Published online Sep 18, 2017. doi: 10.4254/wjh.v9.i26.1081
Peer-review started: January 3, 2017
First decision: February 4, 2017
Revised: April 6, 2017
Accepted: June 6, 2017
Article in press: June 7, 2017
Published online: September 18, 2017
Diffusion-weighted imaging (DWI), a functional imaging technique exploiting the Brownian motion of water molecules, is increasingly shown to have value in various oncological and non-oncological applications. Factors such as the ease of acquisition and ability to obtain functional information in the absence of intravenous contrast, especially in patients with abnormal renal function, have contributed to the growing interest in exploring clinical applications of DWI. In the liver, DWI demonstrates a gamut of clinical applications ranging from detecting focal liver lesions to monitoring response in patients undergoing serial follow-up after loco-regional and systemic therapies. DWI is also being applied in the evaluation of diffuse liver diseases such as non-alcoholic fatty liver disease, hepatic fibrosis and cirrhosis. In this review, we intend to review the basic principles, technique, current clinical applications and future trends of DW-MRI in the liver.
Core tip: This article reviews the current role of diffusion weighted imaging for various oncological and non-oncological applications in the liver.
- Citation: Shenoy-Bhangle A, Baliyan V, Kordbacheh H, Guimaraes AR, Kambadakone A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J Hepatol 2017; 9(26): 1081-1091
- URL: https://www.wjgnet.com/1948-5182/full/v9/i26/1081.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i26.1081
Diffusion-weighted imaging (DWI) is a functional imaging technique, allowing qualitative and quantitative assessment of the diffusion properties of various types of tissues[1,2]. Numerous studies over the past decade have validated the role of DWI in oncologic and non-oncologic applications in the body[1,3-6]. Multiphase contrast enhanced MRI is an established technique for evaluation of a wide spectrum of liver diseases including focal lesions and diffuse parenchymal abnormalities. DWI compliments routine MRI of the liver by providing both qualitative and quantitative assessment for both focal and diffuse hepatic parenchymal processes. Factors such as the ease of acquisition and ability to obtain functional information in the absence of intravenous contrast, especially in patients with abnormal renal function, have contributed to the growing interest in exploring clinical applications of DWI. DWI improves sensitivity in detection of focal lesions, helps differentiate benign from malignant focal hepatic lesions, and also permits evaluation of treatment response to systemic and loco-regional therapies in primary and secondary hepatic malignancies. This review article focused on the basic principles, technique, current clinical applications and recent updates in DWI of the liver.
DWI exploits the regional differences in the motion of water molecules within the extracellular/extravascular compartment of tissues. In highly cellular tissues (e.g., lymphoma, carcinoma and abscess), the compact nature of the extracellular space causes increased impediment to motion of water molecules and the resultant water diffusion in such tissues is said to be “restricted”. On the contrary, in tissues that are necrotic or fluid filled (e.g., cysts), there is unrestricted motion of water molecules and water diffusion in such tissues, which is said to be “free”. Therefore, the diffusion properties in different tissues provide information on tissue cellularity and the integrity of cellular membranes[1,2]. DWI is basically a modified T2 weighted sequence where the signal intensity depicts the tissue diffusion characteristics.
Single-shot spin-echo (SE) echo-planar technique is the most commonly utilized technique to acquire DW-MRI in combination with fat suppression[7]. To obviate the effect of motion, it can be acquired either using breath-hold or free breathing sequences with multiple signal acquisitions (in combination with respiratory and/or cardiac triggering). Free breathing sequences provide improved signal to noise ratios (SNR), thinner image sections, and higher number of b-values obtainable compared to breath-hold sequences. However, these take longer time (3-6 min) to acquire than breath hold sequences to evaluate the liver compared to free breathing EPI which takes (40-60 s)[8]. The free breathing technique has been shown to have better reproducibility of ADC values than other acquisition techniques like breath-hold, respiratory-triggered (RT), and navigator-triggered DWI[9,10]. Although cardiac motion also impacts quantitative ADC measurements, cardiac triggering is not routinely used in clinical practice[11].
Intravoxel incoherent motion (IVIM) imaging is a technique that has been introduced to quantitatively study the effects of tissue perfusion on the signal acquired with DWI and it resolves DWI measurements into true molecular-based (D) and perfusion-related (D*, f) diffusion[12].
In patients with renal failure, gadolinium is contraindicated due to risk for developing nephrogenic systemic fibrosis (NSF)[13]. These patients also have a risk of worsening renal failure with iodinated CT contrast. MRI without contrast is a reasonable option for these patients but non-contrast protocols do not have a diagnostic accuracy comparable to multi-phase contrast MRI. DWI does not require administration of intravenous contrast, and because of its performance in oncological applications in general, it has generated much interest recently. The diagnostic performance of DWI has been tested in metastatic liver disease and HCC, and the results were comparable to contrast MRI[14-16].
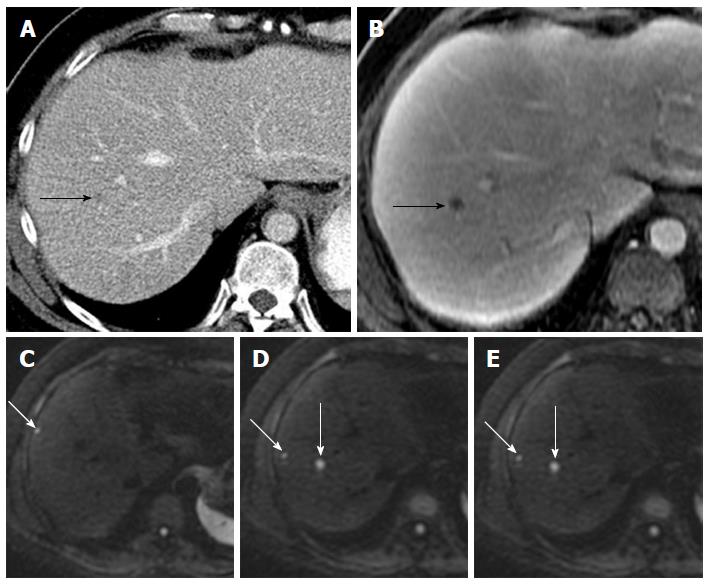
Lesion detection: Multiphase contrast enhanced-MRI is currently the state-of-the-art imaging method for liver lesion detection and characterization. DWI at high b-values (≥ b100) provides a low background signal from normal liver parenchyma and thereby results in increased contrast between the background liver and lesions, enhancing the detection of focal liver lesions[17]. DWI is especially useful in detection of small lesions around vessels and in the periphery of liver which can be challenging to detect on routine T2 weighted images[18,19]. The DW-MRI can be particularly valuable in oncologic patients with compromised renal function who cannot get intravenous gadolinium based contrast agents[14-16]. DWI adds value in oncologic patients (Table 1)[15,20-22] by depicting more metastatic liver lesions when combined with multiphase contrast enhanced-MRI protocols, and improves reader confidence in lesion detection[22-25]. DW-MRI alone is less sensitive than gadoxetic acid-enhanced MRI for detecting liver metastases, but increases the sensitivity of detection for liver metastases (90.6%-95.5%) when combined with multiphase contrast enhanced MRI[25]. A major impact has been noted in the detection of metastases measuring ≤ 10 mm[17,22,24-27] (Figure 1). DWI has been used in detection of metastatic liver lesions from colorectal, pancreatic and neuroendocrine primaries[25,28,29].
| Ref. | b value(s/mm2) | Compared with (Seq) | Sensitivity of DWI vs other sequences | Accuracy of DWI vs other sequences | Advantages of DWI |
| Bruegel et al[27] | 50, 300, 600 | 5 different T2-TSE (Turbo Spin Echo) sequences | 0.88-0.91 compared to 0.45-0.62 | 0.91-0.92 compared to 0.47-0.67 | Better sensitivity and accuracy |
| Zech et al[21] | 50 | Fat suppressed T2WI | 83% vs 61% | - | Better image quality |
| Fewer artifacts | |||||
| Better sensitivity | |||||
| Hardie et al[15] | 0, 50, 500 | Gadolinium enhanced T1WI | 66.3% vs 73.5% | 88.2% and 88.2% for DW-MRI, 90.2% and 92.2% for CE MRI, respectively, for observers 1 and 2 | Not significantly different |
| Donati et al[20] | 0, 150, 500 | Combined (Gd-EOB-DTPA) enhanced MRI/DWI vs Gd-EOB-DTPA enhanced MRI and DWI alone | - | Gd- EOB-DTPA/DWI: 0.84 and 0.83 vs 0.73 and 0.72 for DWI alone | Increase in diagnostic confidence |
| No significant increase in diagnostic accuracy | |||||
| Colagranade et al[22] | 0-500 | Added value of DWI for lesion detection in unenhanced and Gd-EOB-DTPA enhanced MRI | -62.5% for unenhanced MRI w/o DWI | -81.1% for unenhanced MRI w/o DWI | DWI improved all statistical parameters in the unenhanced examinations, as for nodules either smaller or greater than 1 cm. In EOB-enhanced examinations DWI increased specificity/negative predictive value |
| -85.0% for unenhanced MRI+ DWI | -89% for unenhanced MRI + DWI | ||||
| -95.6% for CE MRI | -92.9% for CEMRI | ||||
| -97.3% for CE MRI + DWI | -95.5% for CE MRI + DWI |
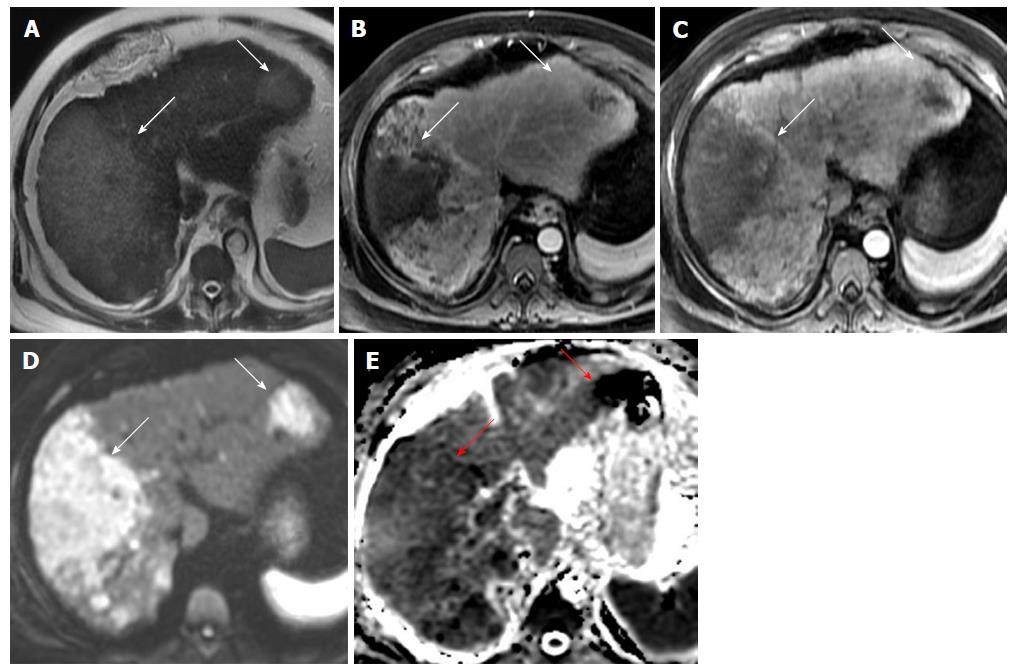
DWI has also been found to be useful in detection of primary hepatic malignancies such as hepatocellular carcinoma (HCC) and cholangiocarcinoma both in cirrhotic and non-cirrhotic livers (Figure 2). A combination of DW hyper-intensity and arterial hyper-enhancement results in increased sensitivity for diagnosis of HCC as compared to traditional criteria, particularly for small HCC < 20 mm[30,31].
A low cost abbreviated MRI (AMRI) protocol for HCC screening and surveillance has been proposed based on a simulation study using DWI and T1-weighted imaging obtained at the hepatobiliary phase (HBP) after gadoxetic acid injection[32]. The AMRI shows sensitivity and negative predictive values of 80.6% and 80% (for DWI + T1W HBP) compared to 90.3% and 94.9% for a full dynamic contrast enhanced data-set[32].
Lesion characterization: Several studies have attempted characterization of liver lesions using DW-MRI[33-38]. A general assumption is that ADC values are higher in benign lesions and lower in malignant liver lesions[33-36]. In fact, studies have found statistically significant difference in ADC values between benign and malignant liver lesions[3]. Different studies have reported variable success using various ADC cut-off values with high variability likely due to the difference in scanners and parameters used to obtain DW-MRI and ADC maps[39-43]. Moreover, there is a high degree of overlap between solid benign and malignant lesions[44,45]. Hence, the use of absolute ADC values or ADC value cut-off for characterization of focal hepatic lesions should be avoided and DWI should always be interpreted as a complimentary technique to conventional MR sequences[42,46,47]. It is also important to note that solid benign lesions such as hemangioma, FNH and hepatocellular adenoma can also show diffusion restriction compared to normal liver parenchyma. ADC values for these lesions are intermediate, generally greater than solid malignant lesions but with a significant degree of overlap[44,45]. Hepatic abscesses show lower ADC values than solid malignant lesions, and restriction pattern may be different from malignant lesions[42] (Table 2).
| Ref. | Lesion type | Mean ADC (10-3mm2/s) | Sample size | b-values | Conclusion |
| Parsai et al[44] | Cyst | 2.66 | 2 | 100, 200, 500, 750, and 1000 mm2/s | ADC cutoff value threshold of 1.6 × 10-3 mm2/s yielded higher accuracy for differentiating benign from malignant lesions. DWI is not reliable to differentiate malignant from benign solid lesions |
| HCC | 1.07 | 26 | |||
| Metastases | 1.04 | 39 | |||
| Taouli et al[98] | Cyst | 3.63 | 52 | 0, 500 | Threshold ADC value of 1.5 × 10-3 mm2/s to differentiate between benign and malignant lesions, but with a significant overlap between benign hepatocellular lesions and HCCs |
| HCC | 1.33 | ||||
| Metastases | 0.94 | ||||
| Parikh et al[35] | Cyst | 2.54 | 211 | 0, 50, 500 | Accuracy of 75.3% for differentiating benign from malignant, by using a threshold ADC of less than 1.60 × 10-3mm2/s . Equivalent performance of DW imaging and T2- weighted imaging for lesion characterization |
| HCC | 1.31 | ||||
| Metastases | 1.5 | ||||
| Bruegel et al[33] | Cyst | 3.02 | 204 | 50, 300, 600 | 88% of lesions were correctly classified as benign or malignant using a threshold value of 1.63 × 10-3 mm2/s. Measurements of the ADCs of focal liver lesions on the basis of a respiratory triggered DW-SS-EPI sequence may constitute a useful supplementary method for lesion characterization |
| HCC | 1.05 | ||||
| Metastases | 1.22 | ||||
| Gourtsoyianni et al[102] | Cyst | 2.55 | 37 | 0, 50, 500, 1000 | Sensitivity and specificity of 100% for differentiating benign from malignant lesions using a cutoff ADC value of 1.47 × 10-3 mm2/s |
| HCC | 1.38 | ||||
| Metastases | 0.99 |
DWI has also been used to assist in differentiation of cirrhotic hepatocellular nodules[48]. Lesion hyperintensity on DWI, especially in association with hypointensity on delayed hepatocellular phase images, and low lesion-to-liver ratios should raise the suspicion of HCC or high-grade dysplastic nodules[49]. The HCCs have a tendency for angio-invasion and can present with filling defects in the portal or hepatic veins. Angio-invasion carries a high risk of distant metastasis and recurrence after transplantation. HCC invasion into the portal vein is considered as a contraindication for liver transplantation. It is important to distinguish tumor thrombus from a bland thrombus that is also common in chronic portal hypertension and has different clinical implications. In patients with locally advanced HCC, DW-MRI has been shown to be useful in characterization of the venous thrombus as bland vs tumor thrombus[50]. The mean ADC ratio of tumor thrombus and HCC has been reported to be < 2 (0.998) as compared to bland thrombus (2.9)[50].
Tumor grade and prognostication: Recently, there have been attempts to predict the histopathological grades of HCC using DWI. ADC values have been found to correlate with histopathological differentiation and microvascular invasion with poorly differentiated HCCs showing significantly lower ADC than well-differentiated and moderately differentiated HCCs[51-54]. A cut-off value of 1.175 × 10-3 mm2/s has been recommended as a predictor of microvascular invasion[52]. Additionally, the recurrence-free survival has been found to be significantly shorter in low-ADC group than in high-ADC group[52].
The association of ADC and histopathological grades has shown conflicting results in few other studies[55,56]. This might be a result of tumor necrosis, as it can result in reduced cellularity and increased ADC in high-grade lesions. Higher signal intensity on DWI has been reported to be associated with higher pathological grades despite insignificant correlation with ADC values[54,56].
Evaluation of NAFLD: Non-alcoholic fatty liver disease (NAFLD) is the most common liver disorder in western industrialized countries with a prevalence of 6%-35% worldwide[57]. The severe form of this disease is steatohepatitis which can progress to cirrhosis in 15% of the patients[58]. Currently, the diagnosis of NAFLD is established based on histopathological evaluation of liver biopsy specimens. Liver biopsy is invasive and has risks of complications and sampling error, and cannot be frequently repeated.
The feasibility of DWI and IVIM was first tested in animal models with early results showing that the IVIM diffusion parameters, in particular the “f” values, might be potential biomarkers of NAFLD[59]. The correlation between histologic features of NAFLD and quantitative measures derived from IVIM-DWI was later tested in humans which showed that the true molecular diffusion was significantly decreased with steatosis[60,61]. ADC was not found to be associated with any histological feature[60]. Although these early results are promising, standardization of acquisition and post-processing techniques of IVIM DW-MRI is needed.
Evaluation of liver fibrosis and cirrhosis: Aubé et al[62] reported early benefits of DWI in the evaluation of diffuse liver diseases, particularly in the detection and quantification of hepatic fibrosis. Several authors thereafter have tried to find a simple, reliable and non-invasive method to detect and monitor hepatic fibrosis, thereby avoiding the existing gold standard involving liver biopsy and its complications[63,64]. A recent meta-analysis suggests that DWI and IVIM parameters can reliably stage hepatic fibrosis[65,66]. However, IVIM measurements and ADC values have been reported to be influenced by presence of fat or iron within the liver that can impact their accuracy for staging of fibrosis[67-69] and ascites[70]. Recent studies comparing MR elastography (MRE) and DWI in characterizing hepatic fibrosis demonstrate higher predictive ability of MRE in distinguishing stages of fibrosis compared to DWI[71,72]. Gadoxetate disodium enhanced liver MRI is also more strongly correlated with fibrosis stage as compared to DWI[73,74]. Considering the conflicting evidence, it can be concluded that at present, DWI cannot replace liver biopsy in liver fibrosis. Further investigations and analysis are needed to increase the reliability of the technique.
There has been a lot of interest in using DWI as an imaging biomarker for monitoring treatment response to various locoregional and systemic therapies in hepatic malignancies (Table 3)[75-79]. In comparison to conventional morphological methods of monitoring response such as RECIST and WHO which rely on changes in tumor dimensions for quantitating tumor response, DW-MRI allows evaluation of treatment response to novel targeted therapies which cause early changes in tumor physiology prior to change in tumor size. The increase in post-treatment ADC values precedes a decrease in size of tumor which has been the traditional method of measurement for post-treatment response, especially in systemic therapy[80-82].
| Ref. | Treatment modality | Tumor type | DW-MR parameter evaluated | Study results/teaching point |
| Chapiro et al[79] | TACE | HCC | (3D) quantitative enhancement-based and DW volumetric MR | High accuracy and intermethod agreement of 3D quantitative techniques in the assessment of tumor necrosis after TACE is clinically relevant |
| High diagnostic performance of qEASL criteria and qADC may help in triaging patients for repeat treatment after a TACE session | ||||
| Mannelli et al[87] | TACE | HCC | ADC measured with DWI in treatment response | Pre TACE ADC obtained at 0, 50, 500 s/mm2b values before and after treatment may be used to predict HCC response to TACE |
| Park et al[42] | Radiotherapy | HCC | DW MR vs conventional MR for treatment response | Improved detection of viable tumor when DW MR is added to conventional sequences |
| Yu et al[76] | Radiation therapy | HCC | DW MR | Change in ADC value before and after RT is related to local progression free survival. Hence ADC value and RECIST may substitute for mRECIST in patients who cannot receive contrast agents |
| Schraml et al[77] | Radiofrequency | n = 16 HCC, 1 = cholangiocarcinoma, and 37 = metastases (28 colorectal cancer, 3 melanoma, 3 breast cancer, 1 pancreatic cancer, 1 gastric cancer, esophageal cancer) | DW MR and mean ADC values | ADC-based evaluation of signal alterations adjacent to the ablation zone may contribute to the identification of local tumor progression and nontumoral post- treatment tissue changes |
| Ablation |
Percutaneous ablation: ADC-based evaluation of signal alterations adjacent to the ablation zone may contribute to the identification of local tumor progression and non-tumoral post-treatment tissue changes after radiofrequency ablation of hepatic primary tumors and metastases[77]. Early post-ablation zone may show heterogeneous signal on non-enhanced T1 and T2 weighted images due to edema, hemorrhage and inflammatory reaction. These changes resolve within 4-6 mo after ablation leaving behind a characteristic homogenous high T1 signal and low T2 signal (coagulation necrosis). Nodular enhancing foci within the ablation zone are considered as a sign of local recurrence. Low ADC values at 1 mo (< 1.145 × 10-3 mm2/s) after RFA have been shown to be associated with an early local recurrence of HCC[83].
Intra-arterial therapies: The utility of DWI has been assessed in treatment response after trans-arterial chemoembolization (TACE) of HCC[84-87]. DWI has been shown to perform equally[78] or better than gadolinium-enhanced MRI in quantifying the area of tumor necrosis after chemoembolization[78,86,88]. Increased ADC values in non-enhancing tumors show a high correlation to the degree of tumor necrosis at pathology[86,88]. Mannelli et al[78] showed excellent performance of ADC for prediction of complete tumor necrosis after TACE (sensitivity of 75% and specificity of 88%) which was comparable to 100% sensitivity, and 58%-79% specificity for contrast-enhanced MRI.
Transarterial radioembolization (TARE) using yttrium-90 (90Y)-loaded resin microspheres is a treatment option for various liver malignancies (including liver-dominant breast metastases). Early arterial blood flow stasis with consecutive incomplete dose administration may occur in 12%-25% of resin-based radioembolization procedures. The perfusion-sensitive IVIM parameter “f” may predict early blood flow stasis in patients undergoing TARE for liver-dominant breast metastases[89].
Image-guided radiation therapy: Image-guided targeted external beam radiation therapy is emerging as an alternative option in the treatment of advanced unresectable HCC. Accurate post-radiation response assessment can be challenging due to the concomitant changes occuring in the radiation zone. MRI is the preferred modality for response assessment. Inclusion of DWI in the imaging protocol has been shown to significantly enhance the diagnostic accuracy (91%-97% vs 72%) for detection of viable tumors after radiation treatment with improved sensitivity, specificity, and negative predictive value as compared to routine MR sequences (90%-97%, 91%-97% and 91%-97% vs 41%-55%, 86%-97% and 67%-70%, respectively)[75]. ADC values have also been shown to correlate with local progression-free survival[76]. Another group demonstrated that ADC values correlate with local progression-free survival and proposed that ADC and RECIST criteria could be substituted for mRECIST in post-radiation evaluation of patients not amenable to receiving contrast agents[76].
Systemic chemotherapy: DWI can detect the effects of chemotherapy combined with antiangiogenetic treatment on liver metastases in patients with advanced colorectal cancer[90]. An increase in ADC values following systemic chemotherapy can be a sign of tumor response with non-responders showing lower ADC values than responders[91]. In addition to monitoring therapeutic response, DWI has also been found to be useful in prediction of response to chemotherapeutic agents[92,93].
Diffusion imaging has several limitations, mostly attributable to the EPI based nature of the sequence[94,95]. SS EPI provides a limited image quality with low spatial resolution and poor SNR and is susceptible to several artifacts, including blurring, ghosting and distortions. Although modern scanners with multichannel coils, strong gradients, high magnetic fields and advanced software have been successful in reducing such effects to a great extent[96]. In addition, parallel imaging techniques improve SNR by allowing a decrease in acquisition time (TE)[97,98]. 3T MRI offers an advantage due to an inherent high SNR, but suffers from several limitations. Uniform fat suppression for liver DWI has always been a challenge with 3 Tesla magnets and susceptibility artifacts are also more pronounced at 3 Tesla scanners[99].
The reproducibility of quantitative ADC values has also been questioned. ADC values have been reported to vary significantly depending on the hardware, human or biologic factors[100]. There has been considerable effort, however, to “industrialize” this important biomarker across vendor platforms[101].
DWI is useful for focal liver lesion detection and is a desirable tool in patients who cannot receive intravenous contrast. In patients receiving systemic and local therapies for hepatic malignancies, DWI acts as a clinical tool for monitoring treatment response and predicting prognosis. Its utility in the assessment of diffuse hepatic parenchymal diseases is still at a research level. Further investigation and analysis are needed to increase the reliability of the technique for these indications. DWI has certain limitations and remains an adjunct and not a replacement to conventional sequences.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jamali R, Karthik SV, Namisaki T, Waszczuk EM S- Editor: Qi Y L- Editor: Ma JY E- Editor: Li D
| 1. | Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 594] [Article Influence: 42.4] [Reference Citation Analysis (2)] |
| 2. | Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169-184. [PubMed] [Cited in This Article: ] |
| 3. | Kaya B, Koc Z. Diffusion-weighted MRI and optimal b-value for characterization of liver lesions. Acta Radiol. 2014;55:532-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, Laurent A, Deux JF, Brugieres P, Rahmouni A. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008;249:891-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 508] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016;8:785-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 166] [Cited by in F6Publishing: 163] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 6. | Sheth RA, Bittencourt LK, Guimaraes AR. Diffusion-weighted imaging of the male pelvis. Magn Reson Imaging Clin N Am. 2014;22:145-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Mürtz P, Flacke S, Träber F, van den Brink JS, Gieseke J, Schild HH. Abdomen: diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology. 2002;224:258-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Schmid-Tannwald C, Oto A, Reiser MF, Zech CJ. Diffusion-weighted MRI of the abdomen: current value in clinical routine. J Magn Reson Imaging. 2013;37:35-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Chen X, Qin L, Pan D, Huang Y, Yan L, Wang G, Liu Y, Liang C, Liu Z. Liver diffusion-weighted MR imaging: reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology. 2014;271:113-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 10. | Choi JS, Kim MJ, Chung YE, Kim KA, Choi JY, Lim JS, Park MS, Kim KW. Comparison of breathhold, navigator-triggered, and free-breathing diffusion-weighted MRI for focal hepatic lesions. J Magn Reson Imaging. 2013;38:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Nasu K, Kuroki Y, Sekiguchi R, Kazama T, Nakajima H. Measurement of the apparent diffusion coefficient in the liver: is it a reliable index for hepatic disease diagnosis? Radiat Med. 2006;24:438-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2349] [Cited by in F6Publishing: 2310] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 13. | Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 629] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 14. | Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging. 2007;25:848-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Hardie AD, Naik M, Hecht EM, Chandarana H, Mannelli L, Babb JS, Taouli B. Diagnosis of liver metastases: value of diffusion-weighted MRI compared with gadolinium-enhanced MRI. Eur Radiol. 2010;20:1431-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Elbarbary AA, Saleh Elahwal HM, Elashwah ME. Role of Diffusion Weighted Magnetic Resonance Imaging in evaluation of hepatic focal lesions. Egypt J Radiol Nucl Med. 2015;46:325-334. [Cited in This Article: ] |
| 17. | Koh DM, Brown G, Riddell AM, Scurr E, Collins DJ, Allen SD, Chau I, Cunningham D, deSouza NM, Leach MO. Detection of colorectal hepatic metastases using MnDPDP MR imaging and diffusion-weighted imaging (DWI) alone and in combination. Eur Radiol. 2008;18:903-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | van den Bos IC, Hussain SM, Krestin GP, Wielopolski PA. Liver imaging at 3.0 T: diffusion-induced black-blood echo-planar imaging with large anatomic volumetric coverage as an alternative for specific absorption rate-intensive echo-train spin-echo sequences: feasibility study. Radiology. 2008;248:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Coenegrachts K, Delanote J, Ter Beek L, Haspeslagh M, Bipat S, Stoker J, Van Kerkhove F, Steyaert L, Rigauts H, Casselman JW. Improved focal liver lesion detection: comparison of single-shot diffusion-weighted echoplanar and single-shot T2 weighted turbo spin echo techniques. Br J Radiol. 2007;80:524-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Donati OF, Fischer MA, Chuck N, Hunziker R, Weishaupt D, Reiner CS. Accuracy and confidence of Gd-EOB-DTPA enhanced MRI and diffusion-weighted imaging alone and in combination for the diagnosis of liver metastases. Eur J Radiol. 2013;82:822-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Zech CJ, Herrmann KA, Dietrich O, Horger W, Reiser MF, Schoenberg SO. Black-blood diffusion-weighted EPI acquisition of the liver with parallel imaging: comparison with a standard T2-weighted sequence for detection of focal liver lesions. Invest Radiol. 2008;43:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Colagrande S, Castellani A, Nardi C, Lorini C, Calistri L, Filippone A. The role of diffusion-weighted imaging in the detection of hepatic metastases from colorectal cancer: A comparison with unenhanced and Gd-EOB-DTPA enhanced MRI. Eur J Radiol. 2016;85:1027-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Kim HJ, Lee SS, Byun JH, Kim JC, Yu CS, Park SH, Kim AY, Ha HK. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radiology. 2015;274:712-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Tanaka M, Kishi Y, Esaki M, Nara S, Miyake M, Hiraoka N, Nagino M, Shimada K. Feasibility of Routine Application of Gadoxetic Acid-Enhanced MRI in Combination with Diffusion-Weighted MRI for the Preoperative Evaluation of Colorectal Liver Metastases. Ann Surg Oncol. 2016;23:3991-3998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26:4595-4615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Kwee TC, Takahara T. Diffusion-weighted MRI for detecting liver metastases: importance of the b-value. Eur Radiol. 2011;21:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Bruegel M, Gaa J, Waldt S, Woertler K, Holzapfel K, Kiefer B, Rummeny EJ. Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusion-weighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol. 2008;191:1421-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | De Robertis R, Tinazzi Martini P, Demozzi E, Dal Corso F, Bassi C, Pederzoli P, D’Onofrio M. Diffusion-weighted imaging of pancreatic cancer. World J Radiol. 2015;7:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | d’Assignies G, Fina P, Bruno O, Vullierme MP, Tubach F, Paradis V, Sauvanet A, Ruszniewski P, Vilgrain V. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology. 2013;268:390-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Vandecaveye V, De Keyzer F, Verslype C, Op de Beeck K, Komuta M, Topal B, Roebben I, Bielen D, Roskams T, Nevens F. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. Eur Radiol. 2009;19:2456-2466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, Cooper N, Knight-Greenfield A, Babb JS, Boffetta P, Padron N. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY). 2017;42:179-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 36. | Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Aisen AM. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad Radiol. 2009;16:1208-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Calistri L, Castellani A, Matteuzzi B, Mazzoni E, Pradella S, Colagrande S. Focal Liver Lesions Classification and Characterization: What Value Do DWI and ADC Have? J Comput Assist Tomogr. 2016;40:701-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Caraiani CN, Marian D, Militaru C, Calin A, Badea R. The role of the diffusion sequence in magnetic resonance imaging for the differential diagnosis between hepatocellular carcinoma and benign liver lesions. Clujul Med. 2016;89:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Emara DMM, Mohamed FSE-D, Abdullah AH, Ibrahim MA-H, Ibrahim ME, Hassouna EM. Is diffusion weighted imaging adding value in diagnosis of focal hepatic lesions? Experience in 50 patients. Alex J Med. 2014;4:287-301. [Cited in This Article: ] |
| 40. | Jahic E, Sofic A, Selimovic AH. DWI/ADC in Differentiation of Benign from Malignant Focal Liver Lesion. Acta Inform Med. 2016;24:244-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Chan JH, Tsui EY, Luk SH, Fung AS, Yuen MK, Szeto ML, Cheung YK, Wong KP. Diffusion-weighted MR imaging of the liver: distinguishing hepatic abscess from cystic or necrotic tumor. Abdom Imaging. 2001;26:161-165. [PubMed] [Cited in This Article: ] |
| 42. | Park HJ, Kim SH, Jang KM, Lee SJ, Park MJ, Choi D. Differentiating hepatic abscess from malignant mimickers: value of diffusion-weighted imaging with an emphasis on the periphery of the lesion. J Magn Reson Imaging. 2013;38:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Karan B, Erbay G, Koc Z, Pourbagher A, Yildirim S, Agildere AM. Utility of Diffusion-Weighted MRI to Detect Changes in Liver Diffusion in Benign and Malignant Distal Bile Duct Obstruction: The Influence of Choice of b-Values. Can Assoc Radiol J. 2016;67:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Parsai A, Zerizer I, Roche O, Gkoutzios P, Miquel ME. Assessment of diffusion-weighted imaging for characterizing focal liver lesions. Clin Imaging. 2015;39:278-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 439] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 46. | Wei C, Tan J, Xu L, Juan L, Zhang SW, Wang L, Wang Q. Differential diagnosis between hepatic metastases and benign focal lesions using DWI with parallel acquisition technique: a meta-analysis. Tumour Biol. 2015;36:983-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Lee NK, Kim S, Kim DU, Seo HI, Kim HS, Jo HJ, Kim TU. Diffusion-weighted magnetic resonance imaging for non-neoplastic conditions in the hepatobiliary and pancreatic regions: pearls and potential pitfalls in imaging interpretation. Abdom Imaging. 2015;40:643-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Nishie A, Tajima T, Ishigami K, Ushijima Y, Okamoto D, Hirakawa M, Nishihara Y, Taketomi A, Hatakenaka M, Irie H. Detection of hepatocellular carcinoma (HCC) using super paramagnetic iron oxide (SPIO)-enhanced MRI: Added value of diffusion-weighted imaging (DWI). J Magn Reson Imaging. 2010;31:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Inchingolo R, De Gaetano AM, Curione D, Ciresa M, Miele L, Pompili M, Vecchio FM, Giuliante F, Bonomo L. Role of diffusion-weighted imaging, apparent diffusion coefficient and correlation with hepatobiliary phase findings in the differentiation of hepatocellular carcinoma from dysplastic nodules in cirrhotic liver. Eur Radiol. 2015;25:1087-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology. 2010;254:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Tang Y, Wang H, Ma L, Zhang X, Yu G, Li J, Ye H. Diffusion-weighted imaging of hepatocellular carcinomas: a retrospective analysis of correlation between apparent diffusion coefficients and histological grade. Abdom Radiol (NY). 2016;41:1539-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Okamura S, Sumie S, Tonan T, Nakano M, Satani M, Shimose S, Shirono T, Iwamoto H, Aino H, Niizeki T. Diffusion-weighted magnetic resonance imaging predicts malignant potential in small hepatocellular carcinoma. Dig Liver Dis. 2016;48:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, Nakanishi K, Kamiyama T, Kubota K, Haga H, Matsuno Y. Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:1302-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, Koh YS, Cho CK, Kang HK. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010;11:295-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Boussouar S, Itti E, Lin SJ, Decaens T, Evangelista E, Chiaradia M, Chalaye J, Baranes L, Calderaro J, Laurent A. Functional imaging of hepatocellular carcinoma using diffusion-weighted MRI and (18)F-FDG PET/CT in patients on waiting-list for liver transplantation. Cancer Imaging. 2016;16:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Nasu K, Kuroki Y, Tsukamoto T, Nakajima H, Mori K, Minami M. Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol. 2009;193:438-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 748] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 58. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 59. | Joo I, Lee JM, Yoon JH, Jang JJ, Han JK, Choi BI. Nonalcoholic fatty liver disease: intravoxel incoherent motion diffusion-weighted MR imaging-an experimental study in a rabbit model. Radiology. 2014;270:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Murphy P, Hooker J, Ang B, Wolfson T, Gamst A, Bydder M, Middleton M, Peterson M, Behling C, Loomba R. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging. 2015;41:1629-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Guiu B, Petit JM, Capitan V, Aho S, Masson D, Lefevre PH, Favelier S, Loffroy R, Vergès B, Hillon P. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012;265:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Aubé C, Racineux PX, Lebigot J, Oberti F, Croquet V, Argaud C, Calès P, Caron C. [Diagnosis and quantification of hepatic fibrosis with diffusion weighted MR imaging: preliminary results]. J Radiol. 2004;85:301-306. [PubMed] [Cited in This Article: ] |
| 63. | Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010;31:589-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 64. | Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Zhang B, Liang L, Dong Y, Lian Z, Chen W, Liang C, Zhang S. Intravoxel Incoherent Motion MR Imaging for Staging of Hepatic Fibrosis. PLoS One. 2016;11:e0147789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Jiang H, Chen J, Gao R, Huang Z, Wu M, Song B. Liver fibrosis staging with diffusion-weighted imaging: a systematic review and meta-analysis. Abdom Radiol (NY). 2017;42:490-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Bülow R, Mensel B, Meffert P, Hernando D, Evert M, Kühn JP. Diffusion-weighted magnetic resonance imaging for staging liver fibrosis is less reliable in the presence of fat and iron. Eur Radiol. 2013;23:1281-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | França M, Martí-Bonmatí L, Alberich-Bayarri Á, Oliveira P, Guimaraes S, Oliveira J, Amorim J, Gonzalez JS, Vizcaíno JR, Miranda HP. Evaluation of fibrosis and inflammation in diffuse liver diseases using intravoxel incoherent motion diffusion-weighted MR imaging. Abdom Radiol (NY). 2017;42:468-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Kocakoc E, Bakan AA, Poyrazoglu OK, Dagli AF, Gul Y, Cicekci M, Bahcecioglu IH. Assessment of Liver Fibrosis with Diffusion-Weighted Magnetic Resonance Imaging Using Different b-values in Chronic Viral Hepatitis. Med Princ Pract. 2015;24:522-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Kahraman AS, Kahraman B, Ozdemir ZM, Gormeli CA, Ozdemir F, Dogan M. Diffusion-weighted imaging (DWI) of the liver in assessing chronic liver disease: effects of the presence and the degree of ascites on ADC values. Abdom Radiol (NY). 2016;41:56-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Park HS, Kim YJ, Yu MH, Choe WH, Jung SI, Jeon HJ. Three-Tesla magnetic resonance elastography for hepatic fibrosis: comparison with diffusion-weighted imaging and gadoxetic acid-enhanced magnetic resonance imaging. World J Gastroenterol. 2014;20:17558-17567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Leitão HS, Doblas S, Garteiser P, d’Assignies G, Paradis V, Mouri F, Geraldes CF, Ronot M, Van Beers BE. Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology. 2017;283:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Watanabe H, Kanematsu M, Goshima S, Kondo H, Onozuka M, Moriyama N, Bae KT. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging--preliminary observations. Radiology. 2011;259:142-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 74. | Ding Y, Rao S, Yang L, Chen C, Zeng M. Comparison of the effect of region-of-interest methods using gadoxetic acid-enhanced MR imaging with diffusion-weighted imaging on staging hepatic fibrosis. Radiol Med. 2016;121:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Park HJ, Kim SH, Jang KM, Lim S, Kang TW, Park HC, Choi D. Added value of diffusion-weighted MRI for evaluating viable tumor of hepatocellular carcinomas treated with radiotherapy in patients with chronic liver disease. AJR Am J Roentgenol. 2014;202:92-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Yu JI, Park HC, Lim DH, Choi Y, Jung SH, Paik SW, Kim SH, Jeong WK, Kim YK. The role of diffusion-weighted magnetic resonance imaging in the treatment response evaluation of hepatocellular carcinoma patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:814-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Schraml C, Schwenzer NF, Clasen S, Rempp HJ, Martirosian P, Claussen CD, Pereira PL. Navigator respiratory-triggered diffusion-weighted imaging in the follow-up after hepatic radiofrequency ablation-initial results. J Magn Reson Imaging. 2009;29:1308-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TW, Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009;193:1044-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, Charu V, Schernthaner R, Wang Z, Tacher V. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273:746-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 80. | Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, Husband JE. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 81. | Schraml C, Schwenzer NF, Martirosian P, Bitzer M, Lauer U, Claussen CD, Horger M. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: initial results. AJR Am J Roentgenol. 2009;193:W301-W307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Tam HH, Collins DJ, Brown G, Chau I, Cunningham D, Leach MO, Koh DM. The role of pre-treatment diffusion-weighted MRI in predicting long-term outcome of colorectal liver metastasis. Br J Radiol. 2013;86:20130281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Barat M, Fohlen A, Cassinotto C, Jannot AS, Dautry R, Pelage JP, Boudiaf M, Pocard M, Eveno C, Taouli B. One-month apparent diffusion coefficient correlates with response to radiofrequency ablation of hepatocellular carcinoma. J Magn Reson Imaging. 2017;45:1648-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, Hsu JS, Liu GC. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006;239:448-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 85. | Goshima S, Kanematsu M, Kondo H, Yokoyama R, Tsuge Y, Shiratori Y, Onozuka M, Moriyama N. Evaluating local hepatocellular carcinoma recurrence post-transcatheter arterial chemoembolization: is diffusion-weighted MRI reliable as an indicator? J Magn Reson Imaging. 2008;27:834-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 87. | Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: Prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur J Radiol. 2013;82:577-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Kamel IR, Bluemke DA, Eng J, Liapi E, Messersmith W, Reyes DK, Geschwind JF. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 89. | Pieper CC, Willinek WA, Meyer C, Ahmadzadehfar H, Kukuk GM, Sprinkart AM, Block W, Schild HH, Mürtz P. Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging for Prediction of Early Arterial Blood Flow Stasis in Radioembolization of Breast Cancer Liver Metastases. J Vasc Interv Radiol. 2016;27:1320-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Anzidei M, Napoli A, Zaccagna F, Cartocci G, Saba L, Menichini G, Cavallo Marincola B, Marotta E, Di Mare L, Catalano C. Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. J Comput Assist Tomogr. 2011;35:690-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 92. | Shirota N, Saito K, Sugimoto K, Takara K, Moriyasu F, Tokuuye K. Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imaging. 2016;16:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Zheng DX, Meng SC, Liu QJ, Li CT, Shang XD, Zhu YS, Bai TJ, Xu SM. Predicting liver metastasis of gastrointestinal tract cancer by diffusion-weighted imaging of apparent diffusion coefficient values. World J Gastroenterol. 2016;22:3031-3037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1418] [Cited by in F6Publishing: 1392] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 95. | Baliyan V, Das CJ, Sharma S, Gupta AK. Diffusion-weighted imaging in urinary tract lesions. Clin Radiol. 2014;69:773-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Chandarana H, Taouli B. Diffusion and perfusion imaging of the liver. Eur J Radiol. 2010;76:348-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Bammer R, Keeling SL, Augustin M, Pruessmann KP, Wolf R, Stollberger R, Hartung HP, Fazekas F. Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE). Magn Reson Med. 2001;46:548-554. [PubMed] [Cited in This Article: ] |
| 98. | Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004;183:677-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 99. | Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. 2011;33:128-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 100. | Sasaki M, Yamada K, Watanabe Y, Matsui M, Ida M, Fujiwara S, Shibata E; Acute Stroke Imaging Standardization Group-Japan (ASIST-Japan) Investigators. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008;249:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 101. | Quantitative Imaging Biomarkers Alliance (QIBA)- Progress Report: as of March 2016[Internet]. Available from: http://qibawiki.rsna.org/images/3/36/HHSN268201500021C_QIBA_Semi-Annual_Progress_Report_14March2016.pdf. [Cited in This Article: ] |
| 102. | Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008;18:486-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |