon JC, Nylander K. Expression of novel p53 isoforms in oral lichen planus. Oral Oncol. 2008;44:156-161. [PubMed] [Cited in This Article: ] |
| 10. | Tabor MP, Brakenhoff RH, van Houten VM, Kummer JA, Snel MH, Snijders PJ, Snow GB, Leemans CR, Braakhuis BJ. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532. [PubMed] [Cited in This Article: ] |
| 11. | Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552-2561. [PubMed] [Cited in This Article: ] |
| 12. | Lindenbergh-van der Plas M, Brakenhoff RH, Kuik DJ, Buijze M, Bloemena E, Snijders PJ, Leemans CR, Braakhuis BJ. Prognostic significance of truncating TP53 mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2011;17:3733-3741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 655] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 14. | Fontemaggi G, Dell’Orso S, Trisciuoglio D, Shay T, Melucci E, Fazi F, Terrenato I, Mottolese M, Muti P, Domany E. The execution of the transcriptional axis mutant p53, E2F1 and ID4 promotes tumor neo-angiogenesis. Nat Struct Mol Biol. 2009;16:1086-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 589] [Cited by in F6Publishing: 621] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 16. | Noll JE, Jeffery J, Al-Ejeh F, Kumar R, Khanna KK, Callen DF, Neilsen PM. Mutant p53 drives multinucleation and invasion through a process that is suppressed by ANKRD11. Oncogene. 2012;31:2836-2848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Mandic R, Schamberger CJ, Müller JF, Geyer M, Zhu L, Carey TE, Grénman R, Dünne AA, Werner JA. Reduced cisplatin sensitivity of head and neck squamous cell carcinoma cell lines correlates with mutations affecting the COOH-terminal nuclear localization signal of p53. Clin Cancer Res. 2005;11:6845-6852. [PubMed] [Cited in This Article: ] |
| 18. | Cabelguenne A, Blons H, de Waziers I, Carnot F, Houllier AM, Soussi T, Brasnu D, Beaune P, Laccourreye O, Laurent-Puig P. p53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: a prospective series. J Clin Oncol. 2000;18:1465-1473. [PubMed] [Cited in This Article: ] |
| 19. | Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [PubMed] [Cited in This Article: ] |
| 20. | Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Kummer JA, Leemans CR, Braakhuis BJ. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin Cancer Res. 2004;10:3607-3613. [PubMed] [Cited in This Article: ] |
| 21. | Schaaij-Visser TB, Graveland AP, Gauci S, Braakhuis BJ, Buijze M, Heck AJ, Kuik DJ, Bloemena E, Leemans CR, Slijper M. Differential Proteomics Identifies Protein Biomarkers That Predict Local Relapse of Head and Neck Squamous Cell Carcinomas. Clin Cancer Res. 2009;15:7666-7675. [PubMed] [Cited in This Article: ] |
| 22. | Koch WM, Brennan JA, Zahurak M, Goodman SN, Westra WH, Schwab D, Yoo GH, Lee DJ, Forastiere AA, Sidransky D. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:1580-1586. [PubMed] [Cited in This Article: ] |
| 23. | Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, Fitzgerald AL, Giri U, Ang KK, Myers JN. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18:290-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012;122:1951-1957. [PubMed] [Cited in This Article: ] |
| 25. | Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 895] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 26. | Loyo M, Li RJ, Bettegowda C, Pickering CR, Frederick MJ, Myers JN, Agrawal N. Lessons learned from next-generation sequencing in head and neck cancer. Head Neck. 2013;35:454-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA. 2010;107:246-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 28. | Devine T, Dai MS. Targeting the ubiquitin-mediated proteasome degradation of p53 for cancer therapy. Curr Pharm Des. 2013;19:3248-3262. [PubMed] [Cited in This Article: ] |
| 29. | Nemunaitis J, Clayman G, Agarwala SS, Hrushesky W, Wells JR, Moore C, Hamm J, Yoo G, Baselga J, Murphy BA. Biomarkers Predict p53 Gene Therapy Efficacy in Recurrent Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2009;15:7719-7725. [PubMed] [Cited in This Article: ] |
| 30. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [PubMed] [Cited in This Article: ] |
| 31. | Nemunaitis J, Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. Head Neck. 2011;33:131-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7:1647-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 167] [Reference Citation Analysis (0)] |
