Published online Aug 10, 2014. doi: 10.5306/wjco.v5.i3.374
Revised: March 10, 2010
Accepted: May 31, 2014
Published online: August 10, 2014
Biotransformation of tamoxifen to the potent antiestrogen endoxifen is performed by cytochrome P450 (CYP) enzymes, in particular the CYP2D6 isoform. CYP2D6*4 is one of the most frequent alleles associated with loss of enzymatic activity. The incidence of CYP2D6*4 among Caucasians is estimated up to 27%, while it is present in up to 90% of all poor metabolizers within the Caucasian population. The hypothesis under question is whether the presence of one or two non-functioning (null) alleles predicts an inferior outcome in postmenopausal women with breast cancer receiving adjuvant treatment with tamoxifen. The numerous existing studies investigating the association of CYP2D6 with treatment failure in breast cancer are inconsistent and give rather conflicting results. Currently, routine CYP2D6 testing among women with breast cancer is not recommended and the significance of CYP2D6 phenotype in decision making regarding the administration of tamoxifen is unclear. The present study summarizes current literature regarding clinical studies on CYP2D6*4, particularly in terms of response to tamoxifen therapy and breast cancer outcome.
Core tip: Currently, routine cytochrome P450 (CYP)2D6 testing among women with breast cancer is not recommended and the significance of CYP2D6 phenotype in decision making regarding the administration of tamoxifen is unclear. The present study summarizes current literature regarding clinical studies on CYP2D6*4, particularly in terms of response to tamoxifen therapy and breast cancer outcome.
- Citation: Markopoulos C, Kykalos S, Mantas D. Impact of CYP2D*6 in the adjuvant treatment of breast cancer patients with tamoxifen. World J Clin Oncol 2014; 5(3): 374-381
- URL: https://www.wjgnet.com/2218-4333/full/v5/i3/374.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i3.374
Tamoxifen (TAM) is a selective estrogen receptor modulator (SERM) and 5 years of Tamoxifen treatment is considered as “standard of care” in the adjuvant setting in most cases of premenopausal patients with estrogen receptor positive (ER+) breast cancer, as well as in postmenopausal patients in different treatment strategies, alone or in combination with aromatase inhibitors. Recently, it was shown that, for women with ER-positive disease, continuing Tamoxifen to 10 years rather than stopping at 5 years produces a further reduction in recurrence and mortality, particularly after year 10. These results, taken together with results from previous trials of 5 years of Tamoxifen treatment vs none, suggest that 10 years of tamoxifen treatment can approximately halve breast cancer mortality during the second decade after diagnosis[1].
Tamoxifen has also been used as prophylactic treatment for those women considered being in high risk for developing breast cancer due to positive family history or the presence of atypia or lobular neoplasia in situ in a breast biopsy. Major side effects associated with tamoxifen use are hot flashes, as well as increased incidence of endometrial cancer and deep venous thrombosis. Besides acting as SERMs, it has been found that some of tamoxifen’s metabolites also act as aromatase inhibitors in vitro[2].
Norendoxifen consists the main metabolite of tamoxifen which is the most potent aromatase inhibitor of the tamoxifen metabolites. It causes the same decrease in vitro in aromatase activity as letrozole, an exclusive aromatase inhibitor.
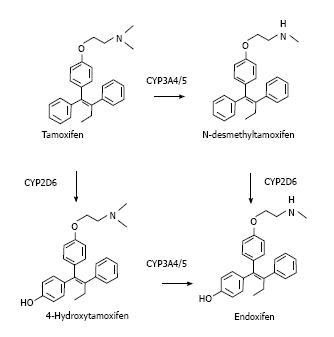
Tamoxifen is actually a pro-drug with a weak affinity to ER that exerts its therapeutical action after been transformed in the liver. Tamoxifen metabolism mostly occurs via two pathways, 4-hydroxylation and N-demethylation, both of which result in the very potent secondary metabolite, endoxifen (Figure 1).
CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. CYP2D6 is highly polymorphic, as 93 alleles with varying functions and distribution among the nations have been reported. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs[3]. This enzyme also metabolizes several endogenous substances such as hydroxytryptamines and neurosteroids[3].
There is considerable variation in the efficiency and amount of CYP2D6 enzyme produced between individuals. Hence for drugs that are metabolized by CYP2D6 (that is CYP2D6 substrates), certain individuals will eliminate these drugs quickly (extensive metabolizers) whilst others will do that slowly (poor metabolizers). If a drug is metabolized too quickly then drug’s efficacy might be reduced, while if the drug is metabolized too slowly, toxicity may result[4]. Therefore, the dose of the drug might have to be adjusted in order to balance the speed at which it is metabolized by CYP2D6[5].
CYP2D6*4 is the most frequent allele associated with loss of enzymatic activity within the Caucasian population (incidence reaching 27%); meaning that its significance in decision making regarding the administration of tamoxifen in breast cancer patients across Europe might be crucial. The clinical efficiency could be even stronger in small countries with a more consistent and homogeneous population.
The numerous existing studies investigating the relevance of CYP2D6 in the treatment of breast cancer are not unique and give conflicting results. The purpose of the present article is to focus especially on studies involving the CYP2D6*4, as this allele is present in up to 90% of all PMs in Caucasians[6].
We performed a preliminary data search in PubMed which revealed 307 abstracts under the general terms “CYP2D6” and “tamoxifen”. Consequently, we tried to narrow our research by excluding abstracts that were outside of clinical trials and the new approach revealed 23 abstracts that met the last criteria. We choose those with exact match in our review, especially involving CYP2D6*4 genotype, while we also selected original articles focused in the treatment of breast cancer with tamoxifen correlated with the CYP2D6 genotype in retrospective series among ER+ patients. We finally selected and took under consideration 31 abstracts as they appear to our references including general articles in order to explain the enzyme that correlates with our review along with tamoxifen usage.
Clinical impact of CYP2D6*4 on breast cancer outcome was the main subject of our review. There are numerous studies performed that provide conflict results to support or not this assumption. Goetz et al[7] performed a retrospective randomised phase III study in postmenopausal women with ER+ breast cancer. They concluded that tamoxifen- treated patients being homozygous for CYP2D6*4 had shorter relapse-free time and decreased DFS but not OS compared to *4/wt and wt/wt carries (P = 0.030, P = 0.020 and P = 0.360 respectively). The same conclusions could be extracted in a follow study for the same cohort evaluating the role of co-administration of CYP2D6 inhibitors[8].
A further retrospective study by Schroth et al[9], compared 206 patients receiving adjuvant tamoxifen and 280 patients not receiving tamoxifen. They tried to extend the assessment of patients with poor metabolic status and assigned more CYP2D6 alleles with impaired activity (*4, *5, *10 and *41). They showed a significant association between CYP2D6-PM and -IM and an unfavourable outcome (more recurrences, shorter relapse-free time and reduced DFS) in patients receiving adjuvant tamoxifen therapy. The incidence of allele *4 has not been reported by the authors. A larger study from the same author refers to results from retrospective analysis of German and US cohorts of 1325 total patients treated with adjuvant tamoxifen. All women were genotyped for CYP2D6 alleles associated with reduced (*10, *41) or absent (*3, *4, *5) activity. Although no difference in overall survival could be observed heterozygous EM/IM and PM had significant higher recurrence rates and worse event-free and disease-free survival. CYP2D6*4 was present in 20% of study cases[10]. A small series of 84 patients under adjuvant treatment with tamoxifen from Spain focused exclusively in allele *4. The authors concluded that combined genotype wt/*4 plus *4/*4 was significant associated with higher risk of disease relapse. This patient group had clearly lower benefit of tamoxifen treatment[11]. Another retrospective study from Spain with 91 patients receiving tamoxifen in the adjuvant setting gave similar results. The genotyping was performed for 11 common CYP2D6 alleles. CYP2D6*4 was present in 22 cases, in 12 of which there was heterozygous in combination with a functional allele (*1, *2 or *35). Poor metabolizers were found to have worse DFS[12]. A further population-based study from the Netherlands examined 85 patients for the clinical affection of allele *4 in breast cancer survival among tamoxifen users. The breast cancer mortality was also found to be significantly increased in patients with *4/*4 genotype[13]. The report of Thompson et al[14], is also of great interest. They genotyped 33 CYP2D6 alleles (including *4) in 618 tamoxifen-treated patients, using a pool of two cohorts from United Kingdom. Patients with at least one reduced function CYP2D6 allele showed a tendency for worse DFS, although not statistically significant. However, analysis restricted to four common variant alleles *4, *5, *10 and *41 showed no significant differences regarding DFS. In a sub-group analysis of post-menopausal women only, decreased metabolizers had a clear higher risk of disease recurrence. The influence of CYP2D6*4 on chemoprevention with tamoxifen was evaluated in a small subset of 46 patients with breast cancer and 136 controls pooled from a large Italian trial. The authors concluded that patients homozygous for allele *4 may be less likely to benefit from tamoxifen in the course of chemoprevention, as the cancer events were significantly higher in this study arm[15]. The effect of variation in CYP2D6 activity on the clinical course of patients with familial breast cancer was approached in another study from United Kingdom. The 115 enrolled patients were genotyped for the CYP2D6 *wt, *3, *4, *5 and *41 alleles. Poor metabolizers showed a significantly worse overall survival, a condition that was also observed in the subset analysis considering only CYP2D6*4, especially in BRCA2 carriers[16]. Karle et al[17] analysed the effect of the CYP2D6 genotyping in a palliative setting, enrolling 88 patients with ER positive advanced breast cancer. Co-medication with known CYP2D6 inhibitors was an exclusion criterion. The overall survival was significantly shorter for the poor or intermediate metabolizers when compared to an extensive group. Allele *4 was the most frequent non-functional variant, being detected in 37 cases. The authors concluded that CYP2D6 testing in advanced breast cancer is reasonable, especially if administration of tamoxifen is considered[17]. A retrospective genetic analysis of patients pooled from the Austrian prospective TIGER study, incorporated 493 women with ER positive tumours and focused exclusively in allele *4. The overall frequency of allele*4 was as high as 31%, with 5.7% of all patients being genotyped as CYP2D6*4/*4. Overall, no significant difference in tumour free survival was reported; however, a subgroup analysis of patients treated with concomitant chemotherapy showed that CYP2D6*4 poor metabolizers had a shorter mean time interval to disease progression. However, It has to be reported that information on co-medication were not available[18]. The impact of CYP2D6 in the outcome of breast cancer was further studied in a population-based cohort trial of 313 women that were adherent to tamoxifen therapy for at least one year (Table 1). It should be mentioned that 22.4% of enrolled patients were premenopausal, while administration of adjuvant chemotherapy was reported in 19,5% of the cases. Ninety seven women were heterozygous and 8 were homozygous for CYP2D6*4 respectively. This incidence was in accordance to the Hardy-Weinberg equilibrium. DNA for genotyping was extracted exclusively from patients’ peripheral blood. CYP2D6 was shown to be an independent predictor of outcome, as its reduced activity was associated with recurrence and breast cancer specific survival. These results were more prevalent in the premenopausal subgroup of this cohort. As a possible explanation for this relationship, the authors proposed the higher endogenous estrogen levels in premenopausal ages that require efficient transformation into anti-estrogenic metabolites[19].
| Ref. | Parameter | Extensive metabolisers | Intermediate metabolisers | Poor metamolisers |
| Goetz et al[8] | RFS DFS OS | NS (P = 0.075) NS (P = 0.097) NS | Worse (P = 0.005) Worse (P = 0.008) NS | |
| Scroth et al[9,10] | RR MR | 14.90% 16.70% | 20.90% 18.00% | 29.00% 22.80% |
| Ramóny Cajal et al[12] | DFS | 118 mo | 114 mo | 98 mo |
| Bijl et al[13] | RBCM | NS | Increased (P = 0.041) | |
| Newman et al[16] | OS | Worse | ||
| Karle et al[17] | PFS | 14 mo | 9 mo | |
| Abraham et al[20] | BCSS | NS | ||
| Nowell[23] | BCR, OS | NS | NS | |
| Rae et al[25] | RR | NS | NS | |
| Regan et al[26] | BCE | NS | NS |
In contrast to the studies mentioned above, Abraham et al[20] published results in the opposite direction. In a large cohort of 3155 patients with confirmed treatment with tamoxifen from United Kingdom, the metabolic status of CYP2D6 was associated with breast cancer survival. In 587 of these patients additional chemotherapy had been administered, while for 1041 patients no data concerning concomitant chemotherapy were available. The authors could prove the absence of relevance between survival and variable CYP2D6 variants and argue therefore against CYP2D6 genetic testing in a clinical setting. A separate subset analysis for allele *4 showed also no statistically significant association regarding breast cancer specific- or overall-survival[20].
On the other hand, some published studies report a protective effect of CYP2D6*4. The relation between CYP2D6*4 genotype and tamoxifen therapy was validated in a cohort of 226 postmenopausal patients participating in the Stockholm Breast Cancer Group clinical trial. Nine patients were genotyped to be homozygous carriers of allele *4, while further 55 were wt/*4. Comparison was made among arms with and without tamoxifen therapy. Tamoxifen-treated patients with at least one CYP2D6*4 allele had a significantly longer survival time than those being homozygous for wildtype genotype[21]. In a different and larger study by the same authors, data of 677 patients from Sweden, all treated with tamoxifen for stage II and III breast cancer, have been analysed. Patients homozygous for allele *4 showed a significant survival advantage when compared to the other two genotype patterns (wt/*4 or wt/wt)[22]. Nowell et al[23] investigated 337 breast cancer patients registered in the Arkansas Cancer Research Center of the USA, performing a CYP2D6*4 genotyping. 162 patients were under tamoxifen treatment, while the remaining 175 received no hormonal therapy. The number of patients with either one or two alleles *4 in the tamoxifen and the non-tamoxifen arm was 48 and 49 respectively. No remarkable impact of CYP2D6*4 on recurrence or overall survival in both study arms could be detected. The authors did actually mention that in all subgroups, the CYP2D6*4 variant seemed to have a slight beneficial role as it was associated with decreased risk of recurrence or death[23]. A case-controled study in a subgroup of patients enrolled in the Women’s Environment Cancer and Radiation Epidemiology (WECARE) study investigated the association between CYP2D6 polymorphisms and risk for contralateral breast cancer among patients receiving tamoxifen. The authors failed to detect such an association in a cohort of 119 and 312 women with contra- and unilateral disease respectively. It should be mentioned that 39 patients had ER negative tumour status, while a small proportion of patients with ER positive cancers was treated with additional chemotherapy. This observation was also issued among CYP2D6*4 carriers[24]. The Arimidex, Tamoxifen Alone or in Combination (ATAC) study was a double-blind randomized clinical trial in which postmenopausal women with early-stage breast cancer were randomly assigned to receive anastrozole alone, tamoxifen alone, or a combination of both agents in a double-blind fashion. Rae et al[25], designed a genetic substudy including 588 patients who enrolled in the tamoxifen group of the ATAC trial only in the United Kingdom. All these women were genotyped for CYP2D6, while 544 of them had hormone receptor-positive cancers. The investigators could not detect any statistically significant association between CYP2D6 activity (EM, IM or PM) and breast cancer outcomes for patients with ER+ early-stage breast cancer treated with adjuvant tamoxifen. They reported that their results could not be ascribed to any clinical pathological factors such as tumor size, grade, nodal status, and age. The Breast International Group (BIG) 1-98 study was a randomized, phase 3, double-blind trial that compared five years of treatment with various adjuvant endocrine therapy regimens in postmenopausal women with ER+ breast cancer. Regan et al[26] investigated the clinical relevance of CYP2D6 phenotype by obtaining tissue samples from 4861 of 8010 postmenopausal women who enrolled in this prospective trial. A specific correlation for allele *4 has been also performed. The CYP2D6 results are interestingly very similar to these from ATAC. Patients who were homozygous (PM phenotype) or heterozygous (IM phenotype) for CYP2D6*4 variant had risk of breast cancer events that were not statistically significantly different from patients who were homozygous for wild-type alleles. CYP2D6*4 status did not influence the outcome in patients under tamoxifen therapy. According to these findings, CYP2D6 genetic testing is not recommended prior to adjuvant administration of tamoxifen. The results of the BIG 1-98 trial have been presented as recommendation at the 33rd annual San Antonio Breast Cancer Symposium, 2010. The results of another large prospective multicenter randomised trial (Austrian Breast and Colorectal Study 8 - ABCSG trial 8) have been newly published. In this cohort 3901 postmenopausal patients with resected ER+ early cancer were randomised to receive either five years tamoxifen or two years tamoxifen followed by anastrazole administration over three more years. The incidence of negative events between carriers of one or two poor alleles (*3, *4, *6, *10, *41) vs patients with two extensive alleles was compared. The possibility of such an event was significantly higher when poor alleles were present and only during the period of tamoxifen administration. It should be noted that *4 was present in 58.45% of all non-EM cases[27].
The advisory Committee for Pharmaceutical Science (Clinical Pharmacology Subcommittee) recommended in 2006 that CYP2D6 variations influence the levels of endoxifen and that patients with ER+ breast cancer who are poor metabolizers (due to genotype or drugs co-administration) have an increased risk for breast cancer recurrence. Contrary to this statement and due to lack of strong evidence, the American Society of Clinical Oncology (ASCO)[28] and the St. Gallen’s expert consensus (2011) do not recommend the routine use of CYP2D6 genotyping in the setting of decision making about administration of tamoxifen.
The analysis of existing literature regarding the clinical importance of CYP2D6 polymorphism in the outcome of breast cancer actually leads to conflicting results. Notably, the contradictory findings on this topic might also be due to the heterogeneity across the studies in terms of data collection, analysis and interpretation.
Interestingly and contrary to the main hypothesis, there are three reports that support improved outcome in patients who are carriers of CYP2D6*4[21-23]. Although the interpretation of these findings remains unclear, these studies do actually support the effectiveness of tamoxifen in the group of intermediate and poor metabolizers. Since then, no other large study could confirm such a favourable impact of allele*4 in the clinical course of ER+ breast cancer.
A large number of published studies show a clear negative relationship between CYP2D6*4 genotype and breast cancer outcome. In five of them the investigation focused exclusively on allele*4, while in the study of Newmann et al[16] performed in patients with familiar breast cancer, a subanalysis for *4 alone has also been performed[7,8,11,13,15]. In six other, the analysis included a larger pool of intermediate/poor metabolizers based on a combined basis of common non-functional alleles, including allele*4[6,7,9,11,14,16]. All of them were unique regarding the worse clinical course of ER+ breast cancer under tamoxifen therapy in non-extensive metabolizers. In the study of Thompson, a higher recurrence risk could be proven, in a sub-group analysis of only post-menopausal women, while Margolin et al[19] described this association mainly in premenopausal subjects.
Furthermore, there are numerous studies supporting the strong relation of CYP2D6 genotype on the circulating serum levels of tamoxifen metabolites. A recent work of Zafra-Ceres et al[29] studied 90 Spanish women with ER positive breast cancer concluded that the CYP2D6*4/*4 genotype was associated with statistically significant lower 4-OH tamoxifen and endoxifen levels. Irvin et al[30] examined a brighter combination of CYP2D6 alleles and reported similar results. It could be additionally demonstrated that a daily dose adjustment from 20 to 40 mg could sufficiently increase the endoxifen concentration in patients with impaired CYP2D6 function. Although this data indirectly support the main hypothesis, there is no strong evidence about the influence of endoxifen levels on the clinical course of breast cancer[30].
On the other hand, a large population based case-cohort study from the United Kingdom showed no association between any CYP2D6 variants and breast cancer specific survival in the setting of tamoxifen therapy. A sub-group analysis regarding allele*4 alone gave consistent results. However, it should be mentioned that a large proportion of included cases had received concomitant chemotherapy[20]. Furthermore, Brooks et.al investigated a cohort of breast cancer patients, pooled from the WECARE study, and showed that the CYP2D6*4 variant is not associated with higher risk of contralateral breast cancer, in the setting of tamoxifen treatment.
All these reported studies implicating allele*4 are retrospective and partially refer to limited cohort numbers. It is reasonable to assume that due to the small number of subjects, the proportion of poor metabolizers is unavoidably limited, a fact that influences the level of evidence of any extracted results regarding this group.
The comparison groups within the existing studies varies, as the heterozygous genotypes wt/*4 (IM) are not consistently stratified in one comparison group. Although most published studies classify them together with the homozygous *4 genotype, in three studies they are incorporated in the homozygous wt arm[7,13,22].
The function of CYP2D6 can be altered or inhibited by several pharmaceutical substances. Agents such as fluoxetine, paroxetine, bupropion quinidine and cinacalcet act as strong inhibitors of CYP2D6 and should therefore be cautiously prescribed in combination with tamoxifen[31]. Unfortunately, there is little and inconsistent data within the majority of available studies, regarding the co-administration of a CYP2D6-inhibitor. This fact strongly insults the reliability and the evidence level of any statistically significant result.
Another important aspect is the adherence of examined population to therapy. It is known that the long lasting administration of tamoxifen is associated with various adverse effects, such as hot flashes, that strongly impair the quality of life of the treated patients. Due to possible side effects, incompliance or therapy discontinuation, especially between extensive metabolizers or non-trial women, remains relatively high[32]. Although data over the adherence to tamoxifen therapy should be known in order to safely validate the existing results, this information is absent in nearly all relevant studies[22].
The estrogen-receptor status of breast malignancies clearly affects the outcome of the administered hormonal therapy. Many of all existing studies analyse cohorts that include a number of patients with ER-negative disease[15,16,20,23,24], while two other provide no sufficient data regarding the hormonal status of the enclosed population[11,13]. This condition represents a strong limitation in the interpretation of the published results.
It is important to mention that the three latest large studies refer exclusively to postmenopausal women with ER positive breast cancer disease[25-27]. Goetz et al[27], have proven a statistically important incidence of negative events within carriers of poor CYP2D6 variants, while the two other studies failed to confirm such an association. Although they favour against a CYP2D6 genetic testing prior to tamoxifen therapy, it should be mentioned that both studies showed a substantial departure from the Hardy-Weinberg equilibrium regarding the frequency of allele*4[25,26].
An important bias of most published trials is the followed genotyping procedure, as the estimation of CYP2D6 variant was performed at paraffin-fixed cancer tissue and not at hosts’ blood-or buccal-derived DNA. However, tumor DNA may show significant differences from germline DNA, considering the frequent occurrence of “loss of heterozygosity” in cancer biology. A possible misclassification leads to a smaller arm of intermediate metabolizers (IM) in favour of extensive metabolizers (EM), a fact that strongly insults the final results and conclusions.
Furthermore and in order to safely evaluate the influence of CYP2D6 genotype on tamoxifen therapy, any performed study should actually enclose patients treated with tamoxifen monotherapy.
These two last conditions are unfortunately not satisfied in all three large prospective trials. It should be mentioned that the examined DNA was extracted from formalin-fixed paraffin-embedded tumor tissue, while concomitant chemotherapy is reported in a large proportion of the cohorts. These strong limitations set the reliability of the various results in doubt.
As a final comment, it remains questionable whether the possible ethnic heterogeneity of the examined population within the existing cohorts has an influence on any extracted results. This assumption makes the evaluation of all this data extremely difficult.
CYP2D6*4 consists the most frequent allele with poor metabolizing function among Caucasians, that possibly affects the clinical course of breast cancer. At present there is an ongoing debate, whether a dose or duration adjustment of administered tamoxifen could guarantee a better outcome in carriers of CYP2D6*4.
The current recommendations regarding breast cancer treatment do not include the routine use of CYP2D6 genotyping in the adjuvant setting of tamoxifen. On the other hand, there is strong evidence supporting a negative impact of allele*4 in the outcome of the disease, that should not be ignored. Large prospective trials, limited exclusively to postmenopausal women with ER positive breast cancer, have been newly completed and offer a huge bank of data eligible to further assessment. These three latest studies lead to conflicting results and are all amenable to criticism due to numerous limitations. In European countries with consistent and homogeneous population the frequency of CYP2D6*4 is expected to be quiet high. For this group of patients, the question about the clinical importance of CYP2D6*4 in the course of breast cancer remains practically unanswered. Future research should explore prospectively the role of CYP2D6 activity in patients receiving tamoxifen, in particular in premenopausal patients for which tamoxifen represents the standard of care in the adjuvant and metastatic setting.
P- Reviewer: Fedele P S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1297] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 2. | Lu WJ, Xu C, Pei Z, Mayhoub AS, Cushman M, Flockhart DA. The tamoxifen metabolite norendoxifen is a potent and selective inhibitor of aromatase (CYP19) and a potential lead compound for novel therapeutic agents. Breast Cancer Res Treat. 2012;133:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Wang B, Yang LP, Zhang XZ, Huang SQ, Bartlam M, Zhou SF. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab Rev. 2009;41:573-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Teh LK, Bertilsson L. Pharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importance. Drug Metab Pharmacokinet. 2012;27:55-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Walko CM, McLeod H. Use of CYP2D6 genotyping in practice: tamoxifen dose adjustment. Pharmacogenomics. 2012;13:691-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312-9318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 563] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 8. | Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 466] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, Simon W, Eichelbaum M, Brauch H. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187-5193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 319] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, Fritz P, Simon W, Suman VJ, Ames MM. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 383] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Gonzalez-Santiago S, ZÐrate R, Haba-RodrØguez J, Gmez A, Bandrs E, Moreno P, Borrega P, Garca-Foncillas J, Aranda E. CYP2D64 polymorphism as blood predictive biomarker of breast cancer relapse in patients receiving adjuvant tamoxifen (abstract 590). J Clin Oncol. 2007;25:25s (June 20 Supplement). [Cited in This Article: ] |
| 12. | Ramóny Cajal T, Altés A, Paré L, del Rio E, Alonso C, Barnadas A, Baiget M. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. 2010;119:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Bijl MJ, van Schaik RH, Lammers LA, Hofman A, Vulto AG, van Gelder T, Stricker BH, Visser LE. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat. 2009;118:125-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Thompson AM, Johnson A, Quinlan P, Hillman G, Fontecha M, Bray SE, Purdie CA, Jordan LB, Ferraldeschi R, Latif A. Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011;125:279-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Bonanni B, Macis D, Maisonneuve P, Johansson HA, Gucciardo G, Oliviero P, Travaglini R, Muraca MG, Rotmensz N, Veronesi U. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol. 2006;24:3708-3709; author reply 3709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Newman WG, Hadfield KD, Latif A, Roberts SA, Shenton A, McHague C, Lalloo F, Howell S, Evans DG. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res. 2008;14:5913-5918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Karle J, Bolbrinker J, Vogl S, Kreutz R, Denkert C, Eucker J, Wischnewsky M, Possinger K, Regierer AC. Influence of CYP2D6-genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res Treat. 2013;139:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Stingl JC, Parmar S, Huber-Wechselberger A, Kainz A, Renner W, Seeringer A, Brockmöller J, Langsenlehner U, Krippl P, Haschke-Becher E. Impact of CYP2D6*4 genotype on progression free survival in tamoxifen breast cancer treatment. Curr Med Res Opin. 2010;26:2535-2542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Margolin S, Lindh JD, Thorén L, Xie H, Koukel L, Dahl ML, Eliasson E. CYP2D6 and adjuvant tamoxifen: possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics. 2013;14:613-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, Luccarini C, Shah M, Ingle S, Greenberg D. CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res. 2010;12:R64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Wegman P, Vainikka L, Stål O, Nordenskjöld B, Skoog L, Rutqvist LE, Wingren S. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7:R284-R290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Wegman P, Elingarami S, Carstensen J, Stål O, Nordenskjöld B, Wingren S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9:R7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C, MacLeod SL, Kadlubar FF, Ambrosone CB. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Brooks JD, Teraoka SN, Malone KE, Haile RW, Bernstein L, Lynch CF, Mellemkjær L, Duggan DJ, Reiner AS, Concannon P. Variants in tamoxifen metabolizing genes: a case-control study of contralateral breast cancer risk in the WECARE study. Int J Mol Epidemiol Genet. 2013;4:35-48. [PubMed] [Cited in This Article: ] |
| 25. | Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, Salter J, Sestak I, Cuzick J, Dowsett M. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:452-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thürlimann B, Lyng MB. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012;104:441-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 27. | Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL, Kuffel M, Jakesz R, Rudas M, Greil R. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19:500-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784-3796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 547] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 29. | Zafra-Ceres M, de Haro T, Farez-Vidal E, Blancas I, Bandres F, de Dueñas EM, Ochoa-Aranda E, Gomez-Capilla JA, Gomez-Llorente C. Influence of CYP2D6 polymorphisms on serum levels of tamoxifen metabolites in Spanish women with breast cancer. Int J Med Sci. 2013;10:932-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Irvin WJ, Walko CM, Weck KE, Ibrahim JG, Chiu WK, Dees EC, Moore SG, Olajide OA, Graham ML, Canale ST. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J Clin Oncol. 2011;29:3232-3239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 31. | Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry. 2009;70:1688-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Rae JM, Sikora MJ, Henry NL, Li L, Kim S, Oesterreich S, Skaar TC, Nguyen AT, Desta Z, Storniolo AM. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9:258-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |