Published online Aug 16, 2012. doi: 10.4253/wjge.v4.i8.362
Revised: July 10, 2012
Accepted: August 8, 2012
Published online: August 16, 2012
AIM: To investigate whether magnifying endoscopy with narrow band imaging (ME-NBI) is useful for evaluating the area of superficial, depressed- or flat-type differentiated adenocarcinoma of the stomach.
METHODS: This procedure was performed in Saitama Medical University International Medical Center, Japanese Red Cross Kumamoto Hospital and Kitakyushu Municipal Medical Center. The subjects were 31 patients in whom biopsy findings, from superficial, depressed- or flat-type gastric lesion, suggested differentiated adenocarcinoma in the above 3 hospitals between January and December 2009. Biopsy was performed on the lesion and non-lesion sides of a boundary (imaginary boundary) visualized on ME-NBI. The results were pathologically investigated. We evaluated the accuracy of estimating a demarcation line (DL) on ME-NBI in comparison with biopsy findings as a gold standard.
RESULTS: The DL that could be recognized at 2 points on the orifice and anal sides of each lesion during ME-NBI was consistent with the pathological findings in 22 patients with 0-IIc lesions, 7 with 0-IIb lesions, and 2 with 0-IIb + IIc lesions, showing an accuracy of 100%.
CONCLUSION: The results suggest the usefulness of ME-NBI for evaluating the area of superficial, depressed- and flat-type differentiated adenocarcinoma of the stomach.
- Citation: Nonaka K, Namoto M, Kitada H, Shimizu M, Ochiai Y, Togawa O, Nakao M, Nishimura M, Ishikawa K, Arai S, Kita H. Usefulness of the DL in ME with NBI for determining the expanded area of early-stage differentiated gastric carcinoma. World J Gastrointest Endosc 2012; 4(8): 362-367
- URL: https://www.wjgnet.com/1948-5190/full/v4/i8/362.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i8.362
Narrow band imaging (NBI) is a new endoscopic technique developed by Olympus Co., Ltd., which facilitates the visualization of microvascular features on the mucosal surface and their fine structure with high-level contrast employing two types of ray (central wavelengths: 415 and 540 nm, respectively)[1]. The endoscopic diagnosis of intramucosal gastric carcinoma of the superficial depressed type or flat type with nonmagnified instrument is often difficult because such carcinoma, so-called “gastritis-like cancer”, are manifest as only subtle changes in color and shape. Yao et al[2] have reported that magnified observation without NBI of the microvascular architecture of intramucosal gastric carcinoma may be useful for characterizing flat carcinoma that exhibit only subtle changes in color and shape at standard endoscopy, and also be useful for determining the extent of intramucosal spread of differentiated carcinomas that have an irregular margin.
Yao et al[3] reported that a regular subepithelial capillary network (SECN) pattern was present in the mucosa around gastric carcinoma, but the pattern was lost in the microvascular architecture of differentiated gastric carcinoma, in which microvascular growth with an irregular morphology and distribution was noted, and a clear demarcation line (DL) was formed at the boundary between the cancerous and non-cancerous regions due to differences in the microvascular architecture between the regular SECN pattern and irregular microvascular pattern. However, they employed magnifying endoscopy (ME) without NBI.
Recent studies have reported that the use of NBI, which facilitates the visualization of microvascular and fine mucosal architectures, contributes to the detection of small, superficial, depressed- or flat-type adenocarcinoma of the stomach and improvement in the diagnostic capacity[4-9]. However, to date, few studies have reported the usefulness of determining of the DL of gastric cancer by ME with NBI (ME-NBI)[7,8,10,11]. In addition, according to recent studies, it is impossible to examine vascular features using this procedure in some patients with differentiated adenocarcinoma of the stomach[12,13]. When evaluating the extent of differentiated adenocarcinoma using NBI, not only differences in the microvascular architecture but also those in the fine structure of mucosa between cancerous and non-cancerous regions must be considered. Furthermore, the usefulness of ME-NBI for establishing/marking the extent of resection has been discussed. However, it is controversial to evaluate whether diagnosis of the extent of cancer using ME-NBI is accurate in a resected specimen involving a mark drawn in an area lateral to the DL visualized on ME-NBI.