| 33. | Farnebo M, Bykov VJ, Wiman KG. The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun. 2010;396:85-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Roh JL, Kang SK, Minn I, Califano JA, Sidransky D, Koch WM. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011;18:1904-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, Moll UM. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res. 2011;9:577-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 37. | Rafferty M, Walker C, Husband D, Helliwell T, Fenton J, Jones A. Retinoblastoma gene abnormalities in early laryngeal cancer. Eur Arch Otorhinolaryngol. 2008;265 Suppl 1:S83-S87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Yokoyama J, Shiga K, Sasano H, Suzuki M, Takasaka T. Abnormalities and the implication of retinoblastoma locus and its protein product in head and neck cancers. Anticancer Res. 1996;16:641-644. [PubMed] [Cited in This Article: ] |
| 39. | Mizokami H, Sawatsubashi M, Tokunaga O, Shin T. Loss of retinoblastoma protein expression in laryngeal squamous cell carcinoma. Mod Pathol. 1999;12:47-53. [PubMed] [Cited in This Article: ] |
| 40. | Morgan DO. Principles of CDK regulation. Nature. 1995;374:131-134. [PubMed] [Cited in This Article: ] |
| 41. | Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682-685. [PubMed] [Cited in This Article: ] |
| 42. | Schwarz S, Bier J, Driemel O, Reichert TE, Hauke S, Hartmann A, Brockhoff G. Losses of 3p14 and 9p21 as shown by fluorescence in situ hybridization are early events in tumorigenesis of oral squamous cell carcinoma and already occur in simple keratosis. Cytometry A. 2008;73:305-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Pérez-Sayáns M, Suárez-Peñaranda JM, Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM, García-García A. p16(INK4a)/CDKN2 expression and its relationship with oral squamous cell carcinoma is our current knowledge enough? Cancer Lett. 2011;306:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Masuda M, Hirakawa N, Nakashima T, Kuratomi Y, Komiyama S. Cyclin D1 overexpression in primary hypopharyngeal carcinomas. Cancer. 1996;78:390-395. [PubMed] [Cited in This Article: ] |
| 45. | Lin RJ, Lubpairee T, Liu KY, Anderson DW, Durham S, Poh CF. Cyclin D1 overexpression is associated with poor prognosis in oropharyngeal cancer. J Otolaryngol Head Neck Surg. 2013;42:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Sturgis EM, Zafereo ME, Wei Q, Li G. p14ARF genetic polymorphisms and susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Cancer. 2011;117:1227-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Liggett WH, Sewell DA, Rocco J, Ahrendt SA, Koch W, Sidransky D. p16 and p16 beta are potent growth suppressors of head and neck squamous carcinoma cells in vitro. Cancer Res. 1996;56:4119-4123. [PubMed] [Cited in This Article: ] |
| 48. | Mobley SR, Liu TJ, Hudson JM, Clayman GL. In vitro growth suppression by adenoviral transduction of p21 and p16 in squamous cell carcinoma of the head and neck: a research model for combination gene therapy. Arch Otolaryngol Head Neck Surg. 1998;124:88-92. [PubMed] [Cited in This Article: ] |
| 49. | Brieger J, Pongsapich W, Mann SA, Hedrich J, Fruth K, Pogozelski B, Mann WJ. Demethylation treatment restores hic1 expression and impairs aggressiveness of head and neck squamous cell carcinoma. Oral Oncol. 2010;46:678-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Brieger J, Mann SA, Pongsapich W, Koutsimpelas D, Fruth K, Mann WJ. Pharmacological genome demethylation increases radiosensitivity of head and neck squamous carcinoma cells. Int J Mol Med. 2012;29:505-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649-661. [PubMed] [Cited in This Article: ] |
| 52. | Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, van De Walle I, Cathelin S, Trimarchi T, Araldi E. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 53. | Westhoff B, Colaluca IN, D’Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci USA. 2009;106:22293-22298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 54. | Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52-59. [PubMed] [Cited in This Article: ] |
| 55. | Chen J, Jette C, Kanki JP, Aster JC, Look AT, Griffin JD. NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia. 2007;21:462-471. [PubMed] [Cited in This Article: ] |
| 56. | Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416-421. [PubMed] [Cited in This Article: ] |
