Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.791
Revised: April 18, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Heart failure (HF) is a leading cause of mortality and morbidity in western countries and occasions major expenses for public health systems. Although optimal medical treatment is widely available according to current guidelines, the prognosis of patients with HF is still poor. Despite the etiology of the disease, increased systemic or cardiac activation of the innate immune system is well documented in several types of HF. In some cases there is evidence of an association between innate immune activation and clinical outcome of patients with this disease. However, the few large trials conducted with the use of anti-inflammatory medication in HF have not revealed its benefits. Thus, greater understanding of the relationship between alteration in the immune system and development and progression of HF is urgently necessary: prior to designing therapeutic interventions that target pathological inflammatory processes in preventing harmful cardiac effects of immune modulatory therapy. In this regard, relatively recently discovered receptors of the innate immune system, i.e., namely toll-like receptors (TLRs) and nod-like receptors (NLRs)-are the focus of intense cardiovascular research. These receptors are main up-stream regulators of cytokine activation. This review will focus on current knowledge of the role of TLRs and NLRs, as well as on downstream cytokine activation, and will discuss potential therapeutic implications.
Core tip: Heart failure (HF) is a leading cause of morbidity and mortality despite of current medical and interventional treatment. Activation of the innate immune system leading to or contribute to advanced HF is focus of intense and growing research. This review will focus on the role of innate immune receptors in HF. We will discuss the current knowledge about the correlation of innate immune activation and the clinical course in HF. In addition, we will comment on potential therapeutic implications of modulating the immune system in this syndrome.
- Citation: Wagner KB, Felix SB, Riad A. Innate immune receptors in heart failure: Side effect or potential therapeutic target? World J Cardiol 2014; 6(8): 791-801
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/791.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.791
Heart failure (HF) is a one of the leading cause of mortality and morbidity. In developed countries, 1% to 2% of the adult population suffers from this syndrome[1]. In patients ≥ 70 years of age, the prevalence increases to more than 10%[2]. Although approximately 50% of HF patients have preserved left ventricular (LV) ejection fraction[1], this review will focus on systolic HF, owing to the lack of data on the influence of the immune system on HF with preserved LV ejection fraction.
The etiology of HF is manifold. Systolic HF arises in more than 60% of cases from coronary artery disease (CAD). Among others, dilated cardiomyopathy, myocarditis, alcohol abuse, and chemotherapy are relevant and often reasons for HF. Current treatment of systolic HF has been documented in a large number of randomized, controlled clinical trials[1]. These studies clearly demonstrate the benefits of drugs such as β-blockers, angiotensin, converting enzyme inhibitors, angiotensin receptor antagonists, mineralocorticoid receptor blockers, and new drugs such as ivabradine. These agents reduce mortality and/or improve clinical symptoms of chronic systolic HF by suppression of the renin-angiotensin, aldosterone system, neurohumoral activation and ion channels. In addition to medical treatment, mechanical interventions such as resynchronization therapy have also proven beneficial in selected patients[3]. However, despite current optimal HF treatment, the prognosis of these patients is still poor and is comparable to neoplastic diseases. This underscores the need for additional therapeutic options. Many different pathophysiological and therapeutic concepts are at the focus of intense current research. Despite various etiologies, there is a growing body of evidence in this context from more than two decades of research for innate immune activation-systemic and/or local-in a significant number of patients and in experimental studies[4]. The innate immune system represents the first line of host defense against pathogens. This system is composed of diverse cellular components including granulocytes (basophils, eosinophils and neutrophils), mast cells, monocytes/macrophages, dendritic cells, and natural killer cells[5]. These cells respond to noxious stimuli and conditions, including infections and tissue injuries that can trigger inflammatory responses[6]. Pro-inflammatory cytokines, which can be excessively produced by immune cells, have been identified over the last decades as “downstream effectors” of the innate immune system[7]. Moreover, several clinical studies that apply pharmacological cytokine inhibition have been carried out for various diseases[4]. However, in HF, suppression of the cytokine tumor necrosis factor (TNF) alpha has failed to show a benefit in patients[8]. One reason for this failure may be a general underestimation of the complexity of the innate immune system. The regulation of cytokines is indeed not well understood[7]. In this regard, the discovery of so-called pattern recognition receptors has substantially enlarged understanding of the innate immune system. Two families of receptors, i.e., toll-like receptors (TLRs) and nod-like receptors (NLRs)-have been relatively recently discovered; they regulate the innate immune response[7,9]. This review will discuss the pathophysiology of TLRs and NLRs and their role as therapeutic targets in systolic HF.
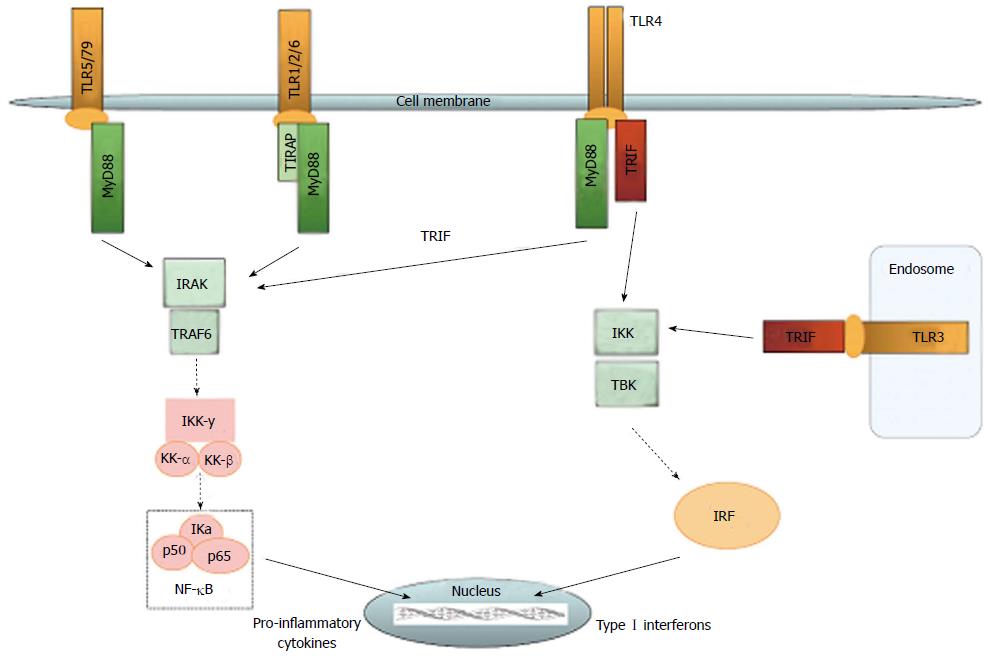
The family of TLRs represents the best known receptor proteins in the innate immune system. The initially discovered TLR4 has by now been known and researched for nearly two decades[10]. Extensive research has led to discovery of ten functional TLRs in humans, and has enabled detailed decoding of the TLR pathway[11]. Still, the role of TLRs in autoimmune diseases has not yet been fully understood. All TLR share a cytoplasmic Toll/IL-R homology (TIR) domain[12]. They reside in different compartments of the cell, with TLR1, 2 and 4-6 on the plasma membrane and TLR3 and 7-10 on intracellular endosomes and lysosomes. In general, cell surface TLRs recognize microbial membrane lipids, and intracellular TLRs respond to microbial nucleic acids[13]. Furthermore, TLR2 recognizes peptidoglycans, TLR3 dsRNA, TLR4 LPS, TLR7 ssRNA, and TLR9 unmethylated bacterial CpG DNA[14]. Beneath their role in immune reaction against pathogens, TLRs can also respond to damage-associated molecular pattern molecules (DAMPs). DAMPs include cell, derived particles such as heat shock proteins (HSP) and high mobility group box (HMGB), particles from the extracellular matrix such as fibronectin, and other substances like oxidized low density lipoprotein and free fatty acids[15]. HSP60 has been shown to activate TLR2 and TLR4 in macrophages[16]. HSP70 also poses an endogenous stimulus to TLR, which leads to release of nitric oxide and tumor necrosis factor[17]. In dendritic cells, TLR2 is activated by hyaluronic acid derived from the extracellular matrix[18]. Upon activation, all TLRs except TLR3 engage the MyD88 pathway. Activated MyD88 forms a complex with IL-1R-associated protein kinases (IRAK4, IRAK1 and IRAK2) (schematic overview see Figure 1). Phosphorylation of IRAK1 leads to activation of tumor necrosis receptor-associated factor (TRAF) 6, which together with IRAK1 forms a new complex. Transforming growth factor-activated kinase (TAK)1, TAK1-binding proteins (TAB)1, TAB2, and TAB3 are recruited to this complex. Upon activation of TAK1 by ubiquitylated TRAF6 IKK-α, IKK-β, and NF-κB essential modulator (NEMO) form a complex, which degrades IκB. This leads to translocation of NF-κB to the nucleus[19]. NF-κB regulates transcription of proinflammatory genes, upregulation cell-adhesion molecules and chemokines, and increasing nitric oxide (NO)[14]. The MyD88-independent pathway is addressed by TLR3 and by TLR4 as an alternative pathway. TLR4 uses the adaptor protein TRIF-related adaptor molecule (TRAM) to activate TIR-domain, containing adapter-inducing interferon-β (TRIF). TRIF can either activate TRAF6-subsequently leading to NF-κB translocation, or can recruit TRAF3, TBK1, and IKKε. This complex phosphorylates interferon regulatory factor (IRF) 3, which induces its translocation to the nucleus and expression of type I interferone genes[19]. Several mechanisms aid in the function of TLR signaling. sCD14 has been known to chaperone lipopolysaccharide (LPS) from LPS binding protein to the TLR4/MD2 complex and thus support induction of TNF-α and interleukin-6 (IL-6) production. Recent research has shown that sCD14 is also capable of promoting internalization of TLR4 and activation of the TRIF-dependent pathway[20]. MHC class II molecules also have the potential of addressing the TLR pathway in a rather unclassical manner. Together with CD40, MHC class II can activate tyrosine kinase Btk, which leads to activation of both the MyD88-and the TRIF-pathways[21]. Another example for support of the proinflammatory TLR pathway is miR-155. This micro RNA interacts with Src homology 2 domain-containing inositol 5-phosphatase-1 (SHIP-1) and thereby restrains it from its control function[22]. A potent system such as the TLR proinflammatory pathway requires not only triggering but, perhaps more importantly, control. Recent years have seen establishment of possible control mechanisms for TLR signaling. SHIP-1 is upregulated after LPS stimulation, owing to increased production of transforming growth factor (TGF)-β, and inhibits PI-3 kinase, which consequently blocks TLR-MyD88 and MyD88 independent pathway[23]. IRAK-M functions as a decoy and prevents IRAK-1 from dissociating MyD88. It suppresses TLR-mediated inflammatory response. IRAK-M knock-out mice demonstrate an increase in inflammatory response and IL-1/TLR-signaling[24]. IRAK-M can also interfere with TLR2, although this downregulation is evidently IRAK-1 independent[25]. Other specific inhibitors are SHP2-which has been shown to inhibit only the TLR3 pathway-and sterile-α and armadillo motif-containing protein (SARM), which blocks only the TRIF-pathway without inhibiting MyD88 signaling[26,27]. An alternative splice variant of MyD88 is expressed after LPS stimulation. This variant, MyD88s, inhibits phosphorylation of IRAK1 by IRAK4, and leads to a suppression of the TLR pathway[28]. While microRNA is involved in the promotion of TLR signaling, it also plays an important role in anti-inflammation. miR-146- and miR-21-levels increase after LPS stimulation. miR-146 interacts with TRAF6 and IRAK1, which leads to decreased mRNA levels of both - whereas miR-21 inhibits PDCD4, which is an inhibitor of IL-10[29]. IKKβ, involved in the TLR-pathway, also has anti-inflammatory capacity by virtue of regulating the activation of the prosurvival kinase Akt1[30]. MHC class I also has a rather untypical function. It can be phosphorylated after TLR activation and can then activate Fps tyrosine kinase, which interferes with TLR signaling[31]. While evidence suggests a possible pro-inflammatory role of MHC class II, MHC class I evidently supports anti-inflammatory effects.
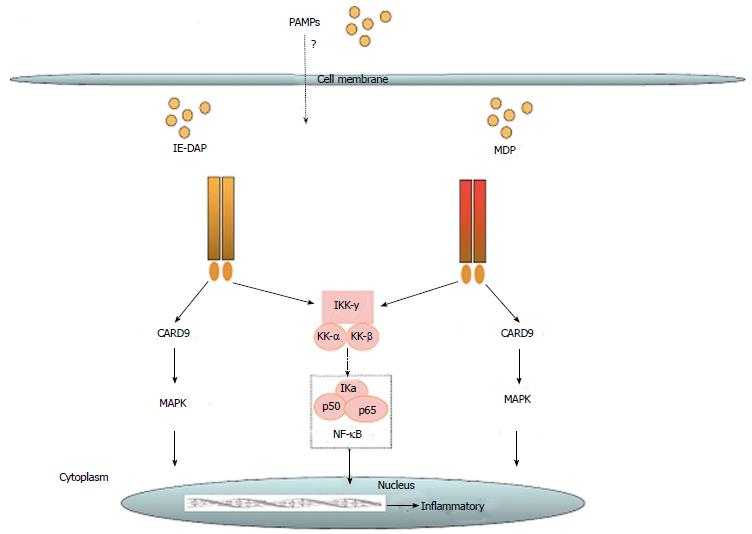
The nucleotide-binding and oligomerization domain NLR family is defined by a common nucleotide binding domain and leucine-rich repeat series. All NLRs have a central ATPase region, which is called the NACHT domain[32]. Until now 22 NLRs have been identified in humans. They differ from each other by heterogeneous N-terminal effector domains and can be divided into four subgroups. Class II transactivator (CIITA) is defined by an acidic transactivation domain (AD), and neuronal apoptosis inhibitor proteins (NAIPs) contain a baculovirus inhibitor of apoptosis protein repeat (BIR). The caspase recruitment domain (CARD) is common for NLRCs, including nod 1 and nod 2 (schematic overview see Figure 2). Finally, NLRPs share a pyridine domain (PYD). NLRs are important pattern recognition receptors in the intracellular compartment. Their activation leads to activation of innate immunity through nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK), and interferon regulatory factors (IRFs)[33]. NLRPs contribute to innate immunity by formation of the inflammasome. This complex is formed by NLRP1, NLRP2, NLRP3, and NLRC4 upon recognition of physical damage to the plasma membrane or certain pathogen-associated molecular particles (PAMPs). Inflammasome formation can directly lead to caspase 1 activation, or it can recruit the adaptor protein ASC (apoptosis-associated speck-like protein containing a casapase recruitment domain) to activate caspase 1[34]. Serving another means of controlling infection, nod 1 and nod 2 can induce autophagy through activation of LC3-positive speckles[35]. In dendritic cells, nod 2-induced autophagy apparently plays a key role for bacterial elimination and antigen presentation[36]. The role of NLR has been implied for some diseases. In patients with early-onset Crohn’s disease, a frameshift mutation of the nod 2 gene has been found, while in early-onset sarcoidosis a gain of function mutation of the NACHT domain has been identified[37]. There also is an indication for activation of NLRP3 in microglia in Alzheimer’s disease by peptide amyloid-β[38].
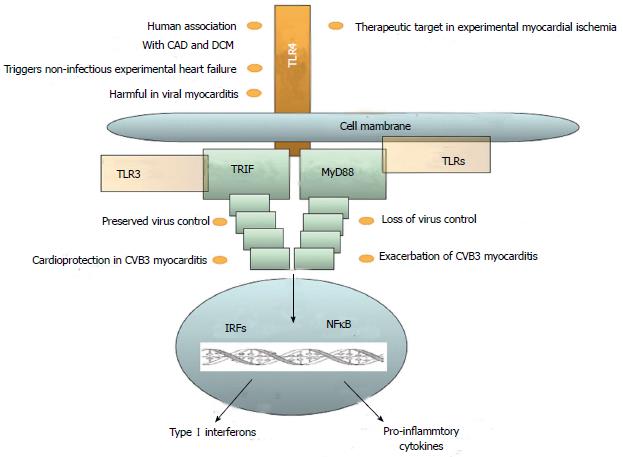
The role of the innate immune system in HF has been controversially discussed. Inflammation plays an important role in most cardiac diseases, and receptor-mediated innate immunity is primarily investigated with respect to TLRs. The role of innate immune cells and NLRs is also subject to current research. All known human TLRs have been found in the heart. However, until yet, the best-characterized TLR in cardiovascular diseases is TLR4 (Figure 3). Their expression level, however, varies greatly. Expression of TLR4, TLR3, and TLR2 is at least 10 times higher than that of any other TLR in the heart[39]. Although TLRs were first known for their role in innate immunity in their action against infection, inflammation in the heart is rarely caused by infectious agents. Other mechanisms lead to an inflammatory response, which often activates TLR pathways. Hemodynamic stress results in inflammation in the myocardium. Myocardial stress increases IL-6 production, which leads to an inflammatory response in the same manner as production of reactive oxygen species (ROS) due to mechanical strain. Macrophage infiltration is triggered by MCP-1 and TGF-β[40]. TNF-α is released by macrophages, mast cells, endothelial cells, and fibroblast. This secretion is triggered not only by infectious agents but also by tissue damage[41]. Necrosis in the myocardium leads to distribution of intracellular particles, which in turn activates the innate immune system. ROS activates innate immune response, but also directly impairs cardiac function. DAMPs activate the complement system and TLRs at the same time[42]. After activation of the TLR pathway, NF-κB induces the expression of pro-inflammatory cytokines and chemokines in endothelial cells, fibroblasts, leukocytes, and vascular cells[43]. Although research has disclosed little for the involvement of NLR in HF, studies have taken place on the effects of the inflammasome in the ischemia-reperfusion model. These results have revealed that mice deficient in caspase-1 or ASC have markedly reduced infarct formation, fibrosis, and cardiac dysfunction. It was further shown that inflammasome activation and IL-1β production occurred primarily in cardiac fibroblasts and leukocytes. This leads to the conclusion that NLRs do play a role in cardiac remodeling and may represent an interesting therapeutic target in the future[34]. Various immune cells evolve to serve functions in primary immune response to tissue damage in the heart, but may also perform a key function in limiting inflammation. Recent investigations have begun to unravel the complex system of macrophage subspecies and functions.
Until now, two different phenotypes have been defined. M1 macrophages are described as first line of defense, with their increased microbicidal capacity as well as production of pro-inflammatory cytokines. M2 macrophages show increased phagocytic activity: they secrete the anti-inflammatory IL-10 and express IL-1 receptor antagonist[44]. The definition of just two subtypes is most likely oversimplified, and a functional perspective could prove more useful in distinguishing pro-inflammatory, regulatory, and reparative macrophages. The phenotype of macrophages is probably defined by a constantly changing variety of cytokines, chemokines, and growth factors, which enable great flexibility in the system[45]. Regulatory T cells (Tregs) have also been reported to possibly influence the macrophage phenotype. These regulatory cells suppress inflammation through IL-10 and TGF-β secretion, or by cell-cell contact. Mice lacking CCR5–which thus reduces Treg infiltration - show increased inflammation and MMP activity[46].
To give some order to these many consequences of cardiac tissue damage, the example of ischemia/reperfusion injury can provide an overview of the immune response. Three phases can be determined that lead to adverse cardiac remodeling. First, neutrophils and pro-inflammatory macrophages migrate to the infarct site, with attraction by chemokines and cytokines secreted upon activation of innate immune pathways. Upon finishing the task of clearing the infarct site of necrotic cells, neutrophils go into apoptosis, which ends the inflammatory phase. Various macrophage subtypes migrate to the infarct in the proliferative phase. They activate endothelial cell growth and myofibroblast formation, with resultant production of a scar. In the final phase, more cells go into apoptosis and collagen cross-links, which possibly leads to ventricle dilation as the infarct matures[47].
During recent decades, intensive research has led to better understanding of ischemia/reperfusion (I/R) injury. I/R injury leads to rapid activation of the immune system, which in turn results in increased expression of TNF, IL-1β, IL-6, NO as well HSP[48,49]. These and other factors lead to infiltration of the infarct with neutrophil granulocytes. In canine and mouse models, infiltration ceased after 3-7 d, and the neutrophils went into apoptosis[50]. Early infiltration of the myocardium can cause more extensive cytotoxic injury to viable cardiomyocytes, which leads in turn to additional damage in the heart[51]. ROS generated by neutrophils may contribute to that adverse effect, as well as interaction with cardiomyocytes through intercellular adhesion molecule-1 (ICAM-1) and integrin[52]. To partially control the immune response, annexin and lactoferrin are transmitted by dying neutrophils to terminate further migration of neutrophils-but at the same time possibly attract macrophages to the site[53]. Furthermore, TNF-α, released at the infarcted area by resident mast cells, also promotes mononuclear cell infiltration[54]. These macrophages begin ingesting the apoptotic cells and, in turn, release cytokines as IL-10, TGF-β proresolving lipoxins, and resolvins[55]. Upregulation of IL-10 and TGF-β suppress production of adhesion molecules. Another process for inhibition of leukocyte adhesion is carried out by endogenous integrin ligands of endothelial cells[56]. Some experiments conducted on knock-out mice have provided further insights on the involved immune cells. The attempt to evade the effect of macrophage I/R injury was analyzed in monocyte chemoattractant protein (MCP) 1/CCL2 knock-out mice. MCP1 recruits pro-inflammatory and phagocytic macrophages to the infarct. Compared to wild-type mice, knock-out mice exhibited reduced dilative remodeling with equal infarct size[57]. Similar results were achieved for IL-1-deficient mice. Although infarct size did not vary, the extent of cardiac remodeling was reduced in deficient mice compared to wild type[58]. Those findings support the theory that the initial immune response does not pose the problem, but that long-term activation causes adverse effects. This could partly explain results for ICAM1-deficient mice. Once again compared to wild type, they showed no difference in infarct size, even after 1-3 wk[59]. The same applies to mice with ICAM1 and P-selectin deficiency. Neutrophil migration was decreased, but infarct size did not vary compared to wild type[60]. These results could also suggest that the role of neutrophils has been overestimated. ROS is a mediator among others secreted by neutrophils. It can activate complements, stimulate P-selectin expression promoting cell migration, and upregulate chemokine and cytokine synthesis through the NF-κB pathway[61]. ROS, as well as ATP and potassium abundance, may activate the inflammasome. The inflammasome is expressed by border-zone cardiomyocytes, white blood cells in the granulation tissue, and cardiac firboblasts. Inflammasome formation can be inhibited by P2X7 and cryopyrin, which leads to a decrease in infarct size[62]. Research on TLRs involved in I/R-injury focusses mainly on TLR2 and TLR4. TLR2 seems to play a key role. TLR2 knock-out mice demonstrate better contractile function after I/R injury, and they show similar infarct size, but less ventricular remodeling compared to wild type. Fibrosis is reduced in the non-infarct area, and TGF-β and collagene type 1 expression are lower in knock-out mice. The recovery of LV-developed pressure is also better in TLR2-deficient mice[63,64]. Further research has focused on the transmission of this effect to determine whether it entailed a central effect using TLR2 in the heart, or a peripheral effect involving white blood cells. Infarct size was compared for TLR2-deficient mice and wild-type mice with TLR2-deficient bone marrow. Infarct size did not differ significantly. When TLR2-deficient mice were injected with wild-type bone marrow, infarct size increased compared to purely TLR2-deficient mice. It was possible to inhibit this effect by administering an TLR2 antagonist-which resulted in smaller infarcts, enhanced overall cardiac function, and reduced inflammation and apoptosis[65]. We and others investigated the role of TLR4 in myocardial infarction. TLR4-deficient mice displayed an improved outcome and decreased cardiac inflammation, as also revealed by others[66,67]. Moreover, pharmacological inhibition of TLR4 using the antagonist eritoran led to beneficial effects, which suggests a potential new therapeutic strategy in myocardial ischemia, at least under experimental conditions[68]. Mice deficient in TLR4 also showed smaller infarct size after I/R injury[69]. Pre-treatment with LPS at 24 h before an I/R injury experiment results in better LV function compared to the sham group[70]. TLR2-TIRAP signaling mediates this effect, in which GSK-3β is subsequently inactivated–which prevents it from destabilizing mitochondria and leading to cell death[71].
The role of the innate immune system in viral cardiomyopathy has been primarily established by experiments using mice infected with coxsackievirus B3 (CVB3). In humans it is known that cardiac CVB3 infection needs intact interferon-I signaling[72]. TLR4-deficient mice exhibited higher titers of coxsackievirus B3 (CVB3) two days after infection, but decreased titers and myocarditis in a 12-d follow up. The cytokines IL-1β and IL-18 were reduced in TLR4-deficient mice[73], Knock-out mice deficient for the TLR downstream adapter protein MyD88 are protected from CVB3 infection[74]. Interestingly, we found in TRIF knock-out mice a much higher susceptibility to CVB3 infection when compared to wild-type mice: to include induction of mortality, loss of virus control, and exacerbation of pro-inflammatory cytokine expression in heart tissue[75]. These data from MyD88 and TRIF knock-out mice suggest not only harmful effects of TLRs, but also cardioprotection in CVB3-induced myocarditis. TLR7 and TLR9 contribute to the susceptibility of MyD88-deficient mice in experimental myocarditis[76]. This is also strengthened by our finding that shows that MyD88 may contribute to the modulation of TLR9 in CVB3-induced myocarditis in mice[77]. In another study, infection of TLR3-deficient mice with encephalomyocarditis virus (EMCV)-a ssRNA virus-interestingly led to earlier death in knock-out mice, combined with increased viral replication and myocardial injury[78]. The mRNA expression of TNF, IL-1β, and IL6 was down-regulated, whereas IFN-β was up-regulated[78]. IRAK-deficient mice and MyD88-deficient mice both exhibit lesser degrees of myocarditis and viral replication after infection, as well as improved survival. Levels of IFN-β were higher in MyD88 knock-out, and IFN-α and IFN-γ were increased in IRAK knock-out. Overall inflammation was reduced[74,79]. Knock-out in cytokines/chemokines led to higher mortality, a greater extent of myocardial injury, higher viral titers for TNF knock-out and EMCV infection, as well as NO knock-out and CVB3 infection[80,81].
Activation of the immune system is widely considered a pathophysiological mechanism in DCM[82-87]. For example, we disclosed that the initial white blood cell count upon initial hospital admission in DCM patients predicts long-term mortality in patients with DCM and severe LV dilation[84]. In addition, genetic variants of TLR4 are significantly associated with cardiac recovery in DCM patients, which suggests a potential role of receptor-mediated innate immunity in this disease[88]. Since TLRs are evidently involved in HF, and although viral or bacterial agents are much less frequently the cause than is ischemia, for example, it is interesting to examine a number of known DAMPs and their link to HF. For HSP60 and HSP70, a possible connection to HF has been evidenced. Both are increased in advanced HF. HSP60 trafficking through the plasma membrane is linked to apoptosis, and serum levels of HSP70 correlate with the severity of cardiac dysfunction[89]. Decreased levels of TLR2 and TLR4 have been defined in all subgroups of cardiomyopathy, ischemic cardiomyopathy (ICM), dilated cardiomyopathy (DCM), and viral cardiomyopathy (VCM), whereas TIRAP and IRAK4 are up-regulated[7]. A much wider overview of genetic alternations in cardiac disease allows fundamental compound analysis of innate immune signaling genes. It has showed that the failing heart shows a different expression plot when compared to non-failing heart tissue. Further gene expression in viral cardiomyopathy and idiopathic dilated cardiomyopathy is similar and is distinguished from ischemic cardiomyopathy. This phenomenon suggests different immunological involvement of VCM and DCM compared to ICM, and supports the theory that DCM may evolve from VCM.
Early studies on the influence of the inflammatory response in HF confirmed the harmful effects of methylprednisolone administration in patients with myocardial infarction[90]. Since that time, many new options have evolved. Nevertheless, it is apparently no less difficult to achieve a positive result, even though current knowledge of innate immunity in HF is much more detailed. These difficulties are obvious in two studies on anti-TNF alpha therapy, with etanercept eventually proving not beneficial and even deleterious[91,92]. Another problem may lie in the limited comparability of humans and animal models. Whereas, in a canine model, antibody-inhibiting leukocyte adhesion acted in a protective manner to limit infarct size by 40% to 50%, there was no effect on infarct size in humans with STEMI administration of CD11b/CD18 integrin receptor inhibition[93,94]. There are, on the other hand, a number of promising substances. TLR4 antagonist eritoran significantly reduces infarct size[68]. New variations of lipid A have been found. They bind to TLR4 but demonstrate reduced agonistic activity (CRX-527, lipad-Iva). TAK-242 also inhibits TLR4 signaling, yet until now its target remains unknown. Ibudilast (AV411) is another TLR 4 antagonist, one that suppresses pro-inflammatory cytokines such as TNF and IL-6. It may induce IL-10 and is currently under trial for opioid dependence. OPN-401, a viral protein-derived peptide, inhibits TLR4 signaling but is still in development. OPN-305 is a promising monoclonal antibody-inhibiting TLR2 and is currently in orphan status for prevention of I/R injury after organ transplantation. AP177-DNA aptamer binds to TLR and antagonizes TLR2 ligand binding[95]. Anakinra, a IL1 receptor antagonist, suppresses post-infarct inflammation and has showed lower incidence of HF[96]. In summary, although knowledge of the pathophysiology of the innate immune system in HF has substantially increased and new therapeutic targets have been addressed under experimental conditions, future investigations, especially clinical trials and experimental research in human tissue–are needed to develop effective innate immune system modulating treatment in HF.
P- Reviewer: Lymperopoulos A, Walker LA S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3411] [Cited by in F6Publishing: 3448] [Article Influence: 287.3] [Reference Citation Analysis (0)] |
| 2. | Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1124] [Cited by in F6Publishing: 1172] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 3. | Jiang C, Xia M, Wang M, Chen S. Dexmedetomidine preconditioning protects isolated rat hearts against ischemia/reperfusion injuries and its mechanism. Zhejiang Daxue Xuebao Yixueban. 2013;42:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1437] [Cited by in F6Publishing: 1438] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 4. | Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Chávez-Sánchez L, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Montoya-Díaz E, Blanco-Favela F. Innate immune system cells in atherosclerosis. Arch Med Res. 2014;45:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3548] [Cited by in F6Publishing: 3908] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 7. | Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol. 2002;86:123-130. [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3900] [Cited by in F6Publishing: 3726] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 11. | Lee C C, Avalos A M, Ploegh H L. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168-179. [Cited in This Article: ] |
| 12. | Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5434] [Cited by in F6Publishing: 6001] [Article Influence: 428.6] [Reference Citation Analysis (0)] |
| 13. | Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Vallejo JG. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond). 2011;121:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Ionita MG, Arslan F, de Kleijn DP, Pasterkamp G. Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. J Innate Immun. 2010;2:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332-31339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 637] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 17. | Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107-15112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 712] [Cited by in F6Publishing: 660] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 18. | Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 534] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 19. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5843] [Cited by in F6Publishing: 6191] [Article Influence: 442.2] [Reference Citation Analysis (0)] |
| 20. | Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 670] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 21. | Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, Yao H, Cao X. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12:416-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106:7113-7118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 644] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 23. | Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Kobayashi K, Hernandez L D, Galan J E, Janeway C A, Jr . Medzhitov R,Flavell R A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191-202. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1069] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 25. | Su J, Xie Q, Wilson I, Li L. Differential regulation and role of interleukin-1 receptor associated kinase-M in innate immunity signaling. Cell Signal. 2007;19:1596-1601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, Xu H, Qian C, Zhou J, Yu Y. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Liang PF, Huang XY, Long JH, Xiao MZ, Yang XH, Zhang PH. Effect of antisense oligonucleotide against Smac/DIABLO on inhibition of hydrogen peroxide induced myocardial apoptosis of neonatal rats. Zhonghua Shaoshang Zazhi. 2006;22:175-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 29. | Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 30. | Ashida N, Senbanerjee S, Kodama S, Foo SY, Coggins M, Spencer JA, Zamiri P, Shen D, Li L, Sciuto T. IKKβ regulates essential functions of the vascular endothelium through kinase-dependent and -independent pathways. Nat Commun. 2011;2:318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Xu S, Liu X, Bao Y, Zhu X, Han C, Zhang P, Zhang X, Li W, Cao X. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat Immunol. 2012;13:551-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Koonin E V, Aravind L. The NACHT family - a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci. 2000;25:223-224. [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Zhong Y, Kinio A, Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;4:333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 34. | Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 633] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 35. | Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 916] [Cited by in F6Publishing: 972] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 36. | Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 775] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 37. | Rosenstiel P, Till A, Schreiber S. NOD-like receptors and human diseases. Microbes Infect. 2007;9:648-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1605] [Cited by in F6Publishing: 1794] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 39. | Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886-892. [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 346] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Coggins M, Rosenzweig A. The fire within: cardiac inflammatory signaling in health and disease. Circ Res. 2012;110:116-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 42. | Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 818] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 43. | Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res. 2011;108:1122-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 406] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 44. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2678] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 45. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5753] [Cited by in F6Publishing: 6320] [Article Influence: 395.0] [Reference Citation Analysis (0)] |
| 46. | Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177-2187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 47. | Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 479] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 48. | Zou N, Ao L, Cleveland JC, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H2805-H2813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Dewald O, Ren G, Duerr G D, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael L H, Entman M L, Frangogiannis N G. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665-677. [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 51. | Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, Smith CW. Inflammation in the course of early myocardial ischemia. FASEB J. 1991;5:2529-2537. [PubMed] [Cited in This Article: ] |
| 52. | Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504-512. [PubMed] [Cited in This Article: ] |
| 53. | Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, Rossi AG, Gregory CD. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20-32. [PubMed] [Cited in This Article: ] |
| 54. | Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 55. | Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 722] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 56. | Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb Haemost. 2009;102:191-197. [PubMed] [Cited in This Article: ] |
| 57. | Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 536] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 58. | Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 59. | Metzler B, Mair J, Lercher A, Schaber C, Hintringer F, Pachinger O, Xu Q. Mouse model of myocardial remodelling after ischemia: role of intercellular adhesion molecule-1. Cardiovasc Res. 2001;49:399-407. [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Briaud SA, Ding ZM, Michael LH, Entman ML, Daniel S, Ballantyne CM. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol. 2001;280:H60-H67. [PubMed] [Cited in This Article: ] |
| 61. | Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456-1462. [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 670] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 62. | Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011;108:19725-19730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 442] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 63. | Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503-H509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905-2910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 65. | Arslan F, Keogh B, McGuirk P, Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010;2010:704202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 66. | Riad A, Jäger S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954-6961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 68. | Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270-I274. [PubMed] [Cited in This Article: ] |
| 69. | Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317-7324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA. 1989;86:2516-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 204] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. Am J Physiol Heart Circ Physiol. 2010;298:H1079-H1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation. 2001;103:756-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731-4737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Fuse K, Chan G, Liu Y, Gudgeon P, Husain M, Chen M, Yeh WC, Akira S, Liu PP. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Riad A, Westermann D, Zietsch C, Savvatis K, Becher PM, Bereswill S, Heimesaat MM, Lettau O, Lassner D, Dörner A. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186:2561-2570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Pagni PP, Traub S, Demaria O, Chasson L, Alexopoulou L. Contribution of TLR7 and TLR9 signaling to the susceptibility of MyD88-deficient mice to myocarditis. Autoimmunity. 2010;43:275-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Riad A, Westermann D, Escher F, Becher PM, Savvatis K, Lettau O, Heimesaat MM, Bereswill S, Volk HD, Schultheiss HP. Myeloid differentiation factor-88 contributes to TLR9-mediated modulation of acute coxsackievirus B3-induced myocarditis in vivo. Am J Physiol Heart Circ Physiol. 2010;298:H2024-H2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Hardarson HS, Baker JS, Yang Z, Purevjav E, Huang CH, Alexopoulou L, Li N, Flavell RA, Bowles NE, Vallejo JG. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251-H258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Valaperti A, Nishii M, Liu Y, Naito K, Chan M, Zhang L, Skurk C, Schultheiss HP, Wells GA, Eriksson U. Innate immune interleukin-1 receptor-associated kinase 4 exacerbates viral myocarditis by reducing CCR5(+) CD11b(+) monocyte migration and impairing interferon production. Circulation. 2013;128:1542-1554. [PubMed] [Cited in This Article: ] |
| 80. | Wada H, Saito K, Kanda T, Kobayashi I, Fujii H, Fujigaki S, Maekawa N, Takatsu H, Fujiwara H, Sekikawa K. Tumor necrosis factor-alpha (TNF-alpha) plays a protective role in acute viralmyocarditis in mice: A study using mice lacking TNF-alpha. Circulation. 2001;103:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Zaragoza C, Ocampo C, Saura M, Leppo M, Wei XQ, Quick R, Moncada S, Liew FY, Lowenstein CJ. The role of inducible nitric oxide synthase in the host response to Coxsackievirus myocarditis. Proc Natl Acad Sci USA. 1998;95:2469-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 83. | Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 433] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 84. | Riad A, Weitmann K, Herda LR, Empen K, Gross S, Nauck M, Dörr M, Klingel K, Kandolf R, Hoffmann W. Initial white blood cell count is an independent risk factor for survival in patients with dilated cardiomyopathy. Int J Cardiol. 2013;168:1207-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Herda L R, Felix S B, Staudt A. Immunoadsorption in patients with dilated cardiomyopathy. Atheroscler Suppl. 2009;10:126-8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Herda LR, Trimpert C, Nauke U, Landsberger M, Hummel A, Beug D, Kieback A, Dörr M, Empen K, Knebel F. Effects of immunoadsorption and subsequent immunoglobulin G substitution on cardiopulmonary exercise capacity in patients with dilated cardiomyopathy. Am Heart J. 2010;159:809-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Staudt A, Staudt Y, Dörr M, Böhm M, Knebel F, Hummel A, Wunderle L, Tiburcy M, Wernecke KD, Baumann G. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Riad A, Meyer zu Schwabedissen H, Weitmann K, Herda LR, Dörr M, Empen K, Kieback A, Hummel A, Reinthaler M, Grube M. Variants of Toll-like receptor 4 predict cardiac recovery in patients with dilated cardiomyopathy. J Biol Chem. 2012;287:27236-27243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238-H2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204-I206. [PubMed] [Cited in This Article: ] |
| 91. | Kwon HJ, Coté TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138:807-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 296] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 92. | Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 857] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 93. | Simpson PJ, Todd RF, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988;81:624-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 536] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 94. | Faxon D P, Gibbons R J, Chronos N A, Gurbel P A, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199-1204. [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 95. | Topkara VK, Evans S, Zhang W, Epelman S, Staloch L, Barger PM, Mann DL. Therapeutic targeting of innate immunity in the failing heart. J Mol Cell Cardiol. 2011;51:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |