Published online Jul 26, 2015. doi: 10.4330/wjc.v7.i7.423
Peer-review started: February 8, 2015
First decision: March 6, 2015
Revised: May 3, 2015
Accepted: May 5, 2015
Article in press: May 6, 2015
Published online: July 26, 2015
AIM: To assess the safety of therapeutic hypothermia (TH) concerning arrhythmias we analyzed serial electrocardiograms (ECG) during TH.
METHODS: All patients recovered from a cardiac arrest with Glasgow < 9 at admission were treated with induced mild TH to 32-34 °C. TH was obtained with cool fluid infusion or a specific intravascular device. Twelve-lead ECG before, during, and after TH, as well as ECG telemetry data was recorded in all patients. From a total of 54 patients admitted with cardiac arrest during the study period, 47 patients had the 3 ECG and telemetry data available. ECG analysis was blinded and performed with manual caliper by two independent cardiologists from blinded copies of original ECG, recorded at 25 mm/s and 10 mm/mV. Coronary care unit staff analyzed ECG telemetry for rhythm disturbances. Variables measured in ECG were rhythm, RR, PR, QT and corrected QT (QTc by Bazett formula, measured in lead v2) intervals, QRS duration, presence of Osborn’s J wave and U wave, as well as ST segment displacement and T wave amplitude in leads II, v2 and v5.
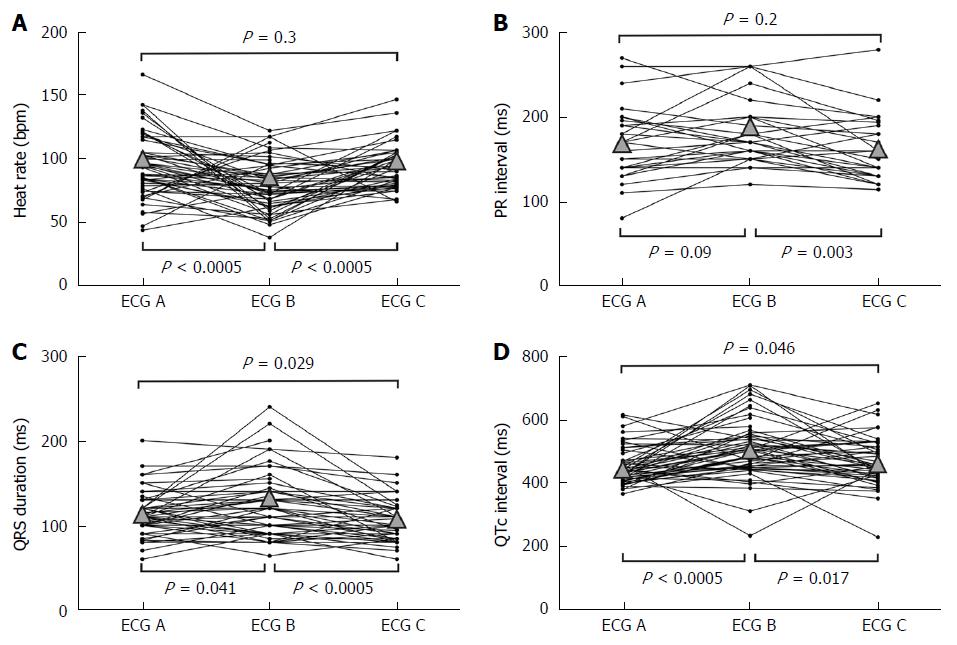
RESULTS: Heart rate went down an average of 19 bpm during hypothermia and increased again 16 bpm with rewarming (P < 0.0005, both). There was a non-significant prolongation of the PR interval during TH and a significant decrease with rewarming (P = 0.041). QRS duration significantly prolonged (P = 0.041) with TH and shortened back (P < 0.005) with rewarming. QTc interval presented a mean prolongation of 58 ms (P < 0.005) during TH and a significant shortening with rewarming of 22.2 ms (P = 0.017). Osborn or J wave was found in 21.3% of the patients. New arrhythmias occurred in 38.3% of the patients. Most frequent arrhythmia was non-sustained ventricular tachycardia (19.1%), followed by severe bradycardia or paced rhythm (10.6%), accelerated nodal rhythm (8.5%) and atrial fibrillation (6.4%). No life threatening arrhythmias (sustained ventricular tachycardia, polymorphic ventricular tachycardia or ventricular fibrillation) occurred during TH.
CONCLUSION: A 38.3% of patients had cardiac arrhythmias during TH but without life-threatening arrhythmias. A concern may rise when inducing TH to patients with long QT syndrome.
Core tip: Induced, therapeutic hypothermia is a treatment for post-cardiac arrest syndrome with a potential survival benefit; however it is not widely used. We aimed to assess the safety of this therapy regarding cardiac arrhythmias through a systematical evaluation of electrocardiograms (ECG) changes during hypothermia and telemetry data. Our conclusions are that therapeutic hypothermia according to current practice is safe with arrhythmias in one third of the patients (38.3%) but no life-threatening arrhythmias. Bradycardia and reversible prolongation of ECG intervals are common findings. A concern may rise when inducing hypothermia to patients with arrhythmias related to long QT syndrome.
- Citation: Salinas P, Lopez-de-Sa E, Pena-Conde L, Viana-Tejedor A, Rey-Blas JR, Armada E, Lopez-Sendon JL. Electrocardiographic changes during induced therapeutic hypothermia in comatose survivors after cardiac arrest. World J Cardiol 2015; 7(7): 423-430
- URL: https://www.wjgnet.com/1949-8462/full/v7/i7/423.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i7.423
In 2002, two randomized clinical trials demonstrated that inducing mild therapeutic hypothermia (TH) between 32 °C and 34 °C Celsius during 12 to 24 h improve survival and neurologic outcome in comatose adults recovered from ventricular fibrillation (VF) cardiac arrest (CA)[1,2]. Thenceforth, the recommendation to induce TH has been extended to non-VF cardiac arrest, and in-hospital CA[3-6]. Today, TH is the only in-hospital treatment that improves survival in comatose patients recovered from a CA[7]. Despite the evidence, TH is still underused nowadays. Some causes have been proposed: technical difficulties, lack of experience with cooling methods, safety concerns and the many gaps on various issues such as optimal target temperature, duration of TH or rewarming rate[8,9].
While there are reports about complications and side effects of hypothermia from more than 50 years ago[10-12], the vast majority of the information on cardiovascular and side effects comes from case reports, accidental deep hypothermia or induced deep hypothermia in cardiac surgery. Known side effects of TH are shivering[13], increased risk of infection[2,14], increased diuresis, electrolyte abnormalities such as hypokalemia, hypophosphatemia and hypomagnesaemia[15], hyperglycemia, coagulopathy with increased risk of bleeding, bradycardia and complex effects in hemodynamics, with small reduction in cardiac output that balances with the decrease of metabolic rate[16]. The randomized clinical trials of TH did not show any differences in arrhythmias between patients assigned to TH or normothermia, but there is paucity of data regarding electrocardiographic abnormalities in humans recovered from a cardiac arrest under controlled mild hypothermia.
In this prospective, observational study we performed a systematic analysis on serial electrocardiograms (ECG) and arrhythmias during TH, in order to describe changes and assess the safety of TH concerning ECG alterations and rhythm disturbances.
We prospectively collected data about every CA admission in the Coronary Care Unit of a Spanish tertiary hospital during a period of 3 years. TH was performed according to current guidelines to those patients recovered from CA with any initial rhythm and Glasgow ≤ 8 at admission. Sedation was obtained with midazolam, fentanyl and muscular relaxation with cisatracurium. All drugs adjusted to body weight and administered by intravenous infusion through a central venous line. Patients where cooled to a target temperature of 32 °C to 34 °C, as soon as possible, with cold fluid infusion. TH was maintained with physical measures (ice packs, isolating blankets) during 24 h in the first 20 patients. The rewarming process was passive, withdrawing cooling measures, during 12 to 24 h. In the last 34 patients an intravascular cooling device (Coolgard 3000®, Zoll medical Corp, Chelmsford, MA) was used to induce, maintain (33 °C for 24 h) and withdraw TH, set to fastest cooling speed at induction and slow rewarming at a rate of 0.08-0.17 °C/h, to slowly rewarm the patient in 12-24 h. Core temperature was measured with a Swan-Ganz catheter or urinary catheter.
During TH, all patients were under mechanical ventilation, muscular relaxation and sedation. Inotropics or vasodilators were used if necessary to maintain a target mean arterial pressure of 80-90 mmHg. Patients underwent urgent coronary angiogram (and percutaneous coronary intervention if necessary) if ST elevation acute coronary syndrome (ACS) or clinical indication. Echocardiogram was performed at admission. Complete 12 lead ECG were recorded at admission (ECG A), during peak hypothermia or minimum stable temperature (ECG B) and after rewarming (but before sedation was withdrawn, ECG C). Continuous ECG telemetry was recorded during TH. The ethical board of the hospital approved TH protocol.
For the present study we selected all consecutive patients (n = 54) that underwent TH. Baseline characteristics of the patients, cooling rates and temperatures of TH protocol, clinical outcome data, ECG telemetry data and original ECG were recorded. ECG analysis was blinded and performed with manual caliper by two independent cardiologists from blinded copies of original ECG, recorded at 25 mm/s and 10 mm/mV. Coronary care unit staff analyzed ECG telemetry for rhythm disturbances. Variables measured in ECG were rhythm, RR, PR, QT and corrected QT (QTc by Bazett formula, measured in lead v2) intervals, QRS duration, presence of Osborn’s J wave and U wave, as well as ST segment displacement and T wave amplitude in leads II, v2 and v5. Quantitative data was obtained through arithmetical mean of 2 measured values. If there was any discordance in rhythm analysis or categorical variables, a final joint decision was reached with a third cardiologist.
Statistical analysis of measured intervals was performed with paired t-tests for related samples. Statistical significance was considered at P < 0.05 (two sided). Continuous variables are represented as means and standard deviation in brackets and categorical variables as percentages. Statistical analysis was performed with SPSS 15 (SPSS Inc, Chicago, IL).
The statistical methods of this study were reviewed by Pablo Salinas, MD, PhD, and bachelor degree in biostatistics.
A total 54 post-CA patients were included in the TH protocol. Of this 54 patients, 7 had one ECG missing (4 of them died before rewarming, 3 had unsatisfactory quality or were missing), therefore a total of 47 patients make the study population. PR interval changes were only considered when the 3 ECG were in sinus rhythm, 29 patients (61.7%).
Baseline characteristics of study population are shown in Table 1. Twenty one percent of the patients were under intraaortic balloon counterpulsation and 10% had a temporary transvenous pacemaker implanted, all of them during coronary angiogram. Two patients (4%) received continuous veno-venous hemofiltration therapy. Three patients (6.4%), already at TH target temperature, required premature protocol termination because of clinical indication, two because of hemodynamical instability and one because of emergent surgery of intraperitoneal hemorrhage, spleen and hepatic lacerations due to traumatic resuscitation. Median hospital stay was 11.5 d, ranging from 2 to 71 d. Mechanical ventilation was maintained for a median of 5.1 d. In-hospital survival rate was 53.2%. Implantable defibrillator was implanted in 23% of survivors.
| Patients | 47 |
| Age (median, range) | 65.9 (19-85) |
| Male | 40 (85.1%) |
| Cardiogenic shock at admission | 15 (31.9%) |
| Urgent coronary angiography | 28 (59.6%) |
| Left ventricular ejection fraction | 43.2 (15.3%) |
| Initial Rhythm, n (%) | |
| Ventricular Fibrillation | 30 (63.8) |
| Asystole | 14 (29.8) |
| Pulseless Electrical Activity | 3 (6.4) |
| Rhythm at admission, n (%) | |
| Sinus rhythm | 31 (66) |
| Atrial fibrillation | 8 (17) |
| AV block/nodal rhythm/paced rhythm | 8 (17) |
| TH protocol | |
| Temperature at admission | 35.7 (0.7) |
| Induction time (from admission to TH, h) | 4.8 (2.6) |
| Time in TH (median, range, h) | 20.8 (5-28.5) |
| Temperature during TH | 32.8 (0.5) |
| Rewarming time (from TH to 36 °C, h) | 11.3 (7.4) |
| Cause of CA, n (%) | |
| Acute coronary syndrome | 21 (44.7) |
| Chronic coronary disease1 | 8 (17.0) |
| Chronic heart failure | 4 (8.5) |
| Others/unknown2 | 14 (29.8) |
Comparison of heart rate, QRS duration and RR, PR, and QTc intervals among ECG at admission (ECG A), during hypothermia (ECG B) and in normothermia after rewarming (ECG C) are shown in Table 2 and Figure 1. Changes form ECG A to ECG B were a statistically significant increase in RR interval (decrease of heart rate of 19.5 bpm, P < 0.0005); a non-significative prolongation in PR interval; a minor significant prolongation of QRS duration of 9.9 ms, P = 0.041; and a significant increase in QTc interval of 57.5 ms (P < 0.0005). Changes from ECG B to ECG C were a statistically significant decrease in RR interval (increase of heart rate of 15.9 bpm, P < 0.0005); a small significant decrease in PR interval of 18.1 ms (P = 0.003); a significant but small shortening of QRS duration of 16.7 ms, P < 0.0005; and a significant shortening of QTc interval of 35.3 ms (P = 0.017). Comparing basal ECG (A) with post-TH ECG (C), there were no significant difference in heart rate or PR interval; but we found a slight significant shortening in QRS duration of 6.8 (P = 0.029) and a significant increase in QTc of 22.2 ms (P = 0.046), with a final mean QTc interval above the upper limit of normal QTc interval (463.9 ms).
| Admission(ECG A) | During MTH(ECG B) | After MTH(ECG C) | P for difference(A to B) | P for difference(B to C) | P for difference(A to C) | |
| RR interval (ms) | 653.8 (174.6) | 818.1 (222.6) | 656.9 (114.4) | < 0.0005a | < 0.0005a | 0.9 |
| Heart rate (bpm) | 97.9 (24.9) | 78.3 (19.8) | 94.2 (17.4) | < 0.0005a | < 0.0005a | 0.3 |
| PR interval (ms) | 169.2 (42.7) | 179.3 (37.5) | 161.2 (37.0) | 0.090 | 0.003a | 0.2 |
| QRS duration (ms) | 108.8 (23.2) | 118.7 (37.9) | 102.0 (22.9) | 0.041a | < 0.0005a | 0.029a |
| QT interval (ms) | 353.8 (58.1) | 448.1 (106.1) | 374.9 (72.0) | < 0.0005a | < 0.0005a | 0.042a |
| QTc interval (ms) | 441.7 (50.7) | 499.2 (95.5) | 463.9 (76.4) | < 0.0005a | 0.017a | 0.046a |
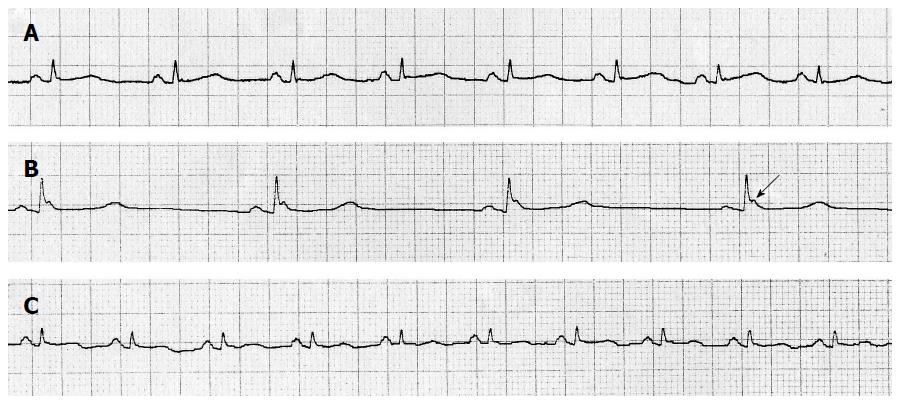
Comparison of T wave amplitude and ST segment deviation are shown in Table 3. On the whole there were no significant changes, except for a progressive decrease in amplitude of T wave in lead v5 through the TH process, a minor descent in ST from ECG B to ECG C in lead v5, and a slight decrease in amplitude of T wave in lead II. Osborn or J wave was observed in 21.3% of the patients in ECG B (Figure 2, arrow) with average amplitude of 0.2 millivolts. All of them appeared with cooling and reverted when patient was rewarmed. No U wave was detected in any ECG.
| Admission(ECG A) | During MTH(ECG B) | After MTH(ECG C) | P for difference(A to B) | P for difference(B to C) | P for difference(A to C) | |
| ST deviation lead II | + 0.05 (1.4) | - 0.20 (1.4) | - 0.12 (0.7) | 0.2 | 0.3 | 0.4 |
| ST deviation lead v2 | + 0.39 (3.2) | + 0.08 (0.6) | + 0.32 (1.0) | 0.4 | 0.06 | 0.9 |
| ST deviation lead v5 | - 0.25 (1.9) | - 0.38 (0.8) | - 0.16 (0.8) | 0.6 | 0.036a | 0.7 |
| T wave lead II | + 1.0 (1.8) | + 0.63 (1.1) | + 0.38 (1.2) | 0.2 | 0.1 | 0.036a |
| T wave lead v2 | + 2.0 (3.6) | + 2.10 (3.2) | + 1.62 (2.4) | 0.8 | 0.3 | 0.5 |
| T wave lead v5 | + 1.60 (2.9) | + 0.84 (2.3) | + 0.04 (2.4) | 0.1 | 0.013a | < 0.0005a |
Arrhythmia analysis is shown in Table 4. Any new arrhythmia occurred in 38.3% of the patients during TH. The most frequent arrhythmia (50% of the patients with arrhythmias) was non-sustained monomorphic ventricular tachycardia (VT), 55% of them in patients with ACS. A 10.6% had severe bradycardia (< 50 bpm) or paced rhythms. An 8.5% had rapid nodal rhythms and 6.4% atrial fibrillation. Neither polymorphic VT, nor sustained VT, nor VF (considered as life-threatening arrhythmias) happened during TH. Twelve percent of the population changed to sinus rhythm after TH induction: half of them were in atrial fibrillation and the other half in accelerated nodal rhythm. Two patients (4.2%) had a reversible change of rhythm with TH: one in sinus rhythm developed an atrial fibrillation during TH and then relapsed to sinus rhythm and the other with an atrial fibrillation at admission had an accelerated nodal rhythm in TH and then reverted to atrial fibrillation with rewarming. No patient needed pacemaker implantation or chronotropic drugs as a result of bradycardia during TH. Supraventricular tachycardias were treated following current guidelines if considered necessary. No treatment was given to non-sustained VT.
| New arrhythmias during TH | 38.3% |
| Non sustained monomorphic VT | 19.1% |
| Bradycardia < 50 bpm/paced rhythm | 10.6% |
| Accelerated nodal rhythm | 8.5% |
| Atrial fibrillation | 6.4% |
| Sustained VT | 0% |
| Polymorphic VT or VF | 0% |
| Change to sinus rhythm with TH | 12.8% |
| Atrial fibrillation to sinus rhythm | 6.4% |
| Accelerated nodal rhythm to sinus rhythm | 6.4% |
In the 1950’s there was a growing interest in hypothermia as a protective measure in the beginnings of open-heart surgery. Some reports from intraoperative ECG obtained during circulatory occlusion and profound hypothermia (reaching 21-23 °C), described a decrease in heart rate and a prolongation in PR, QRS and QT intervals. Arrhythmias were common and were related to core temperature. During mild hypothermia most frequent arrhythmias were ectopic atrial rhythms and nodal rhythms. A remarkable incidence of atrial fibrillation occurred below 30-32 °C[10,11]. VF appeared associated with circulatory occlusion. Other studies in dogs suggested temperature thresholds for VF below 26 °C and asystole below 18 °C[10,11,17]. Changes in ECG and arrhythmias in these reports are subject to multiple confounding factors: very low temperatures, myocardial ischemia, circulatory occlusion, cardioplegic solutions and the open-heart surgery itself. Because of those factors, previously described changes can hardly be applicable to current mild controlled TH.
Since the beginning of our decade, and after a gap in the literature of 40 years, hypothermia has regained interest, partly because the mechanism involved in its therapeutic effect were progressively clarified. Most information about complications and side effects come from old reports, animal experimentation and case reports. In the present study we provide a systematized analysis of cardiac arrhythmias and temperature-dependent, sequential ECG changes during TH performed to an unselected post-CA population.
According to our findings, during TH to a target temperature of 32-34 °C in post-CA patients, some ECG changes may be expected: a considerable decrease in heart rate, a minimum prolongation of PR interval, a slight prolongation of QRS duration and a significant prolongation in QTc interval. All of these changes were reversible, except the prolongation of QTc interval (at least in the first 24 h after rewarming). Temperature-related changes in ST segment and T wave were not conclusive, but there was a trend towards flattening of T waves through TH process. ST segment and T wave changes may be interfered by previous cardiac disease and cause of CA, as almost half of the patients had an ACS.
The Osborn wave, or J wave, first observed in 1938 and fully described in 1953[18], is a frequent ECG feature in deep hypothermia. It can be seen as a notch or hump-like deflection in the terminal forces of QRS or between QRS and ST segment, more visible in precordial leads[19]. The amplitude and duration correlates with temperature, and although literature rarely describes it in mild hypothermia, we found a J wave in 21.3% of the patients (Figure 2, arrow). It is caused by a temperature dependent, transmural voltage gradient of a transient potassium current, more intense in epicardium than endocardium.
On the whole, ECG changes found in our study are concordant with those described previously in deep hypothermia[20-23]. Medical staff as well as nurses working with patients treated with induced TH should be aware of the possible arrhythmias and ECG changes that may occur. An example of ECG changes is shown in Figure 2. These changes are secondary to low body temperature and should not be considered pathological. Prolongation of action potential and decrease of myocardial conduction velocity has been proposed as physiopathological explanations for these phenomena[16]. These changes were reversible with rewarming and did not deteriorate hemodynamic status or clinical situation.
Bradycardia is one of the most disturbing effects of hypothermia because CA-recovered patients are often in cardiogenic shock and cardiac output decreases along with heart rate. In our series, patients with low initial heart rates did not decrease further, but maintained or increased their heart rates (Figure 1A). Besides, some studies suggest that the relation between heart rate and cardiac output inverses with hypothermia and that allowing mild TH to reduce heart rate could actually improve myocardial contractility. This is explained because hypothermia worsens diastolic function in the myocardium, and this is partially balanced by bradycardia[24,25]. External pacing or administration of chronotropic drugs is not recommended during TH to increase cardiac output[16].
The use of TH in post CA patients was safe with no life-threatening arrhythmias that worsened hemodynamic stability or required withdrawing the TH protocol. Non life-threatening arrhythmias were found in less than a half of the patients (38.3%).
The behavior of QTc interval in our TH series was remarkable (Figure 1D). We found a mean baseline QTc interval in the upper normal limits (mean 441 ms), it increased with TH (mean 499 ms), and partially reverted with rewarming, but final QTc interval was still lengthened when compared to initial QTc interval (463 ms) and was above upper normal limits. In spite of that, we had no arrhythmias related to prolongation of QT interval, like polymorphic ventricular tachycardia. We presumably (some patients died before a cause of the CA could be elucidated) did not have any patient with arrhythmic CA caused by long QT, but as QT and QTc intervals lengthen with TH, and remain lengthened afterwards, a concern may raise about safety of hypothermia in patients with long QT CA. Further investigation about this issue is warranted.
There is a concern about whether TH may increase the risk for arrhythmias and that the hypothermic myocardium can be somewhat resistant to antiarrhythmic drugs during hypothermia. It is well known that deep hypothermia under 30° increments the risk for atrial fibrillation and progressively with cooling under 28° the risk for life-threatening arrhythmias as VT and VF is increased[26]. Conversely, controlled mild TH is associated with higher rates of ROSC in animal CA models and is successfully used as a treatment for junctional ectopic tachycardia in infants[27-29]. Our study supports all previous reports that controlled, mild TH, is a safe technique with no increased risk for malignant arrhythmias and a relatively small number of minor arrhythmias that on the other hand can not only be attributed to TH but also to post-CA situation and previous cardiac disease.
Our study has some limitations. Accuracy of manual calipers is limited but represents day-by-day clinical practice. Arrhythmias and ECG changes could be interfered by several confounding factors like electrolyte disturbances. We had no control group, so this point cannot be ruled out in our study. However, our findings are congruent with those previously described in hypothermia and the fact that the changes were reversible with rewarming supports that TH was the cause of these changes. Recent trials show conflicting evidence regarding optimal target temperature, one of them suggests a benefit from deeper hypothermia (32 °C vs 34 °C), while other found no benefit of 33 °C over normothermia (36 °C)[30,31]. It would be relevant to know the “arrythmical” safety of different temperature levels, however our study did not analyzed different target temperatures.
In summary, therapeutic hypothermia according to current practice is safe with a 38.3% of patients having cardiac arrhythmias during TH but without life-threatening arrhythmias. Main ECG changes were bradycardia and prolongation of PR, QRS and QT intervals. A concern may rise when inducing TH to patients with long QT syndrome.
Induced therapeutic hypothermia is currently recommended by most cardiac arrest guidelines, to improve the prognosis of the so-called post-cardiac arrest syndrome. However it is not widely used and has some controversies. Some of the main concerns that prevent intensive care physicians from inducing therapeutic hypothermia are the potential pro-arrhythmic effects of hypothermia. A study regarding cardiac arrhythmias is relevant to reassure patient’s safety, especially for patients with heart disease.
The influence of hypothermia over cardiac rhythm and cardiac conduction system is unknown and main data comes from case reports of accidental deep hypothermia.
This study allows a more comprehensive understanding of the influence of mild hypothermia in cardiac conduction. It shows a reversible prolongation of all cardiac intervals measured by electrocardiograms, suggesting that mild hypothermia slows cardiac conduction speed. The absence of life-threatening arrhythmias is reassuring for using this therapy in cardiac patients.
This study must be interpreted with caution due to the relatively small sample and its observational nature. However, it supports the “electrical” safety of therapeutic hypothermia for cardiac patients. Future lines of research suggested by the study are the potential influence of QT prolongation by hypothermia in long-QT syndromes, and the need for experimental (most probably in animal models) studies on the influence of hypothermia and cardiac conduction speed.
Hypothermia: any temperature below 35.5-36 °C. It may be accidental (cold exposure in winter) or induced (cold fluid or specific devices); Target temperature: The desired temperature in induced hypothermia. Usually 32-34 °C. Some groups are investigating 32 °C vs 34 °C, while others advocate for only preventing hyperthermia (≤ 36 °C).
It is an important topic and well written and well presented.
P- Reviewer: Aggarwal A, Inaba H, Showkat HI S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Group HACAS. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1-203. [PubMed] [Cited in This Article: ] |
| 4. | Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67 Suppl 1:S39-S86. [PubMed] [Cited in This Article: ] |
| 6. | Bernard S. Hypothermia after cardiac arrest: expanding the therapeutic scope. Crit Care Med. 2009;37:S227-S233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452-2483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1061] [Cited by in F6Publishing: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 8. | Abella BS, Rhee JW, Huang KN, Vanden Hoek TL, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Wolfrum S, Radke PW, Pischon T, Willich SN, Schunkert H, Kurowski V. Mild therapeutic hypothermia after cardiac arrest - a nationwide survey on the implementation of the ILCOR guidelines in German intensive care units. Resuscitation. 2007;72:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Hicks CE, Mccord MC, Blount SG. Electrocardiographic changes during hypothermia and circulatory occlusion. Circulation. 1956;13:21-28. [PubMed] [Cited in This Article: ] |
| 11. | Fleming PR, Muir FH. Electrocardiographic changes in induced hypothermia in man. Br Heart J. 1957;19:59-66. [PubMed] [Cited in This Article: ] |
| 12. | Ree MJ. Electrocardiographic changes in accidental hypothermia. Br Heart J. 1964;26:566-571. [PubMed] [Cited in This Article: ] |
| 13. | Mahmood MA, Zweifler RM. Progress in shivering control. J Neurol Sci. 2007;261:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Polderman KH, Peerdeman SM, Girbes AR. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J Neurosurg. 2001;94:697-705. [PubMed] [Cited in This Article: ] |
| 16. | Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186-S202. [PubMed] [Cited in This Article: ] |
| 17. | Covino BG, Wright R, Charleson DA. Effectiveness of several antifibrillary drugs in the hypothermic dog. Am J Physiol. 1955;181:54-58. [PubMed] [Cited in This Article: ] |
| 18. | Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953;175:389-398. [PubMed] [Cited in This Article: ] |
| 19. | Gussak I, Bjerregaard P, Egan TM, Chaitman BR. ECG phenomenon called the J wave. History, pathophysiology, and clinical significance. J Electrocardiol. 1995;28:49-58. [PubMed] [Cited in This Article: ] |
| 20. | Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality--Part 2: Practical aspects and side effects. Intensive Care Med. 2004;30:757-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 287] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Van Mieghem C, Sabbe M, Knockaert D. The clinical value of the ECG in noncardiac conditions. Chest. 2004;125:1561-1576. [PubMed] [Cited in This Article: ] |
| 22. | Slovis C, Jenkins R. ABC of clinical electrocardiography: Conditions not primarily affecting the heart. BMJ. 2002;324:1320-1323. [PubMed] [Cited in This Article: ] |
| 23. | Mattu A, Brady WJ, Perron AD. Electrocardiographic manifestations of hypothermia. Am J Emerg Med. 2002;20:314-326. [PubMed] [Cited in This Article: ] |
| 24. | Lewis ME, Al-Khalidi AH, Townend JN, Coote J, Bonser RS. The effects of hypothermia on human left ventricular contractile function during cardiac surgery. J Am Coll Cardiol. 2002;39:102-108. [PubMed] [Cited in This Article: ] |
| 25. | Mattheussen M, Mubagwa K, Van Aken H, Wusten R, Boutros A, Flameng W. Interaction of heart rate and hypothermia on global myocardial contraction of the isolated rabbit heart. Anesth Analg. 1996;82:975-981. [PubMed] [Cited in This Article: ] |
| 26. | Mortensen E, Berntsen R, Tveita T, Lathrop DA, Refsum H. Changes in ventricular fibrillation threshold during acute hypothermia. A model for future studies. J Basic Clin Physiol Pharmacol. 1993;4:313-319. [PubMed] [Cited in This Article: ] |
| 27. | Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation. 2005;111:3195-3201. [PubMed] [Cited in This Article: ] |
| 28. | Rhee BJ, Zhang Y, Boddicker KA, Davies LR, Kerber RE. Effect of hypothermia on transthoracic defibrillation in a swine model. Resuscitation. 2005;65:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Pfammatter JP, Paul T, Ziemer G, Kallfelz HC. Successful management of junctional tachycardia by hypothermia after cardiac operations in infants. Ann Thorac Surg. 1995;60:556-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Lopez-de-Sa E, Rey JR, Armada E, Salinas P, Viana-Tejedor A, Espinosa-Garcia S, Martinez-Moreno M, Corral E, Lopez-Sendon J. Hypothermia in comatose survivors from out-of-hospital cardiac arrest: pilot trial comparing 2 levels of target temperature. Circulation. 2012;126:2826-2833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197-2206. [PubMed] [Cited in This Article: ] |