| 57. | Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283-2291. [PubMed] [Cited in This Article: ] |
| 58. | Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714-718. [PubMed] [Cited in This Article: ] |
| 59. | Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol. 2007;27:3732-3742. [PubMed] [Cited in This Article: ] |
| 60. | Danilov AV, Neupane D, Nagaraja AS, Feofanova EV, Humphries LA, DiRenzo J, Korc M. DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS One. 2011;6:e26815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Rocco JW, Ellisen LW. p63 and p73: life and death in squamous cell carcinoma. Cell Cycle. 2006;5:936-940. [PubMed] [Cited in This Article: ] |
| 62. | Su X, Cho MS, Gi YJ, Ayanga BA, Sherr CJ, Flores ER. Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf. EMBO J. 2009;28:1904-1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9:749-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 64. | Shim SH, Lee CT, Lee JJ, Kim SY, Hah JH, Heo DS, Sung MW. A combination treatment with SAHA and ad-p63/p73 shows an enhanced anticancer effect in HNSCC. Tumour Biol. 2010;31:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Weichselbaum RR, Dunphy EJ, Beckett MA, Tybor AG, Moran WJ, Goldman ME, Vokes EE, Panje WR. Epidermal growth factor receptor gene amplification and expression in head and neck cancer cell lines. Head Neck. 1989;11:437-442. [PubMed] [Cited in This Article: ] |
| 66. | Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy BA, Netterville J. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170-4176. [PubMed] [Cited in This Article: ] |
| 67. | Chung CH, Zhang Q, Hammond EM, Trotti AM, Wang H, Spencer S, Zhang HZ, Cooper J, Jordan R, Rotman MH. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567-578. [PubMed] [Cited in This Article: ] |
| 69. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2546] [Cited by in F6Publishing: 2389] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 70. | Loeffler-Ragg J, Witsch-Baumgartner M, Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G, Zwierzina H. Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur J Cancer. 2006;42:109-111. [PubMed] [Cited in This Article: ] |
| 71. | Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944-3956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 72. | Benavente S, Huang S, Armstrong EA, Chi A, Hsu KT, Wheeler DL, Harari PM. Establishment and characterization of a model of acquired resistance to epidermal growth factor receptor targeting agents in human cancer cells. Clin Cancer Res. 2009;15:1585-1592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Galer CE, Corey CL, Wang Z, Younes MN, Gomez-Rivera F, Jasser SA, Ludwig DL, El-Naggar AK, Weber RS, Myers JN. Dual inhibition of epidermal growth factor receptor and insulin-like growth factor receptor I: reduction of angiogenesis and tumor growth in cutaneous squamous cell carcinoma. Head Neck. 2011;33:189-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clin Cancer Res. 2011;17:5935-5944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Kondo N, Tsukuda M, Sakakibara A, Takahashi H, Hyakusoku H, Komatsu M, Niho T, Nakazaki K, Toth G. Combined molecular targeted drug therapy for EGFR and HER-2 in head and neck squamous cell carcinoma cell lines. Int J Oncol. 2012;40:1805-1812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | A Study To Assess Absorption Of Study Drug Dacomitinib (PF-00299804), Given As An Oral Tablet Compared To An Intravenous Infusion In Healthy Volunteers. NCT01796327. Available from: http://scibite.com/site/library/2013_2/2/1/NCT01796327.html. [Cited in This Article: ] |
| 77. | Capecitabine and Lapatinib Ditosylate in Treating Patients With Squamous Cell Cancer of the Head and Neck. NCT01044433. Available from: http://clinicaltrials.gov/show/NCT01044433. [Cited in This Article: ] |
| 78. | Evaluation of Afatinib iin Maintenance Therapy in Squamous Cell Carcinoma of the Head and Neck (BIBW2992). NCT01427478. Available from: http://clinicaltrials.gov/show/NCT01427478. [Cited in This Article: ] |
| 79. | Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-1043. [PubMed] [Cited in This Article: ] |
| 80. | Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064-5073. [PubMed] [Cited in This Article: ] |