Unlike the previously reported evaluation of its usefulness in endoscopic submucosal dissection (ESD) specimens[7,9-11], the present study investigated the usefulness of a new method of gastric biopsy performed during ME-NBI.
We used magnifying endoscope (GIF-Q240Z, Olympus Optical Co., Ltd., Tokyo, Japan) combined with NBI system, consisting of an image processor (CV-260SL, Olympus), a light source (CLV-260SL, Olympus) in this study. Before the examination, a hood was mounted on the tip of the endoscope to enable the endoscopist to fix the focal distance at 2 mm between the tip of the instrument and the mucosal surface.
Thirty-one patients with superficial, differentiated carcinoma of the stomach who underwent ME-NBI in 3 hospitals (our hospital, Kitakyushu Municipal Medical Center, and Japanese Red Cross Kumamoto Hospital) between January and December 2009 were enrolled in this prospective, uncontrolled study. Examinations were carried out by 3 endoscopists specializing in NBI (1 per hospital). In all patients, the differentiated adenocarcinoma had been diagnosed previously at conventional endoscopy including biopsies with histopathologic confirmation.
Macroscopic type of the carcinoma was classified according to the classification for early stage gastric cancer of the Japanese Research Society of Gastric Cancer (Type I, protruded; Type IIa, superficial elevated; Type IIb, flat; Type IIc, superficial depressed; and Type III, excavated). Carcinoma of the superficial depressed or flat types as determined at standard endoscopy were included in this study. Protruded and superficial elevated types were excluded as these are easily identified by standard endoscopy alone. Patients were excluded if the endoscopic findings using conventional endoscopy or endoscopic ultrasonography clearly suggested ulceration within the lesion, or obvious submucosal invasion, because both of these findings may influence the microvascular architecture of the lesion. In addition, we excluded patients in whom biopsy findings suggested the coexistence of undifferentiated with differentiated carcinoma.
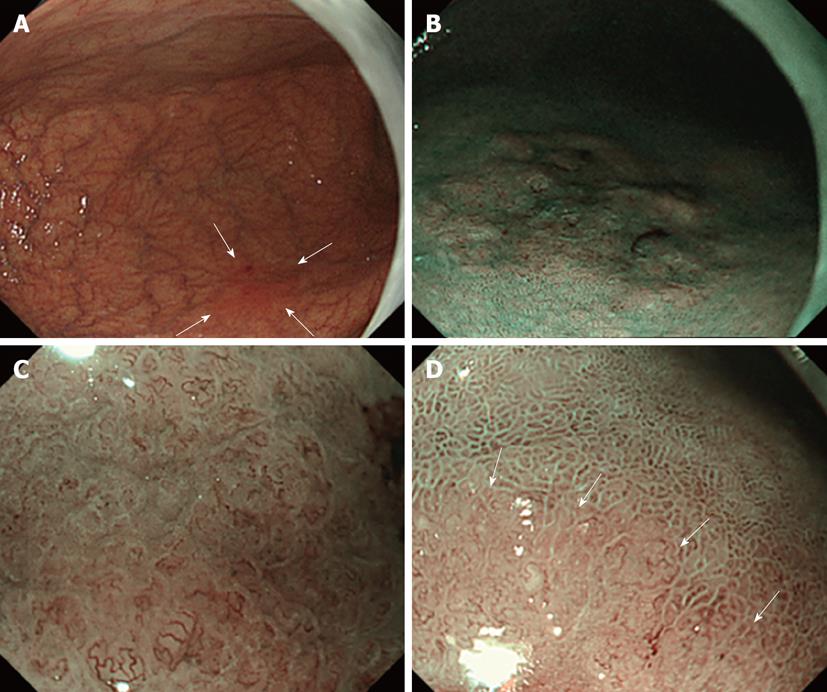
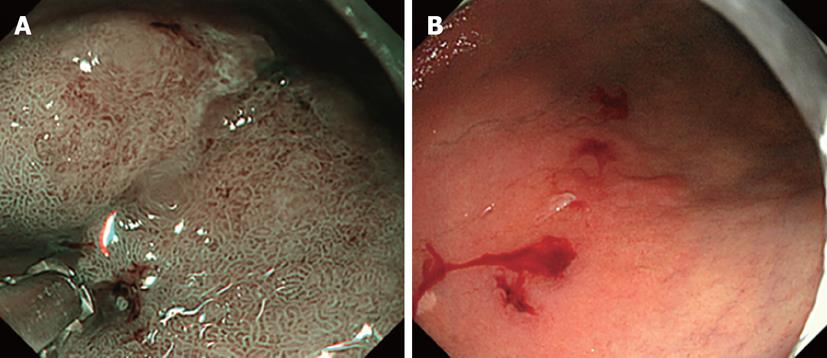
ME-NBI was applied for 31 flat-type and superficial depressed-type lesions with unclear boundaries diagnosed as differentiated gastric carcinoma based on conventional observation and biopsy. The presence or absence of DL was judged on the oral and anal sides of the lesions. The nearest part of the lesion from the esophagogastric junction was determined by endoscopic observation and defined as oral side of the lesions. The nearest part of the lesion from the pyloric ring was also determined by endoscopic observation and defined as anal side of the lesions. Lesions with and without DL identification were presented as DL (+) and (-), respectively. Biopsy was performed, assuming regions sandwiching the DL as cancerous and non-cancerous mucosa (Figures 1 and 2).
For biopsy, we employed biopsy forceps measuring 1.8 mm in tip diameter (FB-21K-1, Olympus Tokyo). To measure the distance from the DL as objectively and accurately as possible, biopsy forceps were used as an indicator. The study used 1.8 mm biopsy forceps, the smallest commercially available, to avoid taking too much tissue and minimize the distance between two biopsy sites sandwiching the DL.
Establishing a distance of approximately 1.8 mm from the boundary (DL) estimated on NBI observation, two regions sandwiching it were assumed as cancerous and non-cancerous. As described above, biopsy was performed on the orifice and anal sides of each lesion. Based on the diagnostic criteria, the assistant doctor recorded the presence or absence of DL during the procedure to ensure the objectivity of the examination. The rate of consistency of the boundary between the cancerous and non-cancerous mucosa on magnified NBI and that identified in the biopsied specimen was investigated. A total of 4 biopsy specimens per lesion were collected. Therefore, 124 biopsy specimens were collected in 31 patients.
All patients gave their written informed consent. Patients who were receiving warfarin or any other anticoagulant treatment were excluded from this study. This study was approved by the Medical Ethics Committee of Saitama Medical University International Medical Center, Japanese Red Cross Kumamoto Hospital and Kitakyushu Municipal Medical Center. The UMIN Clinical Trials Registry identification number for this study is C000001769.
There is no statistical analysis. The calculations were performed by using SAS version 8.0 (SAS Institute Inc., Cary, NC).
In the 31 patients, we investigated the age, gender, lesion site, maximum diameter, morphology, presence or absence of DL at each 2 points on the orifice and anal sides, and proportion of patients in whom NBI findings were consistent with biopsy findings (Table 1). The number of patients did not reach the initial target 60, but enrollment was discontinued because the 1-year enrollment period had ended. The lesion size was measured using resected specimens. In untreated patients from whom no specimen had been resected, measuring forceps for routine observation were employed. The median age was 71 years (57-87 years). The male-to-female ratio was 25:6. Lesions were localized in the upper, middle, and lower areas in 8, 15, and 8 patients, respectively.
| Age (median, yr) | 71 |
| Sex (M:F) | 25:6 |
| Location (U:M:L) | 8:15:8 |
| Tumor size (median) (range) (mm) | 22 (3-72) |
| Macroscopic type [IIb:IIc:(IIb + IIc)] | 7:22:2 |
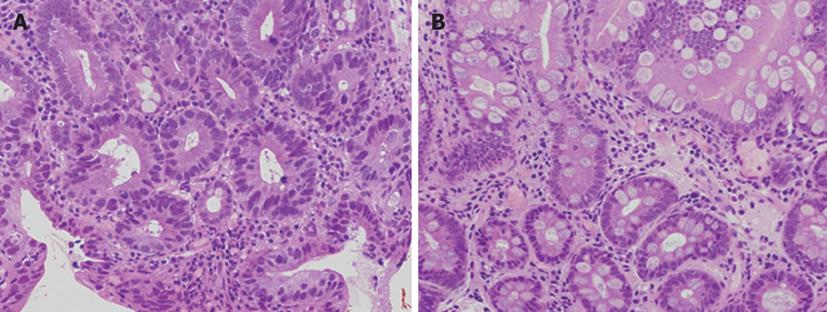
Macroscopically, the morphology was evaluated as IIb in 7 patients, IIc in 22, and IIb + IIc in 2. The median lesion diameter was 22 mm, with an inter-quartile range of 40 mm to 14 mm. On NBI observation, the DL could be recognized at all 62 points. As shown in Figures 1 and 2, biopsy was performed, assuming two regions sandwiching the DL that could be recognized on NBI as cancerous and non-cancerous. In each 2 points in 31 patients, the imaginary boundary was consistent with a pathologically detected border between the cancerous and non-cancerous regions as a gold standard (Figure 3).
An NBI endoscopic system with 415-nm and 540-nm rays has facilitated the visualization of blood vessels in high-level contrast[1]. In this system, an incoming ray penetrates the superficial layer of translucent tissue below the mucosal epithelium, and is strongly absorbed by hemoglobin. As a secondary action, the contrast of vascular features makes it possible to evaluate the fine structure of mucosa, and an incoming ray may be strongly reflected from the mucosal surface, contributing to the visualization of its fine structure.
Based on this principle, the NBI system has commonly been employed for the diagnosis of epithelial and non-epithelial tumors of the digestive tract[12,14-17]. Previous studies involving gastric cancer patients have reported a vascular pattern (fine network pattern) specific to differentiated adenocarcinoma and a corkscrew pattern specific to undifferentiated carcinoma, employing NBI-combined magnified endoscopy. This procedure is routinely used in clinical practice. With respect to fine mucosal structures, Uedo et al[18] proposed a “light blue crest”, Yao et al[12] a “white opaque substance”. Diagnoses are made based on microvascular features and these mucosal structures.
Despite the widespread use of NBI, few studies to date have reported the usefulness of ME-NBI for determining the extent of gastric cancer[7,9-11]. In Japan, the widespread use of ESD has facilitated resection regardless of the tumor size in patients with early gastric cancer[19,20]. Therefore, it is very important to evaluate the extention of the lesions. Kiyotoki et al[11] compared ME-NBI and indigo carmine chromoendoscopy without magnification to determine the gastric tumor margin, and found that the diagnostic accuracy of the former technique was significantly higher, at 97.4%, than that of the latter, at 77.8% (P = 0.009). Their study included 13 patients with adenoma, in only one of whom the extent of tumor invasion by ME-NBI was misdiagnosed. As we previously reported[21], gastric adenoma differs from gastric carcinoma in that, in many cases, microvessels cannot be visualized by NBI, or only microvessels similar to those of the surrounding mucosa can be observed and the mucosal microstructure is very difficult to distinguish from intestinal metaplasia in the surrounding gastric mucosa. Kiyotoki et al[11] considered that these features of gastric adenoma led to the misdiagnosis of the adenoma patient. However, the diagnostic accuracy of ME-NBI in 38 gastric cancer patients was 100%, which was similar to our results.
In a study by Kadowaki et al[10], a group of eight experts and a group of eight non-experts compared the usefulness of four different methods: conventional ME (CME), NE-MBI, enhanced-ME with acetic acid (EME), and ME with NBI and acetic acid (NBI-EME), for determining the extent of gastric cancer using the original scoring system. Both groups found that ME-NBI and NBI-EME were more useful for the diagnosis of the extent of gastric cancer than CME, suggesting that NE-MBI is useful even for non-experts in the diagnosis of gastric cancer invasion.
These two studies were problematic in that they did not include patients with 0-IIb lesions, the extent of which is the most difficult to determine among gastric cancers, but included many with elevated lesions, the margins of which are relatively easily recognizable during routine observation. Yao et al[2] considered that lesions in which it is difficult to determine their extent by routine observation were superficial depressed- (0-IIc) and flat-type (0-IIb) gastric carcinomas (so-called “gastritis-like cancer”), performed magnified observation of these lesions without NBI, and reported the results using the expression “demarcation line”. Based on this first report, we examined patients with 0-IIc or 0-IIb lesions. Although the present study was limited to patients with IIb (flat-type) or IIc (superficial depressed-type) lesions, the diagnostic accuracy for determining the cancer extent was as high as 100%.
Physicians must recognize the limitations of determining the extent of undifferentiated carcinoma, which may extend at the middle part of the lamina propria, showing no abnormal findings in the superficial mucosal layer, as well as the absence of evidence regarding the consistency between NBI-recognized and pathological boundaries in differentiated carcinoma patients who have undergone ESD involving a 2- or 3-mm marginal region via marking at points 2- or 3-mm lateral to the boundary estimated on NBI. In this study, to overcome these limitations, we examined the usefulness of diagnosing the extent using NBI in reference to biopsy findings as a gold standard.
According to Yao et al[2], when employing novel ME without NBI, the DL can be recognized based on differences in microvascular features between the cancerous and non-cancerous regions in patients with differentiated adenocarcinoma of the stomach. Araki et al[22] analyzed NBI-combined ME images of differentiated adenocarcinoma of the stomach using a computer. There were no significant differences in the density or mean diameter of microvessels between the cancerous and non-cancerous regions. However, branching and fusion were significantly more marked in the cancerous region. Therefore, they reported that ME-NBI was useful for evaluating the border between the cancerous and non-cancerous regions. However, actually, it is difficult to recognize microvascular features in some patients. Ezoe et al[13] indicated that there was no abnormal blood vessel in 17% of patients with gastric small depressive cancer. In their study described above, patients in whom no abnormal blood vessel was visually detected were also excluded[22].
ME-NBI has facilitated the visualization of microvascular features and fine mucosal structures, making it possible to recognize a boundary based on differences in the fine structure of mucosa between cancerous and non-cancerous regions. For diagnosis of the extent using NBI, it is important to initially recognize marked vascular abnormalities or those in the fine structure of mucosa at the lesion center and expand the extent of observation toward the lateral side.
In this study, we reviewed serial cases over 1 year, and the results suggested the usefulness of boundary diagnosis using NBI. However, the number of patients was small, and the 3 endoscopists participating in this study were familiar with NBI diagnosis. In the future, a larger number of patients should be investigated, and the results of this procedure carried out by beginners must be reviewed. However, taken together with the report of Kiyotoki et al[11], we can conclude that diagnosis of the extent using NBI is as useful as or more useful than conventional diagnosis based on standard observation. In hospitals in which 4-point biopsy has been performed to evaluate the extension of the lesion, the introduction of this method may eliminate unnecessary biopsy. In our series, biopsy led to a diagnosis of cancer before DL assessment. However, Yao et al[3] reported that 25.3% of patients with gastritis showed a DL on magnifying WLI. Ezoe et al[13] indicated DL presence on magnifying NBI in 42% of patients with non-cancerous gastric small depressive lesions. Their findings must be considered. Briefly, ME-NBI may be very useful for evaluating the extent of differentiated adenocarcinoma, but may not become an absolute diagnostic criterion for cancer.
Recent studies have reported that the use of narrow band imaging (NBI), which facilitates the visualization of microvascular and fine mucosal architectures, contributes to the detection of small, superficial, depressed- or flat-type adenocarcinoma of the stomach and improvement in the diagnostic capacity.
There have been few studies reported the usefulness of determining of the demarcation line (DL) of gastric cancer by magnifying endoscopy with NBI (ME-NBI).
Previous studies have shown the usefulness of ME-NBI for determination of the range of early gastric cancer. However, in each study, endoscopic submucosal dissection (ESD) specimens were used for evaluation. The authors consider it difficult to discuss the usefulness of this method using ESD specimens obtained by marking a few mm outside the line recognized as the lesion border and incision a few mm outside the marking. This problem could be overcome in the present study.
In hospitals in which 4-point biopsy has been performed to evaluate the extention of the lesion, the introduction of this method may eliminate unnecessary biopsy.
DL was formed at the boundary between the cancerous and non-cancerous regions due to differences in the microvascular architecture between the regular subepithelial capillary network pattern and irregular microvascular pattern.
The authors prospectively studied usefulness of ME-NBI on diagnosis of differentiated early stage of gastric adenocarcinoma. This is a very interesting and novel topic. Information of this study is very important for future progress of gastrointestinal endoscopy field.
Peer reviewers: Carlos Robles-Medranda, MD, Head of the Endoscopy Division, Ecuadorian Institute of Digestive Disease, San Antonio Clinic, Av. Reales Tamarindo y Tennis Club, Portoviejo-Manabi-Ecuador, Casilla 13-01-266, Ecuadorian; Shuhei Yoshida, MD, PhD, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215, United State
S- Editor Song XX L- Editor A E- Editor Zheng XM
| 1. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 2. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [PubMed] [Cited in This Article: ] |
| 3. | Yao K, Matsui T, Iwashita A. [Clinical application of magnification endoscopy with NBI for diagnosis of early gastric cancer]. Nihon Shokakibyo Gakkai Zasshi. 2007;104:782-789. [PubMed] [Cited in This Article: ] |
| 4. | Ang TL, Fock KM, Teo EK, Tan J, Poh CH, Ong J, Ang D. The diagnostic utility of narrow band imaging magnifying endoscopy in clinical practice in a population with intermediate gastric cancer risk. Eur J Gastroenterol Hepatol. 2012;24:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhang J, Guo SB, Duan ZJ. Application of magnifying narrow-band imaging endoscopy for diagnosis of early gastric cancer and precancerous lesion. BMC Gastroenterol. 2011;11:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017-2025.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Hirasawa D, Fujita N, Yamagata T, Suzuki T, Noda Y. A case of early gastric cancer in which the degree of histological atypia was correctly predicted by magnifying endoscopy combined with narrow band imaging. Dig Endosc. 2011;23 Suppl 1:92-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Morita Y, Fujiwara S, Tanaka S, Toyonaga T, Azuma T. A case of small early gastric cancer that was successfully detected by narrow band imaging magnifying endoscopy. Dig Endosc. 2011;23 Suppl 1:89-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Takeuchi M, Kobayashi M, Hashimoto S, Narisawa R, Aoyagi Y. Usefulness of magnifying narrow band imaging for assessing lateral tumor extent of early gastric cancer: a case report. Dig Endosc. 2011;23 Suppl 1:86-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kadowaki S, Tanaka K, Toyoda H, Kosaka R, Imoto I, Hamada Y, Katsurahara M, Inoue H, Aoki M, Noda T. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009;24:1625-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kiyotoki S, Nishikawa J, Satake M, Fukagawa Y, Shirai Y, Hamabe K, Saito M, Okamoto T, Sakaida I. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010;25:1636-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Ezoe Y, Muto M, Horimatsu T, Minashi K, Yano T, Sano Y, Chiba T, Ohtsu A. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010;71:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 476] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 15. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Nonaka K, Ishikawa K, Shimizu M, Sakurai T, Nakai Y, Nakao M, Yoshino K, Arai S, Kita H. Education and Imaging. Gastrointestinal: gastric mucosa-associated lymphoma presented with unique vascular features on magnified endoscopy combined with narrow-band imaging. J Gastroenterol Hepatol. 2009;24:1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Nonaka K, Ishikawa K, Arai S, Shimizu M, Sakurai T, Nishimura M, Nakao M, Sasaki Y, Kita H. Magnifying endoscopic observation of mantle cell lymphoma in the stomach using the narrow-band imaging system. Endoscopy. 2010;42 Suppl 2:E94-E95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 21. | Nonaka K, Arai S, Ban S, Kitada H, Namoto M, Nagata K, Ochiai Y, Togawa O, Nakao M, Nishimura M. Prospective study of the evaluation of the usefulness of tumor typing by narrow band imaging for the differential diagnosis of gastric adenoma and well-differentiated adenocarcinoma. Dig Endosc. 2011;23:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Araki Y, Sasaki Y, Hanabata N, Yoshimura T, Sawaya M, Hada R, Fukuda S. Morphometry for microvessels in early gastric cancer by narrow band imaging-equipped magnifying endoscopy. Dig Endosc. 2011;23:233-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |