Published online Jun 27, 2013. doi: 10.4254/wjh.v5.i6.340
Revised: May 19, 2013
Accepted: June 1, 2013
Published online: June 27, 2013
Focal nodular hyperplasia (FNH) is a benign condition that affects normal liver with low prevalence. Recently, the extensive use of oxaliplatin to treat patients with colorectal cancer has been reported to be associated with the development of different liver injuries, as well as focal liver lesions. The present work describes two patients with multiple bilateral focal liver lesions misdiagnosed as colorectal liver metastases, and treated with liver resection. The first patient had up to 15 small bilateral focal liver lesions, with magnetic resonance imaging consistent with colorectal liver metastases (CLM), and fluorodeoxyglucose (FDG)-positron emission tomography (PET) negative. The second patient had up to 5 small focal liver lesions, with computed tomography consistent with CLM, and FDG-PET negative. They had parenchyma sparing liver surgery, with uneventful postoperative course. At the histology the diagnosis was multiple FNHs. The risks of oxaliplatin-based chemotherapy regimens in development of liver injuries, such as FNH, should not be further denied. The value of the modern multidisciplinary management of patients with colorectal cancer relies also on the precise estimation of the risk/benefit for each patient.
Core tip: This report describes two interesting cases of patients who developed multiple focal nodular hyperplasias during oxaliplatin-based therapy for colorectal cancer. Such multiple bilateral focal liver lesions were misdiagnosed as colorectal liver metastases, and treated with liver resection. A review of the cases revealed that in both cases the fluorodeoxyglucose-positron emission tomography was negative. A brief review of the literature together with the authors’ comments is included.
- Citation: Donadon M, Di Tommaso L, Roncalli M, Torzilli G. Multiple focal nodular hyperplasias induced by oxaliplatin-based chemotherapy. World J Hepatol 2013; 5(6): 340-344
- URL: https://www.wjgnet.com/1948-5182/full/v5/i6/340.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i6.340
Focal nodular hyperplasia (FNH) is a benign condition that affects normal liver with prevalence up to 2.6% in autopsy studies[1,2]. Most of the patients are asymptomatic, while few of them may develop portal hypertension[3,4]. The physiopathology of FNH remains unknown, but non-specific chronic distortion of the intrahepatic blood flow has been considered a potential cause[5]. Recently, the extensive use of oxaliplatin to treat patients with colorectal cancer has been reported to be associated with the development of different liver injuries[5,6]. The present work describes two patients with multiple bilateral focal liver lesions misdiagnosed as colorectal liver metastases (CLM), and treated with liver resection.
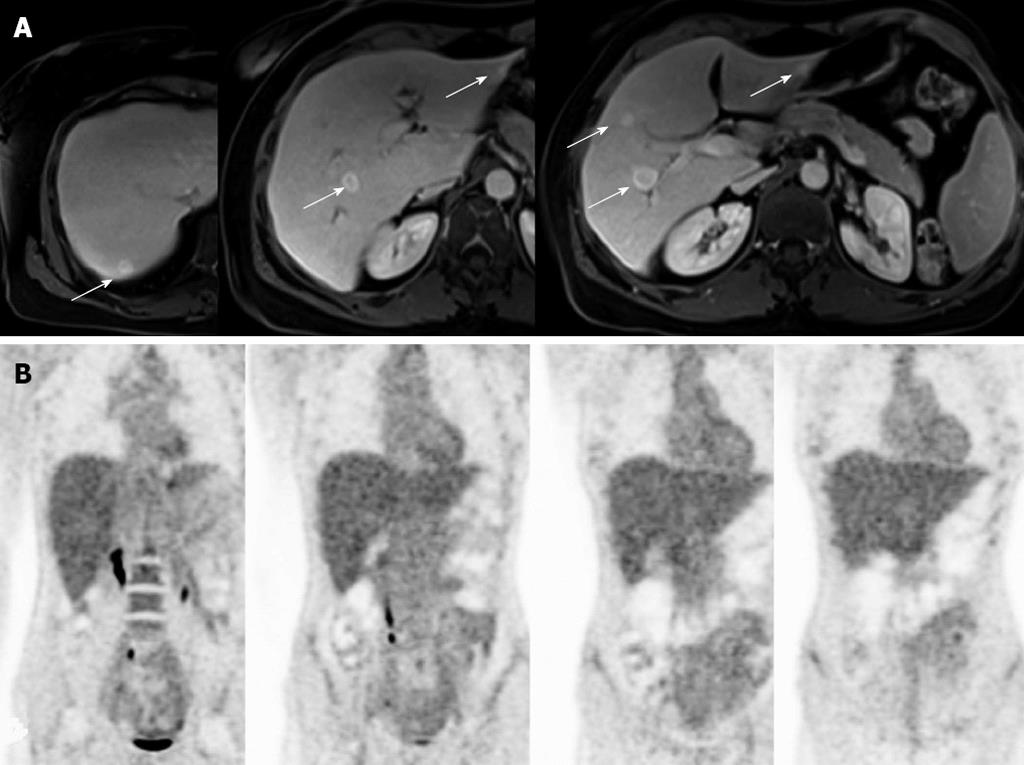
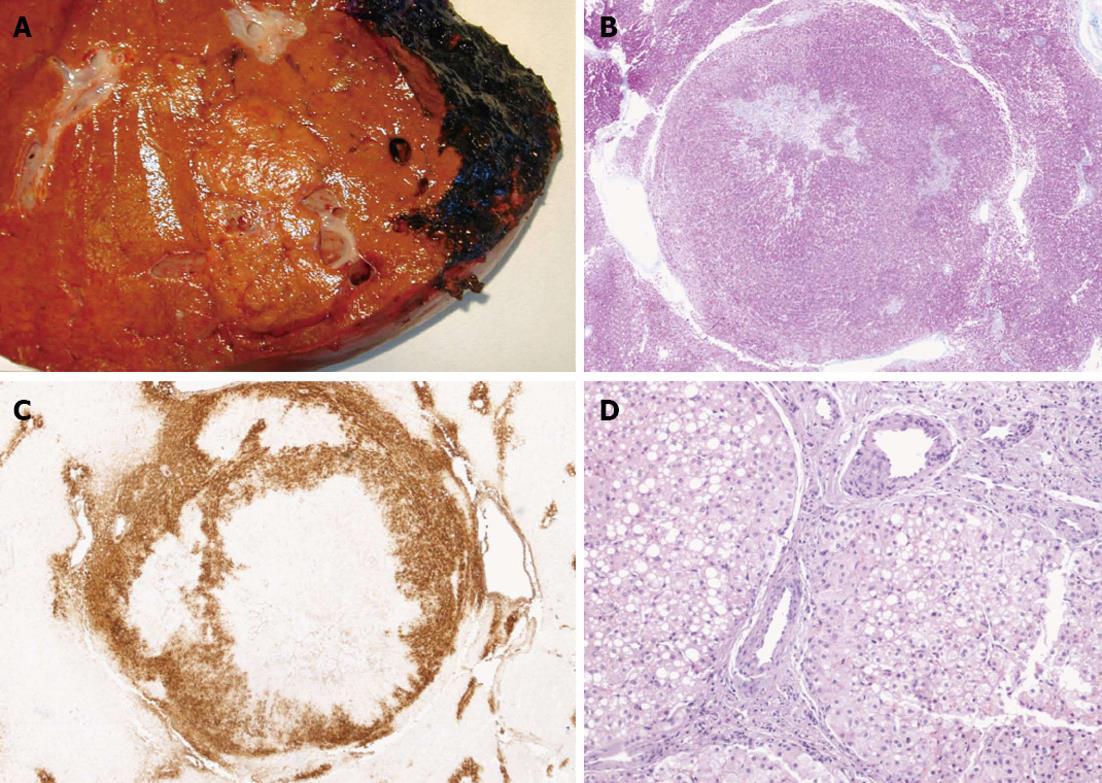
A 53-year-old woman presented at the liver surgery unit because of multiple focal liver lesions. In December 2007 in another institution after a preoperative staging comprehensive of an abdominal computed tomography (CT), which resulted negative for liver lesions, the patient had laparoscopic right colectomy because of colonic adenocarcinoma pathologically staged as pT3N2M0-G2. Then, she had 8 courses of systemic chemotherapy with FOLFOX regimen (oxaliplatin, leucovorin, and fluorouracil) followed by 4 courses of De Gramont regimen (fluorouracil and folinic acid). The modification of the chemotherapy regimen was due to oxaliplatin side effects, such as peripheral neuropathy, and thrombocytopenia. Then the follow-up, based on quarterly CT, was regular and negative till January 2010, when she developed up to 15 bilateral focal liver lesions. She was than referred to our institution where she underwent a diagnostic work-up comprehensive of abdominal magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan. MRI was consistent with the diagnosis of CLM, while FDG-PET was negative (Figure 1). Both the carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) serum values were normal. The CEA was elevated before the colonic resection. Based on our weekly multidisciplinary meeting the patient was candidate to surgical resection. Therefore, after 14 mo from the end of the adjuvant chemotherapy, in March 2010 she had multiple ultrasound-guided liver resections for the removal of all 15 lesions. The postoperative course was uneventful. At gross inspection the lesions sized between 0.5 and 2 cm and were characterized by well-defined margins, lobulated appearance, and a central scar (Figure 2A). At histology the lesions were characterized by several fibrous septa highlighted by special stains (Figure 2B, Masson staining), and they showed a typical patter at glutamine synthetase immunostaining (Figure 2C). Yet, at higher magnification they contained several dystrophic vessels (Figure 2D). All these findings were in keeping with a diagnosis of multiple FNH without any evidence of malignant cells. She did not receive postoperative chemotherapy. After 32 mo the patient is alive, and free of tumoral recurrence. However, this patient developed 4 new small liver lesions consistent with multiple FNHs, which are stable after 13 mo of follow-up.
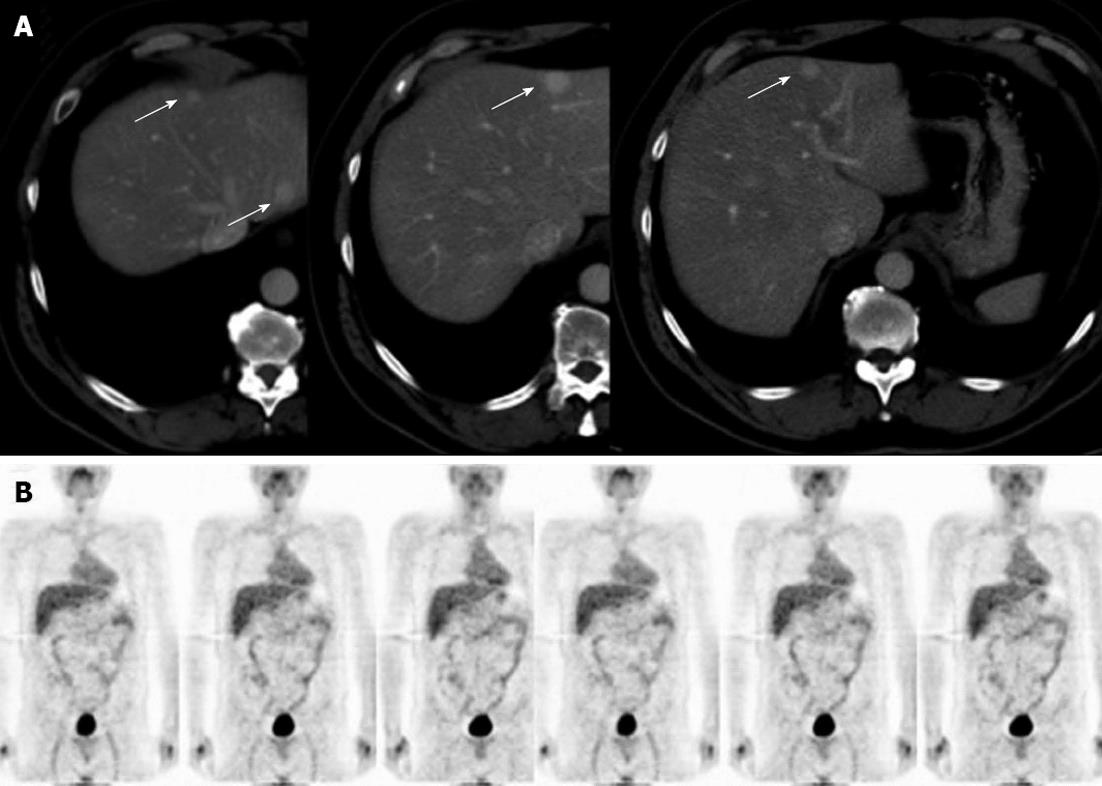
A 56-year-old man presented at the liver surgery unit because of multiple focal liver lesions. In July 2008 after a preoperative staging comprehensive of an abdominal CT, which resulted negative for liver lesions, the patient had laparoscopic left colectomy because of colonic adenocarcinoma pathologically staged as pT3N1M0-G2. Then, the patient had 12 courses of systemic chemotherapy with FOLFOX regimen. The subsequent follow-up was regular and negative till March 2011, when he developed up to 5 bilateral focal liver lesions and was referred to out institution where he had abdominal CT and FDG-PET scan. CT was consistent with the diagnosis of CLM, while FDG-PET was negative (Figure 3). Both the CEA and CA19-9 serum values were normal, and they were within the normal range even before the colonic resection. Based on our weekly multidisciplinary meeting the patient was candidate to surgical resection, and in June 2011, 16 mo the end of adjuvant chemotherapy, he had multiple ultrasound-guided liver resections for the removal of all 5 lesions. The postoperative course was uneventful. Similarly to the previous patient, at gross inspection the larger lesion was 2 cm in diameter. They were characterized by well-defined margins, and the histology review showed some typical findings consistent with the diagnosis of multiple FNHs. He did not receive postoperative chemotherapy. After 20 mo the patient is alive and free of recurrence.
The presented cases showed two patients affected by colorectal cancer with multiple FNHs potentially inducedby oxaliplatin-based chemotherapy. Both of them had no history of chronic liver disease, and the chronological correlation between the chemotherapy, its duration, and the appearance of the liver lesions suggests a cause-effect association.
There is a burgeoning use of preoperative chemotherapy in patients with CLM, and a corresponding burgeoning literature reporting non-tumoral liver lesions induced by different chemotherapy regimens[6]. Some direct correlations between chemotherapy agents, specific liver toxicity and postoperative morbidity have been also reported[7,8]. In particular, the development of FNH during oxaliplatin-based systemic chemotherapy has been reported up to 15% of the patients treated with preoperative chemotherapy[9]. Based on a recent review on such topic the damages associated with oxaliplatin-based chemotherapy are complex and heterogeneous. Both erythrocyte extravasation and hepatocytic plate disruption have been reported being signs of sinusoidal wall rupture[5,10]. However, the pathogenesis of FNH remains unclear. Changes in intrahepatic blood flow are supposed to be the primary cause. Such changes may be due to portal vein injuries at the level of the sinusoids, and the resulting portal hypertension plays an important role. The natural history of FNH remains unknown, too. Few spontaneous regressions after the suspension of the chemotherapy have been reported as well as some protective effects of bevacizumab on the development of this toxicity[5,11,12].
From the clinical standpoint their proper diagnosis is a delicate matter, since they occur in oncological patients with generally well-documented, and extensive negative imaging. In our two patients to these consideration the multinodular pattern has further biased the diagnostic conclusion. Indeed, only Hubert et al[11] previously reported two cases of multiple FNHs occurred in similar circumstances while other authors described patients with single lesions[5,6,9]. Our experience is conveying a further peculiarity, which could be useful for further discussion and experiences in this sense. Indeed, to our knowledge no previous studies on the use of FDG-PET in such specific clinical setting have been reported. Even if no conclusions can be drawn based on two cases, and considering also the tendency of lower accuracy of FDG-PET after chemotherapy[13], a potential value of such imaging modality should be further investigated, and taken into account during the workup in such circumstances.
In conclusion, an increasing proportion of patients with CLM nowadays receive oxaliplatin-based chemotherapy, including postoperative treatment after stage III colon cancer, induction therapy in patients with extensive metastases, and perioperative treatment in patients with resectable metastases. The risks of such chemotherapy regimens in development of FNHs, as well as of other forms of liver injuries, should not be further denied. The value of the modern multidisciplinary management of patients with colorectal cancer relies also on the precise estimation of the risk/benefit for each patient.
P- Reviewers Figueiredo P, Nielson HJ, SiposF, Yu B S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Nakanuma Y. Nodular regenerative hyperplasia of the liver: retrospective survey in autopsy series. J Clin Gastroenterol. 1990;12:460-465. [PubMed] [Cited in This Article: ] |
| 2. | Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 367] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Molina E, Reddy KR. Noncirrhotic portal hypertension. Clin Liver Dis. 2001;5:769-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Dachman AH, Ros PR, Goodman ZD, Olmsted WW, Ishak KG. Nodular regenerative hyperplasia of the liver: clinical and radiologic observations. AJR Am J Roentgenol. 1987;148:717-722. [PubMed] [Cited in This Article: ] |
| 5. | Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey JN. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 731] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 970] [Cited by in F6Publishing: 928] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 8. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Wicherts DA, de Haas RJ, Sebagh M, Ciacio O, Lévi F, Paule B, Giacchetti S, Guettier C, Azoulay D, Castaing D. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2011;18:659-669. [PubMed] [Cited in This Article: ] |
| 10. | Bioulac-Sage P, Laumonier H, Cubel G, Rossi JZ, Balabaud C. Hepatic resection for inflammatory hepatocellular adenomas: pathological identification of micronodules expressing inflammatory proteins. Liver Int. 2010;30:149-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Hubert C, Sempoux C, Horsmans Y, Rahier J, Humblet Y, Machiels JP, Ceratti A, Canon JL, Gigot JF. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int. 2007;27:938-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767. [PubMed] [Cited in This Article: ] |
| 13. | Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, Even-Sapir E, Figer A, Ben-Haim M. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg. 2007;11:472-478. [PubMed] [Cited in This Article: ] |