| 81. | Chau NG, Perez-Ordonez B, Zhang K, Pham NA, Ho J, Zhang T, Ludkovski O, Wang L, Chen EX, Tsao MS. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071-4076. [PubMed] [Cited in This Article: ] |
| 83. | 111-In-ch806 in Patients With Advanced Tumours Expressing the 806 Antigen. NCT00291447. Available from: http://clinicaltrials.gov/show/NCT00291447. [Cited in This Article: ] |
| 84. | A Study of ABT-806 in Subjects With Advanced Solid Tumor Types. NCT01255657. Available from: http://clinicaltrials.gov/show/NCT01255657. [Cited in This Article: ] |
| 85. | Basu D, Nguyen TT, Montone KT, Zhang G, Wang LP, Diehl JA, Rustgi AK, Lee JT, Weinstein GS, Herlyn M. Evidence for mesenchymal-like sub-populations within squamous cell carcinomas possessing chemoresistance and phenotypic plasticity. Oncogene. 2010;29:4170-4182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Bruzzese F, Leone A, Rocco M, Carbone C, Piro G, Caraglia M, Di Gennaro E, Budillon A. HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol. 2011;226:2378-2390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 87. | Wheeler DL, Iida M, Kruser TJ, Nechrebecki MM, Dunn EF, Armstrong EA, Huang S, Harari PM. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009;8:696-703. [PubMed] [Cited in This Article: ] |
| 88. | Basu D, Montone KT, Wang LP, Gimotty PA, Hammond R, Diehl JA, Rustgi AK, Lee JT, Rasanen K, Weinstein GS. Detecting and targeting mesenchymal-like subpopulations within squamous cell carcinomas. Cell Cycle. 2011;10:2008-2016. [PubMed] [Cited in This Article: ] |
| 89. | Saranath D, Chang SE, Bhoite LT, Panchal RG, Kerr IB, Mehta AR, Johnson NW, Deo MG. High frequency mutation in codons 12 and 61 of H-ras oncogene in chewing tobacco-related human oral carcinoma in India. Br J Cancer. 1991;63:573-578. [PubMed] [Cited in This Article: ] |
| 90. | Xu J, Gimenez-Conti IB, Cunningham JE, Collet AM, Luna MA, Lanfranchi HE, Spitz MR, Conti CJ. Alterations of p53, cyclin D1, Rb, and H-ras in human oral carcinomas related to tobacco use. Cancer. 1998;83:204-212. [PubMed] [Cited in This Article: ] |
| 91. | Paterson IC, Eveson JW, Prime SS. Molecular changes in oral cancer may reflect aetiology and ethnic origin. Eur J Cancer B Oral Oncol. 1996;32B:150-153. [PubMed] [Cited in This Article: ] |
| 92. | Quinlan MP, Settleman J. Isoform-specific ras functions in development and cancer. Future Oncol. 2009;5:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11-22. [PubMed] [Cited in This Article: ] |
| 94. | Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386:193-205. [PubMed] [Cited in This Article: ] |
| 95. | Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr Top Microbiol Immunol. 2010;346:143-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Qiu W, Schönleben F, Li X, Ho DJ, Close LG, Manolidis S, Bennett BP, Su GH. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441-1446. [PubMed] [Cited in This Article: ] |
| 97. | Qiu W, Tong GX, Manolidis S, Close LG, Assaad AM, Su GH. Novel mutant-enriched sequencing identified high frequency of PIK3CA mutations in pharyngeal cancer. Int J Cancer. 2008;122:1189-1194. [PubMed] [Cited in This Article: ] |
| 98. | Shao X, Tandon R, Samara G, Kanki H, Yano H, Close LG, Parsons R, Sato T. Mutational analysis of the PTEN gene in head and neck squamous cell carcinoma. Int J Cancer. 1998;77:684-688. [PubMed] [Cited in This Article: ] |
| 99. | Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 100. | Kurasawa Y, Shiiba M, Nakamura M, Fushimi K, Ishigami T, Bukawa H, Yokoe H, Uzawa K, Tanzawa H. PTEN expression and methylation status in oral squamous cell carcinoma. Oncol Rep. 2008;19:1429-1434. [PubMed] [Cited in This Article: ] |
| 101. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [PubMed] [Cited in This Article: ] |
| 102. | Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335-348. [PubMed] [Cited in This Article: ] |
| 103. | Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926-1945. [PubMed] [Cited in This Article: ] |
| 104. | Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, Naing A, Falchook GS, Moroney JW, Piha-Paul SA. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 105. | Rapamycin Therapy in Head and Neck Squamous Cell Carcinoma. NCT01195922. Available from: http://clinicaltrials.gov/show/NCT01195922. [Cited in This Article: ] |
| 106. | MK2206 in treating patients with recurrent or metastatic head and neck cance. NCT01349933. Available from: http://clinicaltrials.gov/show/NCT01349933. [Cited in This Article: ] |
