INTRODUCTION
Breast cancer is the most common cancer diagnosis in women, and one in eight women will be diagnosed with breast cancer during her lifetime[1]. Treatment, both surgical and chemotherapeutic, has evolved from the Halsted radical mastectomy to less invasive surgical techniques, combination treatment with focused radiation, and more effective adjuvant therapeutic options including tamoxifen and aromatase inhibitors (AIs)[2]. These advances in early detection and effective therapies have led to a growing number of cancer survivors worldwide. As vital as these interventions are for a woman’s ultimate survival, the overall quality of life of a woman with breast cancer encompasses additional issues to be addressed by the health care provider caring for her during and after the diagnosis and treatment. These issues are often only elicited with compassionate, yet direct inquiry. Adequately addressing these concerns may ultimately make a significant difference in the general health, adherence to recommended therapy, and overall well-being of the woman and her loved ones. In this review, we focus on issues of contraceptive options, fertility and pregnancy after breast cancer treatment, management of bothersome vasomotor symptoms (VMS), vulvovaginal atrophy (VVA) and other sexual health issues, prevention of bone loss, and evidence-supported surveillance for breast cancer recurrence. Ideally, coordination of care with specialists in oncology, reproductive endocrinology, women’s health, breast health, gynecology, and primary care to address psychological and medical needs can provide a sense of a “medical home” extending beyond a woman’s cancer diagnosis, and can contribute to her overall quality of life.
SURVEILLANCE AFTER BREAST CANCER
Breast cancer survival has been attributed to advances in screening mammography and adjuvant therapy. In the United States, this survival benefit has led to a growing population of women who are living with a history of breast cancer[3]. With the new focus on patient centeredness, a multidisciplinary approach is the expectation for survivorship care in order to best address the woman’s needs. A survey study demonstrated that breast cancer survivors reported a high rate of distress, neuropathy, chest wall, and arm pain. The majority of survivors stated that their medical needs were met, but only 49% reported that their psychological and spiritual needs were met following completion of cancer treatment[4]. Surveillance to monitor for recurrence, management of treatment related adverse effects, and promotion of preventive and general health care are all integral components of improving quality of care of breast cancer survivors. The Institute of Medicine published a report in 2005, “From Cancer Patient to Cancer Survivor: Lost in Transition,” which was instrumental in promoting awareness of the importance of standardization of survivorship care, development of cancer treatment plans, and improvement in the quality of care of breast cancer survivors[5].
The American Society of Clinical Oncology (ASCO) has outlined evidence-based recommendations for survivorship care[6]. A study comparing intensive vs standard surveillance for early-stage breast cancer demonstrated no difference in the disease-free or overall survival[7]. Recommendations for follow-up care after breast cancer include taking a history of symptoms and performing a physical examination every 3-6 mo for 3 years, then every 6-12 mo for 2 years, and then annually. Women are encouraged to perform monthly breast awareness and promptly report new findings to their health care provider. Breast imaging includes an annual mammogram for women with remaining breast tissue. Routine laboratory testing and radiologic studies are not recommended. Preventive health and screening guidelines for other cancers should follow average-risk recommendations. Women are advised to maintain a healthy lifestyle with regular exercise, avoidance of alcohol, and maintenance of a healthy weight[8]. Those with a hereditary predisposition for breast cancer and those with a known breast cancer mutation are advised to have an annual breast MRI in conjunction with mammography[9].
Adjuvant hormonal therapy has been shown to decrease breast cancer recurrence for hormone-dependent breast cancer[10]. Both AIs and tamoxifen are typically prescribed for 5 years for estrogen receptor-positive breast cancer. There is further evidence that longer therapy is beneficial for estrogen receptor-positive disease. A recent large study, the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial, comparing 5 vs 10 years of tamoxifen demonstrated a further reduction in recurrence and mortality after 10 years of tamoxifen in women with early-stage estrogen receptor-positive breast cancer[11].
However, common side effects of antiestrogen therapies such as exacerbation of VMS, vaginal dryness, vaginal bleeding or spotting, and arthralgias can negatively impact quality of life for many women. These adverse effects can result in early discontinuation and nonadherence to adjuvant hormonal therapy[12]. Clinicians can be proactive in assessing and counseling patients experiencing medication-related side effects. Various management options are available to provide relief of bothersome symptoms and evaluation of worrisome findings, such as postmenopausal bleeding in the setting of tamoxifen therapy, and can improve therapy adherence and survival.
MANAGEMENT OF VASOMOTOR SYMPTOMS
Vasomotor symptoms are among the most common bothersome symptoms associated with the menopausal transition, occurring in up to 80% of women[13]. Though the experience of VMS varies, recent evidence suggests that VMS begin before the final menstrual period and may last for over a decade[14]. Further, up to 10% of women in a Scandinavian study continued to experience VMS well into their 70s[15]. Women undergoing treatment for breast cancer may also experience VMS as a consequence of therapy, specifically tamoxifen or AIs[16].
Hormone therapy (HT) whether estrogen alone, estrogen plus progestin, or progestin alone effectively treats VMS[17,18]. However, systemic hormone therapy has been associated with an increased recurrence risk in breast cancer survivors in some but not all studies[19,20]. In a review of 15 studies between 1967 and 2001 by Batur et al[21], menopausal HT (estrogen plus progestin in 14 of the 15) was not associated with increased cancer recurrence, cancer-related mortality, or total mortality. Nonetheless, synthetic progestins demonstrate proliferative effects in the breast and may augment carcinogenesis by stimulating conversion of differentiated cancer cells to cancer stem cells[22]. In addition, the 13-year follow-up of the Women’s Health Initiative showed increased risk of breast cancer after approximately 5 years of therapy in the estrogen plus progestin group and not in the estrogen alone group, adding to the concern that certain progestins may increase breast cancer risk[22,23]. There is no current data to support differential management of VMS for women with different receptor-positive tumor types (i.e., ER, PR, and HER2neu).
In short, women on HT diagnosed with breast cancer need to discontinue therapy. There is no evidence-based guidance as to whether a taper is preferable to abrupt discontinuation, though a slow taper may be preferable based on expert opinion[24]. Approximately 50% of women who discontinue HT will experience recurrence of VMS[25].
NONHORMONAL TREATMENT OPTIONS FOR VASOMOTOR SYMPTOMS
Given the safety concerns with HT use in breast cancer survivors, nonhormonal treatments are often considered[26]. Lifestyle modifications for management of VMS are recommended as first-line interventions. They include avoidance of triggers (caffeine, alcohol, tobacco, warm beverages, spicy foods), dressing in layers, and the use of cooling or wicking clothing and bed linen. The results of studies regarding soy, exercise, and acupuncture have been mixed[27-29], whereas paced respirations may be of some benefit for VMS management[30,31]. Caution should be exercised with nonprescription products claiming efficacy for VMS, as robust scientific evidence is lacking. Further, these products are not regulated by the Food and Drug Administration (FDA) raising questions about safety.
A 2010 Cochrane review of 16 randomized controlled trials of nonhormonal interventions for VMS management in breast cancer survivors showed a mild-to-moderate effect with selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), gabapentin, clonidine, and relaxation therapy[32]. Limitations of existing studies include significant placebo effect, differing measures of efficacy, and in many, short duration of treatment. Areas of uncertainty are potential long-term drug effects, optimal duration of therapy, and symptom recurrence upon discontinuation[31].
Gabapentin has been shown to reduce VMS in breast cancer survivors[33,34]. However, extended-release gabapentin did not meet FDA approval for this indication in 2013, due to concerns regarding marginal effectiveness and adverse effects such as dizziness.
Low-dose mesylate salt of paroxetine (Brisdelle 7.5 mg) modestly reduces VMS frequency in clinical trials and is the first FDA-approved nonhormonal option for VMS management[35]. In a recent year-long randomized, double-blind, placebo-controlled trial, desvenlafaxine (Pristiq) was found to be associated with a statistically significant and clinically meaningful VMS reduction[36]. However, desvenlafaxine did not receive FDA approval for this indication in 2011 due to concerns regarding the risk-benefit profile.
Sexual side effects of antidepressants, including difficulty with arousal and orgasm, are associated with many antidepressants with some exceptions (bupropion and mirtazapine)[37]. In fact, bupropion has been used in the setting of sexual side effects associated with other antidepressants, though it has not been studied specifically in women with breast cancer[38].
In breast cancer patients, an interaction between tamoxifen and SSRIs that strongly inhibit cytochrome P450 2D6 (CYP2D6) may exist, potentially reducing endoxifen, the bioactive form of tamoxifen. Newer evidence is concerning for a potential correlation between tamoxifen efficacy or risk of breast cancer recurrence with an inherited variant of the CYP2D6 allele or with the use of medications which may inhibit the CYP2D6 enzyme[39]. Therefore, caution is currently advised when using strong inhibitors of CYP2D6 in breast cancer patients on tamoxifen.
Though some data suggest short-term improvement in VMS with stellate ganglion block in breast cancer survivors, results have been mixed and further investigation is needed[40,41].
Providers may choose a VMS management option based on potential side effects, medical comorbidities, and patient preference. For example, if a patient suffers from migraine, gabapentin may be reasonable. If a mood disorder is present, an antidepressant may be most appropriate.
SEXUAL HEALTH CONCERNS IN THE BREAST CANCER SURVIVOR
While sexual health is increasingly identified as an important issue in cancer survivors, providers don’t always query patients about sexual function, and patients are often reluctant to bring up concerns, fearing nothing can be done or dissuaded by provider discomfort[42]. Most patients are, however, interested in discussing sexual function and want their providers to broach the topic. Survivors of breast cancer face well-described sexual health challenges, including changes in body image, loss of fertility, impairment of relationships, lack of libido, VVA, and dyspareunia. Both the experience of cancer and its treatment can have a significant impact on sexual functioning. Chemotherapy-induced ovarian insufficiency (or risk-reducing oophorectomy) as well as medications used to treat or control disease may result in hormonal loss or blockade, potentially leading to changes in libido, VVA, dyspareunia, and decreased quality of life[43].
MANAGEMENT OF VULVOVAGINAL ATROPHY IN THE BREAST CANCER SURVIVOR
In contrast with VMS, which tend to improve with time, symptoms associated with VVA progress over time. In women with VVA, meticulous vulvar care is important, and products with perfumes or dyes should be avoided (toilet tissue, soaps, fabric softeners, stimulating lubricants, vaginal hygiene products)[44]. Vaginal moisturizers can be used on a regular basis to replace vaginal moisture, whereas lubricants are needed with sexual activity to reduce friction[45]. First-line therapies for VVA include not only vaginal moisturizers and lubricants, but also regular sexual activity (with partner, device, or solo) to stimulate blood flow to the area. Though data is lacking, expert opinion favors proactively educating postmenopausal women about vulvovaginal health in order to preserve sexual function[46]. This concept is perhaps even more pertinent for the breast cancer survivor. Pelvic floor physical therapy may be utilized to facilitate pelvic floor relaxation, and may include education, relaxation techniques of the pelvic floor muscles, deep breathing, biofeedback, and instruction on the use of lubricated vaginal dilators in graduated sizes to gently stretch vaginal tissues[47]. Psychosocial interventions, including individual or couple counseling, cognitive behavioral therapy, and mindfulness-based interventions are utilized in breast cancer survivors to improve coping strategies. These also serve to address a number of concerns that impact sexuality including altered body image, anxiety, fear of recurrent disease, changes in relationships, and dyspareunia, though supportive evidence is sparse[43].
In particular, breast cancer survivors on AI therapy experience profound estrogen deprivation, vaginal dryness, and dyspareunia[48]. A few small studies have demonstrated significant increases in plasma estradiol concentrations in postmenopausal breast cancer patients on AIs treated with local vaginal estrogen therapy (LVET). However, no studies to date have revealed an increased risk of breast cancer recurrence with LVET use[49]. It is becoming increasingly evident that severe VVA can have a negative impact on quality of life and may result in medication noncompliance. LVET for severe symptoms is available in ring, cream, or tablet form, but the long-term safety and systemic absorption from these various preparations are still largely unknown. Newer evidence has demonstrated that serum pharmacokinetics can be used to determine the maximum annual dose delivered and serum estradiol levels of various LVETs. Vaginal estrogen tablets (10 μg) prescribed twice a week demonstrated the lowest annual delivered systemic dose as compared to other LVET[44]. Providers should be aware of barriers to breast cancer treatment adherence and take into account individual patient’s symptom severity, preference, and potential cancer recurrence risk when considering LVET.
A trial utilizing topical testosterone vaginal cream in 20 breast cancer patients on AIs revealed improvements in vaginal dryness, dyspareunia, and vaginal maturation index (VMI) without a change in estradiol levels. The significance of increased reported testosterone levels is unclear[50]. Likewise, intravaginal dehydroepiandrosterone (DHEA) has been associated with improvements in bothersome vaginal symptoms, VMI, and vaginal pH without significant changes in serum estrogen or androgen levels[51]. A trial of vaginal DHEA in 465 breast cancer survivors with VVA is underway, with results expected in 2014[52].
Ospemifene is a selective estrogen receptor modulator (SERM) FDA-approved for treatment of moderate-to-severe dyspareunia. While it has demonstrated antiestrogenic effects in preclinical models of breast cancer, it has not been studied in breast cancer survivors and is not approved for use in this population or in those at high risk of breast cancer[53].
FERTILITY AFTER BREAST CANCER
While many women are diagnosed with breast cancer after menopause, a 20-year-old woman in the United States has about a 0.5% chance of developing breast cancer in her reproductive years, bringing up the impact of cancer therapy on future fertility, and of pregnancy on cancer recurrence[54]. In general, the impact of chemotherapy on fertility is related to age-related baseline fertility as well as the type and dose of chemotherapy used[55,56]. In women who desire pregnancy after breast cancer, fertility preservation should be discussed at the time of diagnosis. While a full discussion is beyond the scope of this review, both embryo and oocyte cryopreservation are considered standard options. With cycle-day-independent ovarian stimulation protocols, the timing of cancer therapy initiation can be minimally affected. In order to reduce potential risks associated with increased estrogen levels at ovarian stimulation, many regimens now utilize letrozole. Gonadotropin-releasing hormone analogs should not be used for fertility preservation due to unproven efficacy[57].
While women were previously counseled to wait two years prior to conceiving after breast cancer diagnosis, newer published data does not demonstrate an alteration in survival with breast cancer to pregnancy intervals longer than 10 mo[58]. Estrogen-receptor status does not impact breast cancer recurrence with pregnancy[59]. Initial studies noting improved outcome in women who become pregnant after breast cancer were attributed to better baseline status in women who chose pregnancy, the so-called “healthy mother effect”[60]. A meta-analysis designed to correct for the “healthy mother effect” included over 1000 women with pregnancy after breast cancer diagnosis and over 13000 health-matched controls. This study noted improved survival in women who became pregnant after breast cancer treatment as compared to those who did not, hazard ratio 0.51 [95% confidence interval (CI), 0.42-0.62] suggesting that pregnancy at least 10 mo post breast cancer treatment in women < 45 years does not adversely affect prognosis and may in fact significantly improve survival[60].
About 50% of women can lactate after breast cancer therapy, but breast milk volume tends to be reduced[61].
ASSOCIATION BETWEEN HORMONAL CONTRACEPTION AND BREAST CANCER
Though data is difficult to interpret given varying hormonal doses and lengths of follow-up, there is no clear evidence that hormonal contraceptive use (oral contraceptive have been most thoroughly studied), past or present, is associated with a significantly increased breast cancer risk[62]. Importantly, older studies reporting a weak association contain data pertaining to older, higher dose contraceptive formulations not in use today[63,64]. Therefore, patients can be reassured of the unlikely contribution of prior hormonal contraception to the breast cancer diagnosis.
CONTRACEPTION OPTIONS DURING BREAST CANCER TREATMENT AND BEYOND
Safe, effective, and convenient contraception should be discussed and made available to all women undergoing diagnosis, treatment, and surveillance for breast cancer. Women not wishing further fertility may consider male or female sterilization with inherent failure rates < 1%[65,66]. A number of minimally invasive options for sterilization now exist including laparoscopic tubal ligation and hysteroscopic sterilization (Essure). It is important to note that the latter does not have immediate efficacy, and tubal occlusion must be confirmed by hysterosalpingogram 12 wk following the procedure[67]. Therefore, as with male sterilization, another form of contraception should be used until efficacy can be assured[65,67].
During the evaluation for breast cancer, a woman on hormonal contraception, including combined hormonal contraception, should continue using this method until she receives appropriate counseling regarding future reproductive goals, an assessment of medical needs beyond the breast cancer, and, most importantly, until a new method is initiated. The United States Medical Eligibility Criteria for Contraceptive Use (US MEC) considers all hormonal contraception as “advantages generally outweigh theoretical or proven risks” in the setting of an undiagnosed breast mass[68]. Nonhormonal methods including the copper T380 (CuIUD or ParaGard) and barrier methods have “no restriction in use” in this setting. However, even with perfect use, barrier method alone is unlikely to provide sufficient efficacy and convenience to most women, given its 15% failure rate[69]. An unplanned pregnancy at a time when breast cancer treatment should be initiated can lead to needlessly difficult choices about pregnancy termination or treatment delay.
The Cu-IUD is the only hormone-free, long-acting reversible contraceptive (LARC) currently available in the United States and has “no restrictions for use” in the setting of current breast cancer[68]. Pregnancy rates are < 1% per year with this method indicated for up to 10 years[70]. In addition, a Cu-IUD can be placed at any time that pregnancy can be reasonably ruled out, as well as within 5 d of unprotected intercourse if emergency contraception is desired. Additional back-up contraception is not required after insertion. Further, due to its non-systemic mechanism of action, chemotherapy-associated nausea and vomiting does not alter efficacy.
While the Cu-IUD represents the first-line reversible contraception in women with breast cancer, concurrent medical issues such as endometrial proliferation on tamoxifen, anemia, menorrhagia, and dysmenorrhea may warrant consideration of local progestin therapy, especially in women with hormone receptor-negative tumor types. These considerations need to be balanced with US MEC rating of “unacceptable health risks, method not to be used” for all hormone-containing contraceptives. The newer 13.5 mg levonorgestrel releasing system (low dose LNG-IUS) (Skyla) has lower circulating levels than the 52 mg LNG-IUS (Mirena) used in prior studies of LNG-IUS and breast cancer[71-74]. In addition to being an extremely effective reversible contraception that is effective for up to 3 years, low dose LNG-IUS may improve anemia, menorrhagia, and dysmenorrhea that can be associated with Cu-IUD, while exposing a woman to lower circulating levels of levonorgestrel than LNG-IUS (Mirena). However, there is currently no evidence regarding low dose LNG-IUS efficacy for menorrhagia treatment or its safety in the setting of breast cancer.
A small case-control study of women using LNG-IUS compared 79 women, who started or continued using LNG-IUS after diagnosis of breast cancer, and 120 controls. While there was no increased risk of recurrence overall, there was concern about the 3.39 adjusted hazard ratio of breast cancer recurrence (95%CI, 1.01-11.35) in a subgroup who developed breast cancer while using LNG-IUS and continued its use[74]. This finding contrasts with population data that has not found an increased risk of breast cancer in LNG-IUS users as compared to Cu-IUD users in 5100 breast cancer patients and 20000 controls[73].
LNG-IUS is indicated for the treatment of menorrhagia and can decrease menstrual blood loss by up to 90%[75]. This is compared with a 40% reduction in menstrual blood loss with antifibrinolytics and about 25% with NSAIDs[76,77]. Unfortunately, there is virtually no data on the contraceptive implant (Implanon, Nexplanon) in the setting of breast cancer[78]. As with all other hormonal LARC methods, the US MEC rates the implant as “unacceptable health risk, method should not be used” with current breast cancer and as “theoretical or proven risks usually outweigh the advantages” after 5 years without breast cancer recurrence[68].
When determining the need for contraception after breast cancer diagnosis, amenorrhea and elevated gonadotropins such as follicular stimulating hormone (FSH) are unreliable markers of infertility in women who have received chemotherapy[55,79]. The incidence of chemotherapy-induced amenorrhea is reported to be 53%-89%, and is more likely to be reversible in women under 40 years than older women[80].
EMERGENCY CONTRACEPTION
The World Health Organization (WHO) has determined that there is no medical condition wherein the risks of emergency contraception outweigh its benefits[81]. Therefore emergency contraception should be available to women diagnosed with and undergoing treatment for breast cancer.
The Cu-IUD can serve as 96% effective emergency contraception if inserted within 5 d post-unprotected intercourse, and can remain as primary hormone-free, yet reversible contraception for up to 10 years. In one study, over 80% of women who received Cu-IUD for emergency contraception continued using it for primary contraception thereafter[82].
Additional emergency contraception methods include levonorgestrel (LNG or PlanB one step) and Ulipristal (UPA or Ella)[83,84]. Plan B is now available in the United States without a prescription regardless of patient age, and is indicated for use within 72 h of unprotected intercourse[84]. An efficacy rate of 85% has been reported. LNG alone is more effective than the combined Yuzpe regimen of oral contraceptives and is associated with fewer side-effects[85]. UPA is indicated in a single dose for emergency contraception up to 5 d post-unprotected intercourse[83]. Lower failure rates than LNG have been reported with UPA, (OR, 0.35, 0.58, 0.55 at 24, 72, and 120 h respectively)[86]. Differences in efficacy of emergency contraception have been reported in women with normal vs elevated body mass index (BMI). As compared to women with a BMI < 25 kg/m2, women with a BMI of 25-29 kg/m2 had a 1.5-fold increased risk of pregnancy whereas those with a BMI > 30 kg/m2 had a 3.6-fold increased risk with LNG EC vs UPA[86]. Therefore, UPA or Cu-IUD, which do not have BMI-related efficacy differences, should be considered over LNG in women with elevated BMI.
BONE HEALTH
Bone health has been increasingly recognized as a significant issue for breast cancer survivors from the standpoint of osteoporosis prevention as well as its diagnosis and treatment[87-89]. A recently published survey study found that women aged 65 and older with a breast cancer diagnosis had a higher prevalence of osteoporosis and falls. However, their risk was not more likely to have been identified by their health care provider, and bone health or fall prevention discussed[90].
There are multiple mechanisms by which breast cancer treatment impacts bone health. Primary ovarian insufficiency or premature menopause often results from treatment with gonadotropin-releasing hormone agonists or chemotherapeutic agents in previously premenopausal women and increases risk of osteoporosis[88]. The use of antiestrogen therapies can cause estrogen deficiency resulting in bone loss and reduced bone integrity[91]. Tamoxifen has different effects on bone in pre- vs postmenopausal women. In premenopausal women, tamoxifen has been shown to cause a 1%-2% bone loss over 1-2 years, but experts note that this is not a clinically significant change, and that monitoring or treatment solely based on tamoxifen use is not indicated. In contrast, tamoxifen is associated with increased bone density in postmenopausal women[92]. Of greater concern for a negative impact on bone health and fracture risk is the use of AIs. AIs are used in postmenopausal women with hormone receptor-positive breast cancer to reduce recurrence risk with a demonstrated survival benefit[93,94]. However, AIs result in substantial reduction in estrogen production and estradiol levels, and are associated with decreased bone mineral density (BMD) and higher rate of fracture[95].
Strategies for prevention of bone loss in all women, including those receiving antiestrogen therapies, include counseling on the importance of adequate calcium (1200 mg per day) and vitamin D (800-1000 IU per day) through diet or supplement, regular exercise including both weight-bearing and muscle strengthening, advice on fall prevention, smoking cessation, and avoidance of excess alcohol[96].
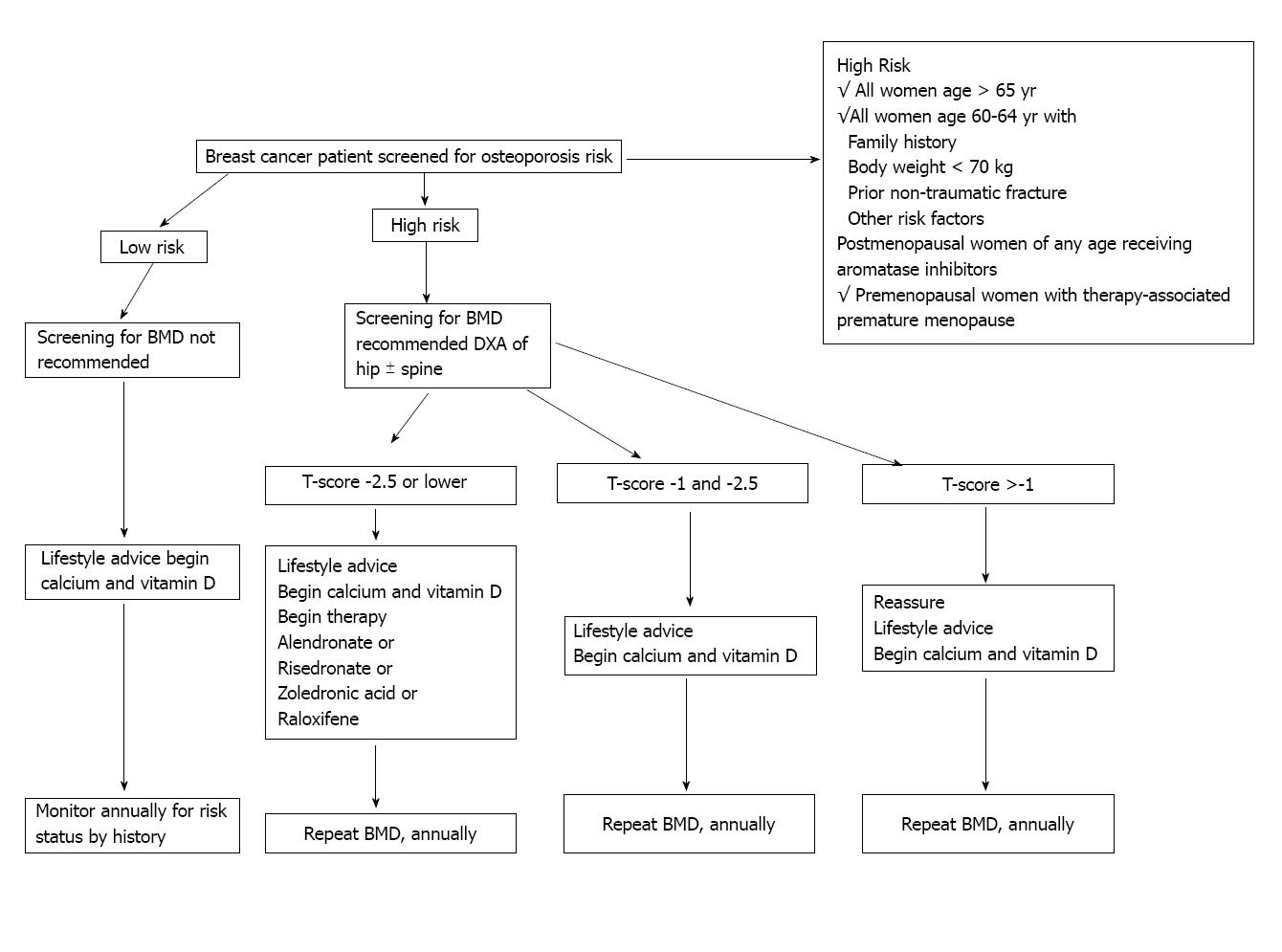
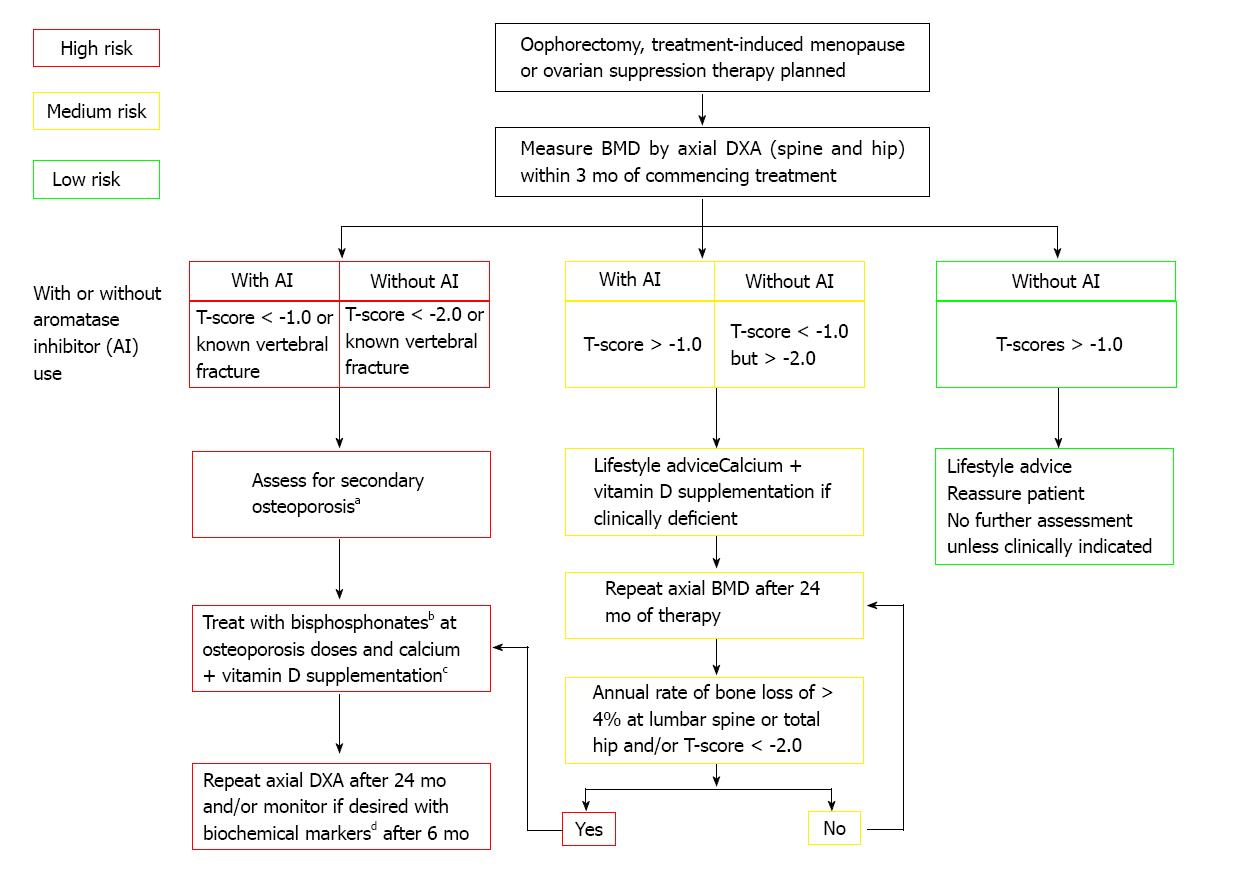
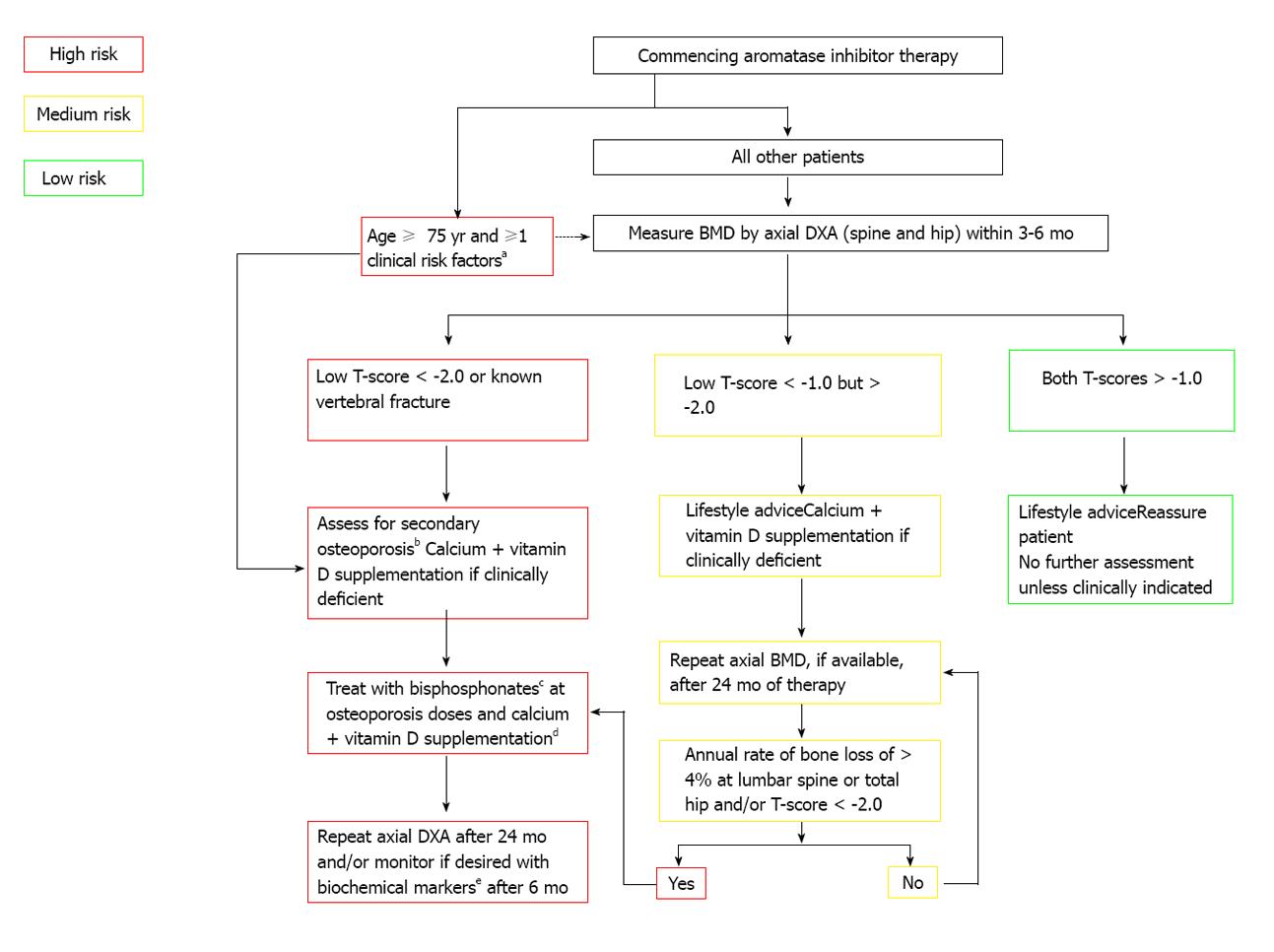
In 2003 the ASCO published an algorithm (Figure 1) for screening and treatment specifically for breast cancer patients that recommended BMD screening annually for all postmenopausal women on AIs and for premenopausal women with treatment-induced premature menopause, as well as for all breast cancer patients aged 65 years and older or those aged 60-64 years with risk factors. The treatment guideline at that time only called for bisphosphonate or raloxifene for women with a T-score of -2.5 or lower[97]. A consensus statement from a UK Expert Group in 2008 divided that algorithm into specific guidelines for formerly premenopausal women with treatment-induced premature menopause (defined in the publication as < 45 years old) and for postmenopausal women treated with AIs (Figures 2 and 3). For women with continued menstruation or postmenopausal women older than 45 years old and either on tamoxifen or not on AIs, no specific recommendation is given for screening. Any woman with a T-score < -2.0 or with a history of vertebral fracture is advised to have evaluation for secondary causes of osteoporosis[92].
Figure 1 Recommended management strategy for patients with diagnosed nonmetastatic breast cancer[97].
This management strategy is largely based on influence from results in non-breast cancer populations. BMD: Bone mass density; DEXA: Dual energy X-ray absorptiometry bone scan. Reprinted with permission. © (2003) American Society of Clinical Oncology. All rights reserved.
Figure 2 Adjuvant treatment associated with ovarian suppression/failure with or without concomitant aromatase inhibitor use in women who experience premature menopause[92].
aErythrocyte sedimentation rate, full blood count, bone and liver function (calcium phosphate, alkaline phosphatase, albumin, AST/γGT), serum creatinine, endomysial antibodies, serum thyroid stimulating hormone; bAlendronate 70 mg per week, risedronate 35 mg per week, ibandronate (150 mg po monthly or 3 mg iv 3-monthly), zoledronic acid 4 mg iv 6-monthly; cTo be given as ≥ 1 g of calcium + ≥ 800 IU of vitamin D; dBiochemical markers such as serum C-terminal telopeptide of type I collagen or urinary N-telopeptide of type I collagen. Reprinted with permission. © (2008) Elsevier.
Figure 3 Postmenopausal adjuvant treatment with aromatase inhibitors[92].
aPrevious low-trauma fracture after age 50, parental history of hip fracture, alcohol intake of ≥ 4 units/day, diseases associated with secondary osteoporosis, prior corticosteroids for > 6 mo, low BMI (< 22); bErythrocyte sedimentation rate, full blood count, bone and liver function (calcium phosphate, alkaline phosphatase, albumin, AST/γGT), serum creatinine, endomysial antibodies, serum thyroid stimulating hormone; cAlendronate 70 mg per week, risedronate 35 mg per week, ibandronate (150 mg po monthly or 3 mg iv 3-monthly), zoledronic acid 4 mg iv 6-monthly; dTo be given as ≥ 1 g of calcium + ≥ 800 IU of vitamin D; eBiochemical markers such as serum C-terminal telopeptide of type I collagen or urinary N-telopeptide of type I collagen. Reprinted with permission. © (2008) Elsevier.
For breast cancer patients with treatment-induced early menopause < 45 years old, a BMD measurement by spine and hip dual energy X-ray absorptiometry (DXA) is recommended. If a woman not on an AI had a T-score < -2.0, bisphosphonate treatment is recommended with a follow up DXA in 24 mo. With a score of -1.0 or higher, no further testing is indicated. If the T-score is between -1.0 and -2.5, a repeat DXA in 24 mo is advised. For the woman on AI, the threshold for treatment drops to a T-score of < -1.0 with repeat DXA recommended at 24 mo post bisphosphonate initiation. For a woman on AI and in premature menopause at < 45 years old, a T-score > -1.0 results in a recommendation for DXA in 24 mo[92].
For postmenopausal women starting AI therapy, DXA of the spine and hip is recommended. If the T-score is < -2.0 or if the woman is 75 years or older with any osteoporosis risk factors, a bisphosphonate is recommended with follow-up DXA in 24 mo. Similar to the algorithm for breast cancer patients with early menopause, a T-score of -1.0 or higher requires no further screening unless indicated by a change in the clinical situation. For a T-score between -1.0 and -2.5, a follow-up DXA in 24 mo is recommended[92].
All breast cancer patients with known vertebral fractures should be considered for treatment per the UK Expert Group guidelines. Another indication for treatment is an annual bone loss rate of > 4% in both the premature menopause and postmenopausal AI-treated groups[92].
CONCLUSION
In focusing on the woman undergoing treatment and surveillance for breast cancer, attention to a number of concurrent issues beyond the breast cancer itself will impact her satisfaction with treatment and overall care. Appropriate counseling and evidence-supported surveillance strategy along with age-appropriate testing will promote overall health and perhaps a sense of some control over her care. Careful attention to medication-related side effects including chemotherapy-induced VVA with resultant dyspareunia can certainly affect her well-being and relationship with her partner. The availability of nonhormonal treatment options for VVA and VMS can help her focus on her recovery. If future fertility is a concern, she should ideally be evaluated by a reproductive endocrinologist prior to initiation of chemotherapy and counseled regarding fertility preservation options. Pregnancy is generally not advised for at least 10 mo after breast cancer therapy, but advancing maternal age and other factors need to be considered in individual counseling. If contraception is desired, women should be counseled about both reversible and permanent hormone-free options, but they should not discontinue current contraception until the initiation of an alternate method. Chemotherapy-induced amenorrhea should not be considered permanent ovarian insufficiency, especially in women younger than 40 years. Likewise, elevated FSH in this setting is not an indicator of permanent ovarian insufficiency. Attention to bone health is important in breast cancer survivors, particularly in the context of chemotherapy-induced premature menopause or AI use. A multidisciplinary, comprehensive, and holistic approach to the woman with breast cancer can facilitate her transition from breast cancer patient to breast cancer survivor.