| 107. | Study of BKM120 in Advanced Squamous Cell Carcinoma of Head and Neck. NCT01527877. Available from: http://clinicaltrials.gov/show/NCT01527877. [Cited in This Article: ] |
| 108. | Study of Efficacy and Safety of Buparlisib (BKM120) Plus Paclitaxel Versus Placebo Plus Paclitaxel in Recurrent or Metastatic Head and Neck Cancer Previously Pre-treated With a Platinum Therapy. NCT01852292. Available from: http://clinicaltrials.gov/show/NCT01852292. [Cited in This Article: ] |
| 109. | PI3K Inhibitor BKM120 and Cetuximab in Treating Patients With Recurrent or Metastatic Head and Neck Cancer. NCT01816984. Available from: http://clinicaltrials.gov/show/NCT01816984. [Cited in This Article: ] |
| 110. | Study of PX-866 and docetaxel in solid tumors. NCT01204099. Available from: http://clinicaltrials.gov/show/NCT01204099. [Cited in This Article: ] |
| 111. | Phase 1 and 2 study of PX-866 and cetuximab. NCT01252628. Available from: http://clinicaltrials.gov/show/NCT01252628. [Cited in This Article: ] |
| 112. | Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1183] [Cited by in F6Publishing: 1221] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 113. | van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, Hardy C, O’Meara S, Teague J. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 114. | Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, Bignell G, Butler A, Cho J, Dalgliesh GL, Galappaththige D, Greenman C, Hardy C, Jia M, Latimer C, Lau KW, Marshall J, McLaren S, Menzies A, Mudie L, Stebbings L, Largaespada DA, Wessels LF, Richard S, Kahnoski RJ, Anema J, Tuveson DA, Perez-Mancera PA, Mustonen V, Fischer A, Adams DJ, Rust A, Chan-on W, Subimerb C, Dykema K, Furge K, Campbell PJ, Teh BT, Stratton MR, Futreal PA. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 939] [Cited by in F6Publishing: 965] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 115. | Takahashi H, Murai Y, Tsuneyama K, Nomoto K, Okada E, Fujita H, Takano Y. Overexpression of phosphorylated histone H3 is an indicator of poor prognosis in gastric adenocarcinoma patients. Appl Immunohistochem Mol Morphol. 2006;14:296-302. [PubMed] [Cited in This Article: ] |
| 116. | Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225-4231. [PubMed] [Cited in This Article: ] |
| 117. | Yang J, Ji WY, Qu YR, He LX, Zhao XD, Jin MZ. [DNA methylation and histone modification relate to RASSF1A gene deletion in laryngeal carcinoma tissues]. Zhonghua Erbi Yanhou Toujing Waike Zazhi. 2011;46:308-312. [PubMed] [Cited in This Article: ] |
| 118. | Hatziapostolou M, Iliopoulos D. Epigenetic aberrations during oncogenesis. Cell Mol Life Sci. 2011;68:1681-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 119. | Bogdanović O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118:549-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 120. | Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630-3633. [PubMed] [Cited in This Article: ] |
| 121. | Ogi K, Toyota M, Ohe-Toyota M, Tanaka N, Noguchi M, Sonoda T, Kohama G, Tokino T. Aberrant methylation of multiple genes and clinicopathological features in oral squamous cell carcinoma. Clin Cancer Res. 2002;8:3164-3171. [PubMed] [Cited in This Article: ] |
| 122. | Youssef EM, Lotan D, Issa JP, Wakasa K, Fan YH, Mao L, Hassan K, Feng L, Lee JJ, Lippman SM. Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res. 2004;10:1733-1742. [PubMed] [Cited in This Article: ] |
| 123. | Shaw RJ, Liloglou T, Rogers SN, Brown JS, Vaughan ED, Lowe D, Field JK, Risk JM. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: quantitative evaluation using pyrosequencing. Br J Cancer. 2006;94:561-568. [PubMed] [Cited in This Article: ] |
| 124. | Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003;105:41-46. [PubMed] [Cited in This Article: ] |
| 125. | Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486-1500. [PubMed] [Cited in This Article: ] |
| 126. | Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA. 2006;103:14825-14830. [PubMed] [Cited in This Article: ] |
| 127. | Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI, Lin SC, Liu CJ, Hu WY, Yu YH. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer Res. 2011;71:1912-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 128. | Liu J, Guo Y, Fu S, Yang M, Sun KL, Fu WN. Hypomethylation-induced expression of S100A4 increases the invasiveness of laryngeal squamous cell carcinoma. Oncol Rep. 2010;23:1101-1107. [PubMed] [Cited in This Article: ] |
| 129. | Koutsimpelas D, Pongsapich W, Heinrich U, Mann S, Mann WJ, Brieger J. Promoter methylation of MGMT, MLH1 and RASSF1A tumor suppressor genes in head and neck squamous cell carcinoma: pharmacological genome demethylation reduces proliferation of head and neck squamous carcinoma cells. Oncol Rep. 2012;27:1135-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 130. | Gan CP, Hamid S, Hor SY, Zain RB, Ismail SM, Wan Mustafa WM, Teo SH, Saunders N, Cheong SC. Valproic acid: growth inhibition of head and neck cancer by induction of terminal differentiation and senescence. Head Neck. 2012;34:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Kothari V, Joshi G, Nama S, Somasundaram K, Mulherkar R. HDAC inhibitor valproic acid enhances tumor cell kill in adenovirus-HSVtk mediated suicide gene therapy in HNSCC xenograft mouse model. Int J Cancer. 2010;126:733-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Valproic Acid and Platinum-based Chemoradiation in Locally Advanced Head and Neck Squamous Cell Carcinoma. NCT01695122. Available from: http://clinicaltrials.gov/show/NCT01695122. [Cited in This Article: ] |
| 133. | Ph I Vorinostat in the Treatment of Advanced Staged Oropharyngeal Squamous Cell Carcinoma. NCT01064921. Available from: http://clinicaltrials.gov/show/NCT01064921. [Cited in This Article: ] |
| 134. | Capecitabine and Vorinostat in Treating Patients With Recurrent and/or Metastatic Head and Neck Cancer. NCT01267240. Available from: http://clinicaltrials.gov/show/NCT01267240. [Cited in This Article: ] |
| 135. | Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhøi BP, Overgaard M, Specht L, Andersen E, Johansen J, Andersen LJ. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6& amp; 7 trial. Radiother Oncol. 2011;100:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 136. | Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 137. | Available from: http://clinicaltrials.gov/show/NCT01530997. [Cited in This Article: ] |
| 138. | Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 139. | Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 140. | Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101:412-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 141. | Baleja JD, Cherry JJ, Liu Z, Gao H, Nicklaus MC, Voigt JH, Chen JJ, Androphy EJ. Identification of inhibitors to papillomavirus type 16 E6 protein based on three-dimensional structures of interacting proteins. Antiviral Res. 2006;72:49-59. [PubMed] [Cited in This Article: ] |
| 142. | Anchoori RK, Khan SR, Sueblinvong T, Felthauser A, Iizuka Y, Gavioli R, Destro F, Isaksson Vogel R, Peng S, Roden RB. Stressing the ubiquitin-proteasome system without 20S proteolytic inhibition selectively kills cervical cancer cells. PLoS One. 2011;6:e23888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 143. | Chung CH, Aulino J, Muldowney NJ, Hatakeyama H, Baumann J, Burkey B, Netterville J, Sinard R, Yarbrough WG, Cmelak AJ. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21:864-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Gilbert J, Lee JW, Argiris A, Haigentz M, Feldman LE, Jang M, Arun P, Van Waes C, Forastiere AA. Phase II 2-arm trial of the proteasome inhibitor, PS-341 (bortezomib) in combination with irinotecan or PS-341 alone followed by the addition of irinotecan at time of progression in patients with locally recurrent or metastatic squamous cell carcinoma of the head and neck (E1304): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2013;35:942-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 145. | Wu A, Zeng Q, Kang TH, Peng S, Roosinovich E, Pai SI, Hung CF. Innovative DNA vaccine for human papillomavirus (HPV)-associated head and neck cancer. Gene Ther. 2011;18:304-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 146. | Wick DA, Webb JR. A novel, broad spectrum therapeutic HPV vaccine targeting the E7 proteins of HPV16, 18, 31, 45 and 52 that elicits potent E7-specific CD8T cell immunity and regression of large, established, E7-expressing TC-1 tumors. Vaccine. 2011;29:7857-7866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Wansom D, Light E, Worden F, Prince M, Urba S, Chepeha DB, Cordell K, Eisbruch A, Taylor J, D’Silva N. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136:1267-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |