Published online Aug 16, 2010. doi: 10.4253/wjge.v2.i8.278
Revised: June 22, 2010
Accepted: June 29, 2010
Published online: August 16, 2010
Endoscopic ultrasound (EUS) provides relevant information when an ampullary or periampullary tumor is suspected. Early detection, T and N staging and Fine Needle Aspiration plus cithologic confirmation, are some of the expected benefits. Exclusion of benign findings like choledocholithiasis or chronic pancreatitis is also important. A correct understanding of the complex ampullary and periampullary anatomy is needed. Knowledge of the individual clinical history and other previous diagnostic images all contribute to a successful EUS examination. Radial and lineal EUS images are uniquely detailed and, at the moment, it seems to be the best way to exclude or confirm malignant or benign findings. We propose a procedural algorithm, including EUS, for suspected ampullary or periampullary tumors. This review summarizes the vast amount of information to be found spread in the literature, and recognizes this small anatomic area as the origin for a clinical entity with proper clinical presentation, proper imaging and proper therapeutic resolutions. The benefits of performing EUS for its study are highlighted.
- Citation: Castillo C. Endoscopic ultrasound in the papilla and the periampullary region. World J Gastrointest Endosc 2010; 2(8): 278-287
- URL: https://www.wjgnet.com/1948-5190/full/v2/i8/278.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i8.278
Tumors of the papilla and the periampullary region are rare and often malignant. Their prognosis is generally better than for other digestive malignancies, due to their different histology and because the clinical manifestations tend to manifest themselves earlier. Detection of small tumors and proper staging are therefore important.
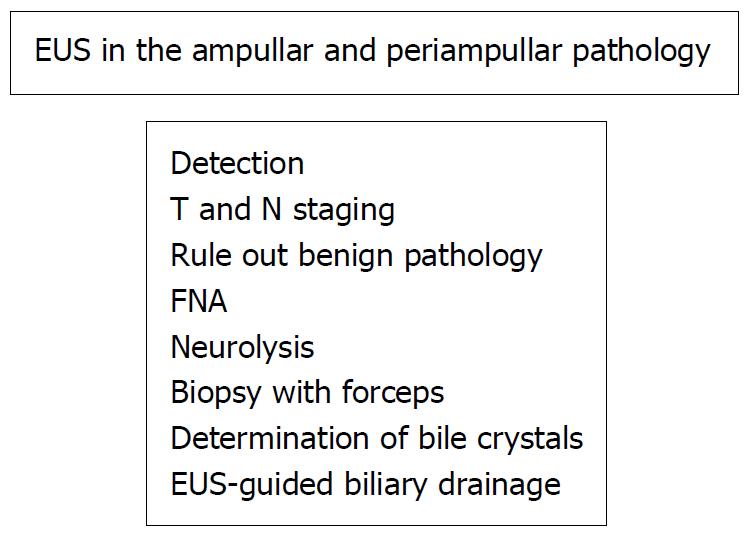
Two particular features make EUS useful in the investigation of ampullary and periampullary pathologies: the first is its capacity to identify small lesions more effectively than other imaging technologies, and the second, the possibility of puncturing these lesions as well as neighboring lymph nodes for cytohistologic confirmation[1]. EUS is considered one of the optimal indications for evaluating the papilla and the periampullary region, although there is no final consensus on this[2,3].
Tumors in the periampullary region arise in the papilla of Vater and the two centimeters surrounding it. Histologically, they could originate in the duodenal wall, pancreatic tissue, the wall of the distal bile duct or the structures of the ampullary complex. The papilla of Vater is formed by the confluence of the pancreatic duct and the bile duct and by the sphincter of Oddi that surrounds it. The sphincter of Oddi also has components for the bile duct and pancreatic duct which are outside the papilla. The primary ampullary tumors originate in the epithelium of the bile duct, the pancreatic duct or the duodenal mucosa[4].
Ampullary and periampullary tumors are infrequent, but have a malignancy rate of more than 90%[4]. Periampullary tumors comprise 5% of malignant gastrointestinal tumors, while ampullary tumors comprise less than 1%[5].
The overall prevalence of resected periampullary cancers show in 50%-70%, cancer of the head of the pancreas, ampullary cancer in 15%-25%, biliary cancer in 10% and duodenal cancer in 10%. The prognosis and survival of patients depends on the tissue of origin and the tumor stage. Survival of these patients is greatest for ampullary and duodenal tumors (4 to 5 years), intermediate for bile duct tumors (3 years) and lowest for pancreatic tumors (less than 1 year)[5-7].
Accurate histological classification is not always possible, even after careful histopathological sample review[5]. All periampullary cancers arise from their respective epithelia and almost all are adenocarcinomas.
Other tumors in the ampullary and periampullary region are basically ampullary villous adenomas or tubulo-villous adenomas, hemangiomas, leiomyomas, leiomyofibromas, lipomas, lymphangiomas and neuroendocrine tumors[4,5].
The benign pathology we must rule out upon examining the ampullary and periampullary region is also diverse: choledocholithiasis or microlithiasis, chronic pancreatitis, dysfunction of the sphincter of Oddi and the presence of alterations in biliopancreatic drainage as a periampullary diverticulum, choledochocele or pancreas divisum.
The clinical manifestations of tumors in this region can appear early on, due to small neoplasias that obstruct either the bile duct or the pancreatic duct or both, and which may be discovered incidentally or as a result of symptoms.
Symptoms may be similar in patients with ampullary or periampullary pathology and these symptoms can be diverse. They may be insidious, as in silent obstructive jaundice or ferropenic anemia. They may manifest as acute pancreatitis, upper digestive hemorrhage or a duodenal obstruction[8].
The most frequent isolated symptoms of ampullary and periampullary neoplasias are obstructive jaundice and clinical or laboratory cholestasis (50% to 80%)[4,8]. Usually there is no pain; rather, certain unspecific symptoms occur, such as nausea, dyspepsia or vague discomfort in the upper hemiabdomen. Obstruction of the bile duct can manifest as pruritus or appear as cholangitis. In ampullary tumors, the jaundice is usually fluctuant, due to the erosion and intermittent permeability of the bile duct. Upon ulceration, upper digestive hemorrhage may present, causing anemia. Tumor markers may be helpful in some cases (CA19-9).
Benign diseases may present a very characteristic symptomatology, such as a choledocholithiasis. However, it is precisely the complex cases, with superimposed clinical symptoms, with unclear or non-conclusive findings in the laboratory or conventional images, which require the contribution of the endosonography.
The clinical manifestations and their diagnosis may be troublesome: an impacted stone may present clinical symptoms of silent obstruction; an ampullary or periampullary tumor can be the cause of acute pancreatitis. In addition, in some patients, lithiasis coexists in an obstructed bile duct, with symptoms in addition to the symptoms of the obstruction.
The principal incidental finding in abdominal ultrasound (US), computerized tomography (CT) or magnetic resonance imaging (MRI) is bile-duct or pancreatic dilatation or dilatation of both ducts, as indirect signs of obstruction. The cause of the obstruction is not always identifiable in these images.
Another frequent incidental finding is that of ampullary growth during an upper digestive tract endoscopy or during evaluation procedures for at-risk patients. The prevalence of ampullary lesions increases 200 to 300 times in patients with familial adenomatous polyposis and also in patients with hereditary nonpolyposis colorectal cancer. These two genetic conditions require follow-up screening, even among young patients.
There are some endoscopic characteristics that could help in the diagnosis of malignant transformation of an ampullary adenoma: induration or rigidity, the presence of ulcerations, lack of elevation after submucosal injection or the presence of a submucosal mass. Some ampullary tumors could grow without invading the mucosa, thus simulating a submucosal tumor with large and convex papillae.
It is important to know the history and clinical evolution of the patient’s symptoms, their laboratory and image data, as well as their personal and family background and associated risk factors, prior to performing EUS. The objective is to understand what is being studied, and the possible procedures derived from the findings. By bearing this background information in mind, we will know what it is we are looking for, and the possibility of finding lesions, especially small ones, will increase. If we are not aware of this information, our findings will be incidental, mistaken or we will only find obvious lesions[9].
The equipment we use to evaluate the ampullary and periampullary region will depend on what we are looking for and the operator’s expertise in radial and/or linear techniques. With both we can obtain images which are uniquely precise and detailed, both of the papilla and of the periampullary region and the opening of the bile duct and pancreatic duct.
The recognized advantages of using radial equipment include the possibility of visualizing the extrahepatic biliary tract in one section, and for the linear equipment, the feasibility of complementing the exam with fine needle aspiration (FNA) for cytohistology.
Probes for performing intraductal sonography (IDUS) are not widely used. In expert hands, IDUS is the most precise method for in-depth evaluation of the severity or “T”, of the ampullary tumors[10-12]. Because of its lack of ultrasound penetration, it is not used to establish the presence of lymph nodes.
The endosonography exam begins with a conventional evaluation of the patient’s pancreas, gallbladder and bile duct. It is useful to know the structure of the pancreatic parenchyma in the body and tail prior to evaluating the head, in order to have a comparison pattern for its echogenicity and thus to identify any focal lesion with greater certainty.
The endoscopic image of the papilla and the periampullary region provides us with a very valuable semiology which could include duodenal compressions or stenosis, the quality of the mucosa, the size and form of the papilla, pore aspect, leakage (or not) of bile, mucous or blood, and the presence or lack of diverticula. Staining and magnification can also help with the diagnosis.
The papilla and the periampullary region are examined from the second portion of the duodenum. Better images are obtained by using duodenal paralysis with Buscopan or Glucagon and by adding water to the duodenum so that the papilla is submerged and free of bubbles. The techniques for radial and linear transducers are similar. Once the papilla is identified by means of endoscopy, we set the small wheel, and free up the large wheel. Our position should be such that as we bring the large wheel toward us, the transducer moves closer to the papilla. We then begin the endoscope withdrawal, maintaining a distance from the papilla so as not to compress it and to be able to identify its various characteristics. Once we are able to see the papilla through endoscopy, we should withdraw the endoscope 1 to 2 cm in order to position the papilla in front of the ultrasound transducer. We can then obtain the best image by making small up-and-down movements with the large wheel and small lateral movements with the shoulders.
The papilla may easily be compressed by the transducer, so it is important to carefully maintain the proper distance in order not to deform it. The lesion may be delicate and could bleed during the exploration.
The vascular and lymph node exam is described further on.
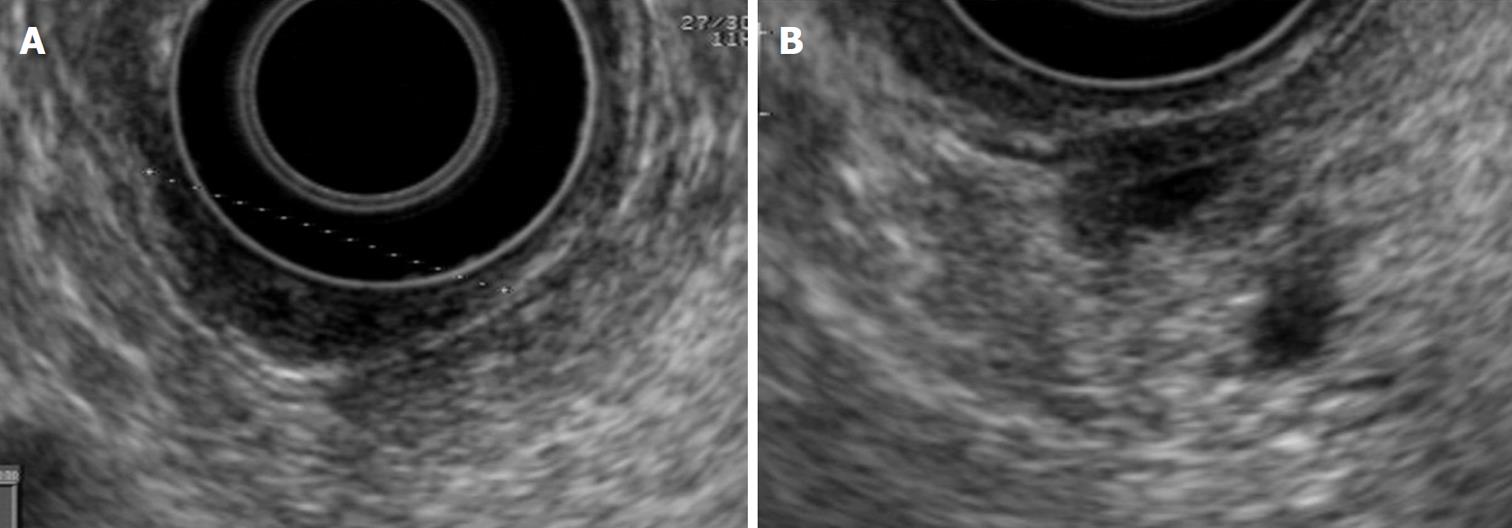
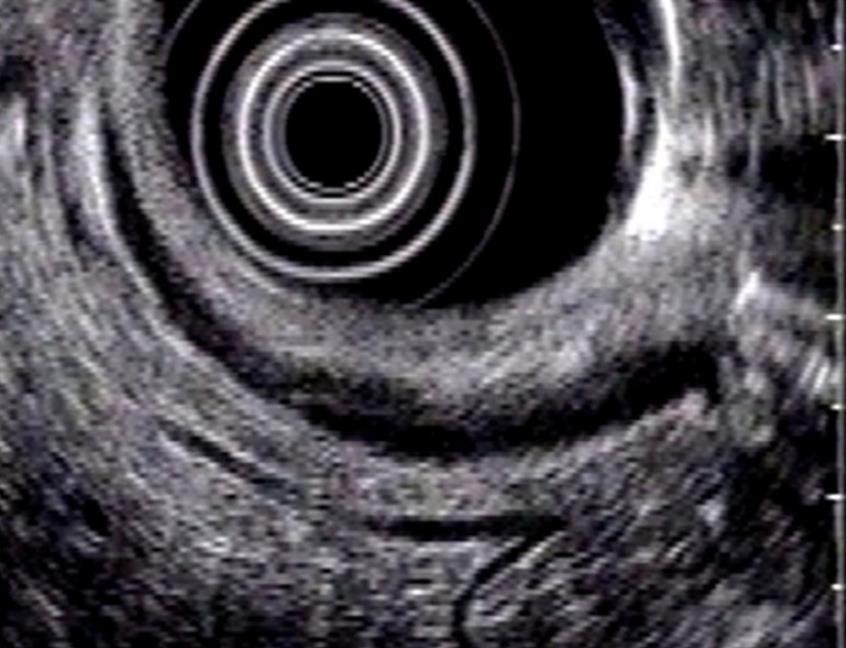
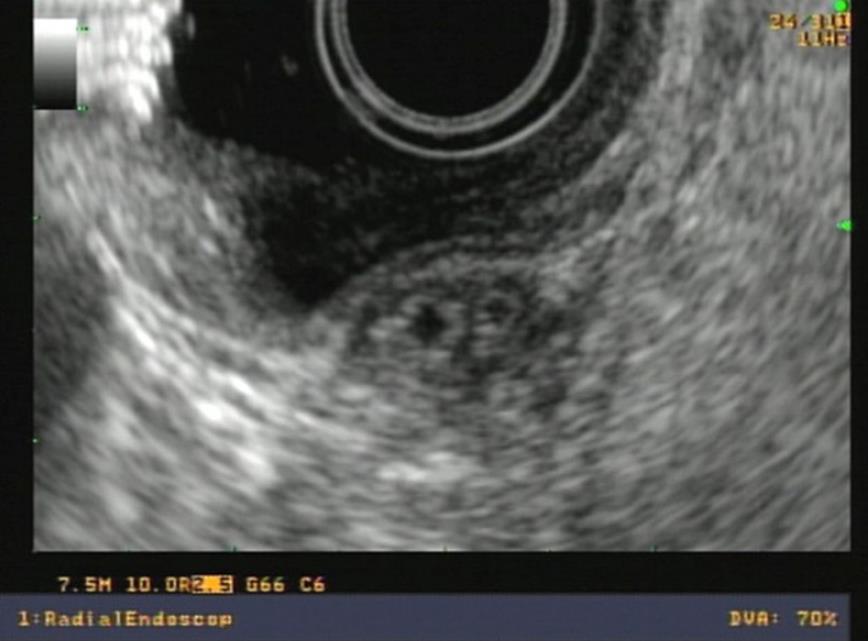
The normal papilla is visualized with radial equipment as a hypoechoic, homogeneous thickening, with a crescent moon shape, well demarcated by the duodenal wall. With the linear equipment, the boundaries of the papilla are less clear. However, the opening and the tract of the bile and pancreatic ducts through the papilla can be better observed than with the radial equipment (Figures 1, 2 and 3).
TNM classification is used for ampullary tumors. For periampullary tumors, the pancreas TNM classification, which is the most frequent etiology, is used. Strictly speaking, the classification that should be used is the one that corresponds to the organ of origin, that is, the extrahepatic bile duct, the pancreas or the duodenum.
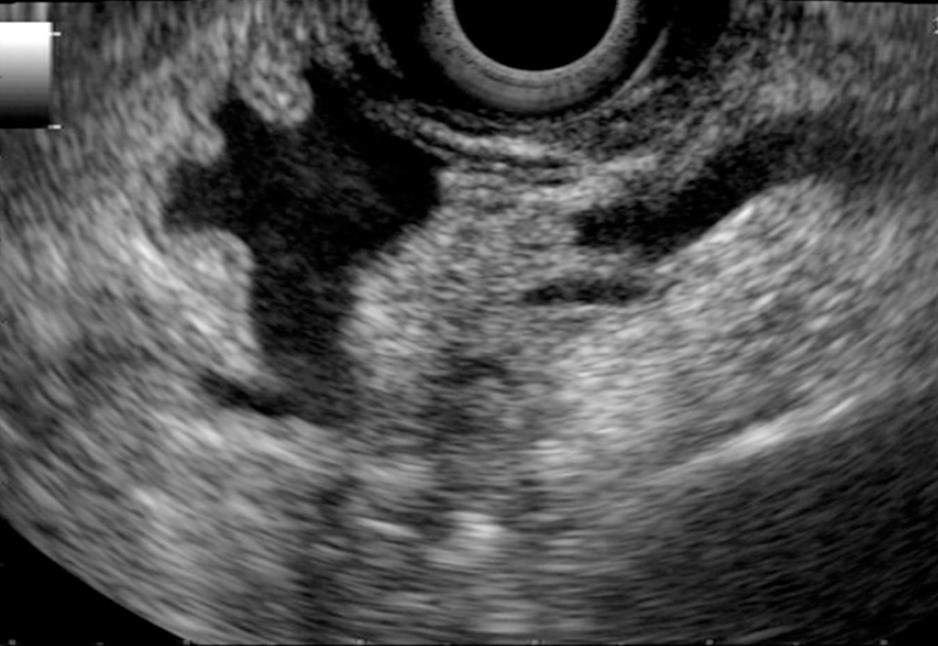
The presence of a stent in the bile duct produces acoustic interference that makes it difficult to interpret the images and diminishes the accuracy of the diagnosis both of T and N by approximately 10%[13-15]. Whenever possible, it is preferable to perform EUS prior to the endoscopic bile duct exploration. Occasionally it is recommended that the endoprosthesis be withdrawn and re-implanted once the EUS exam has been done (Figure 4).
The EUS image in the ampullary tumor varies, depending on whether it is an adenoma or an adenocarcinoma.
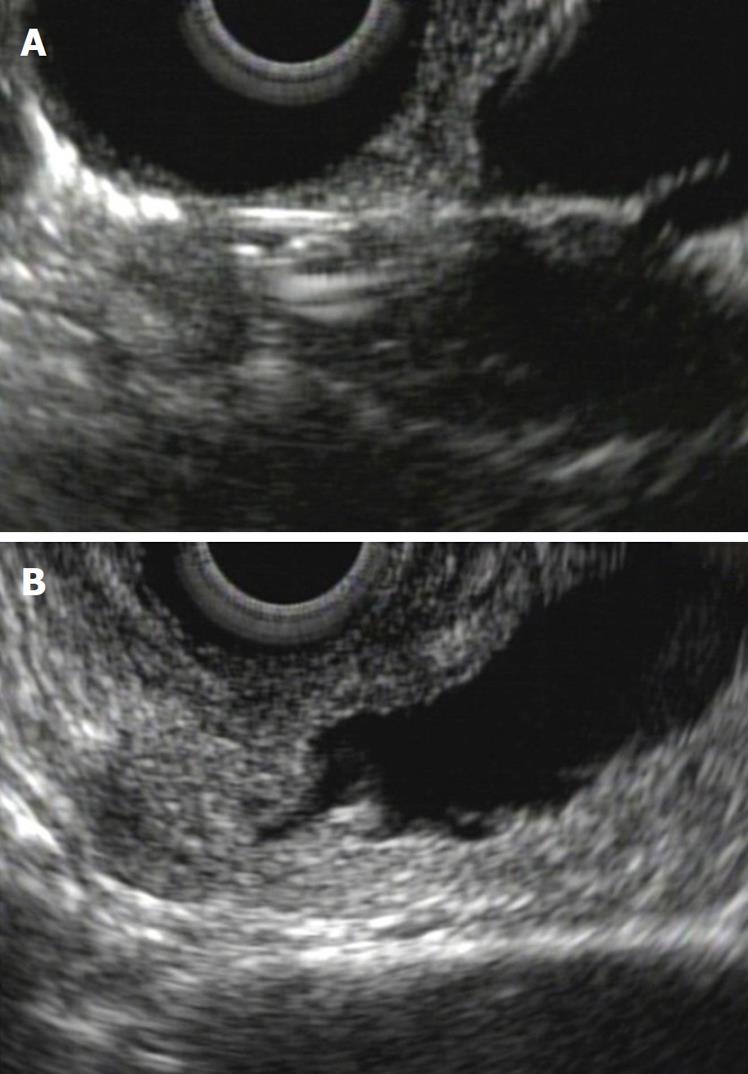
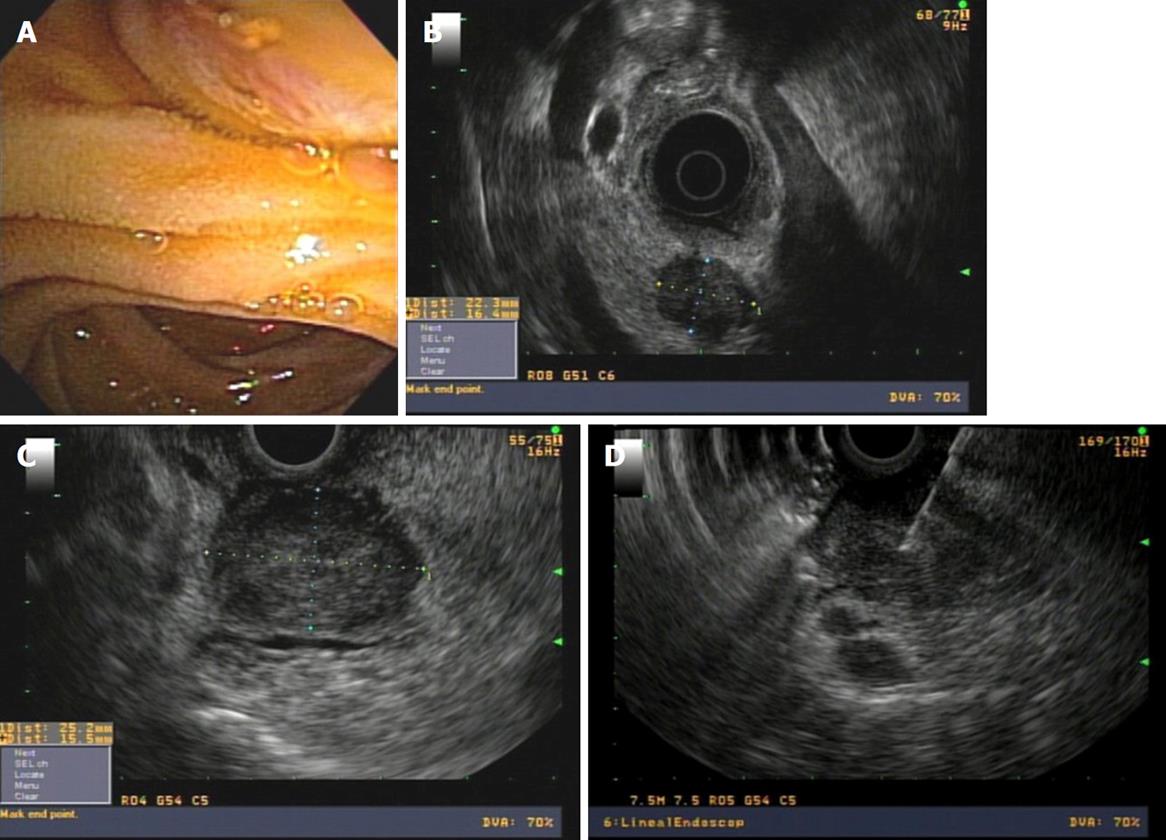
The adenoma is a benign tumor that is visualized as a hypoechoic and homogenous thickening of the papilla, without invasion of the duodenal wall. If the view is optimal, we can recognize harmless submucosal and duodenal muscularis propria layers. The adenoma can grow toward the lumen of the bile duct and/or the pancreatic duct. This information is very valuable for planning therapy, as progress greater than 1 cm toward the ducts excludes the possibility of complete endoscopic resection therapy (Figure 5).
It is difficult for the EUS to identify a focal malignancy within an ampullary adenoma, however, invasive carcinoma can be ruled out, and thus an unnecessary endoscopic procedure can be avoided[4].
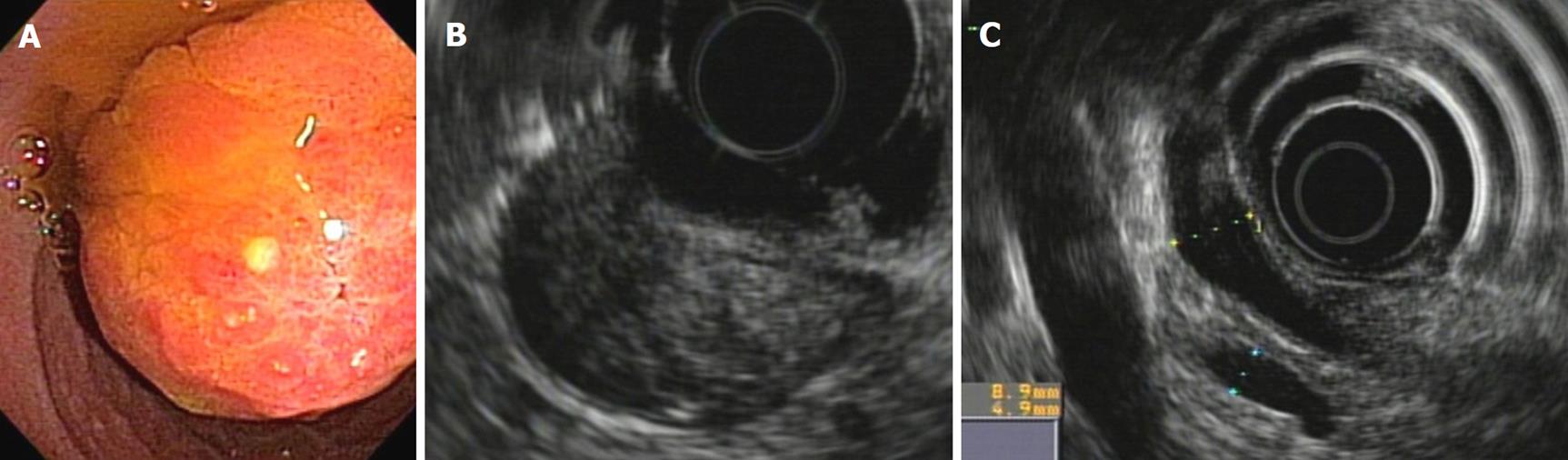
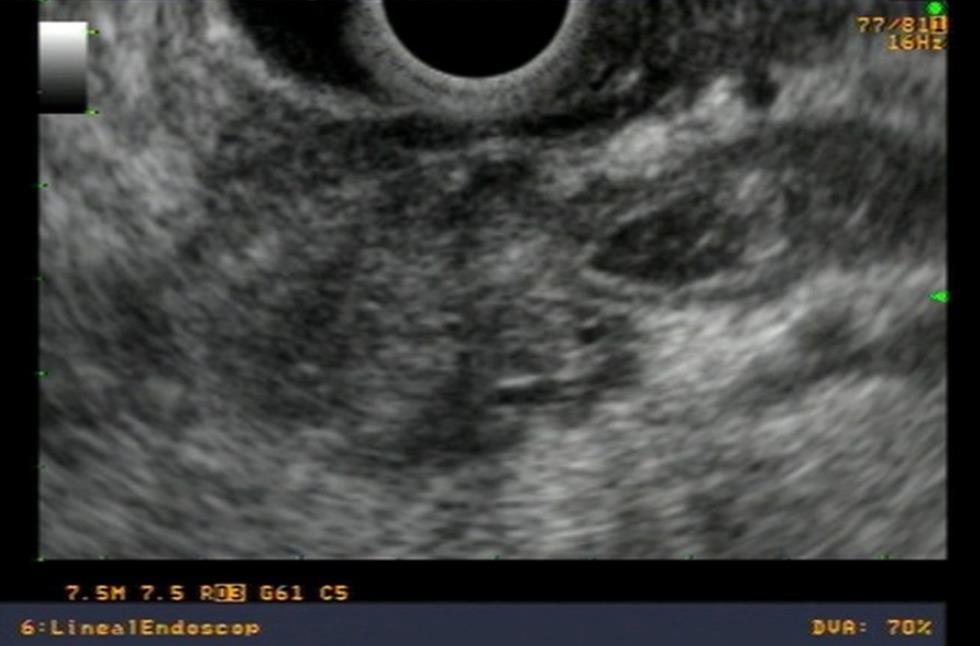
In an ampullary adenocarcinoma, the echogenicity is generally more hypoechoic and heterogeneous. One must define the relationship of this image to the duodenal wall. This relationship is quite subtle, and it is not always possible to distinguish it precisely with conventional endosonography equipment. If there is effacement of the interface between the ampullary tumor and the duodenal wall, this is a T2 in the TNM classification. Invasion of an ampullary carcinoma in the periampullary pancreatic tissue is generally easier to see. If this invasion is less than 2 cm, it is a T3, and if it is greater than 2 cm or invades other structures, it is a T4[16] (Figures 6A and B).
The staging of ampullary and periampullary tumors includes examination of the portal vein and mesenteric vessels. In order to obtain an adequate vascular evaluation, this must be complemented with images from the stomach, bulb, and 2nd, 3rd and 4th duodenal portions[17,18].
The vascular invasion is defined using the following criteria[19]: (1) Presence of venous collaterals around a pancreatic mass that obliterates the usual anatomic location of a portal vessel; (2) Presence of tumor in the vascular lumen; and (3) Abnormal vascular outline due to compression of a vessel by the mass as well as loss of the hyperechoic interface between the vessel and the parenchyma.
These criteria have been standardized for the venous infiltration and not for the arterial infiltration. Whichever one is present, it has a precision rate of 87.5% to predict vascular invasion[20-22]. This is very well evaluated for the portal vein, superior mesenteric vein and splenic vein. The loss of the interface between the vessel and the tumor does not in itself confirm the invasion if it is not accompanied by an anomaly in the vessel outline. Compromise of the superior mesenteric artery and the celiac trunk can usually be better visualized in multislice CT and MRI angiography[19].
Finally, regional and remote lymph nodes are examined, in order to complete the staging. The courses of lymphatic drainage of the papilla and the periampullary region move toward the chains of the posterior and anterior pancreatoduodenal arteries, the hepatic artery and the superior mesenteric artery. The lymph nodes which are furthest away, the splenic ones or those of the celiac trunk, are considered distant metastases[23].
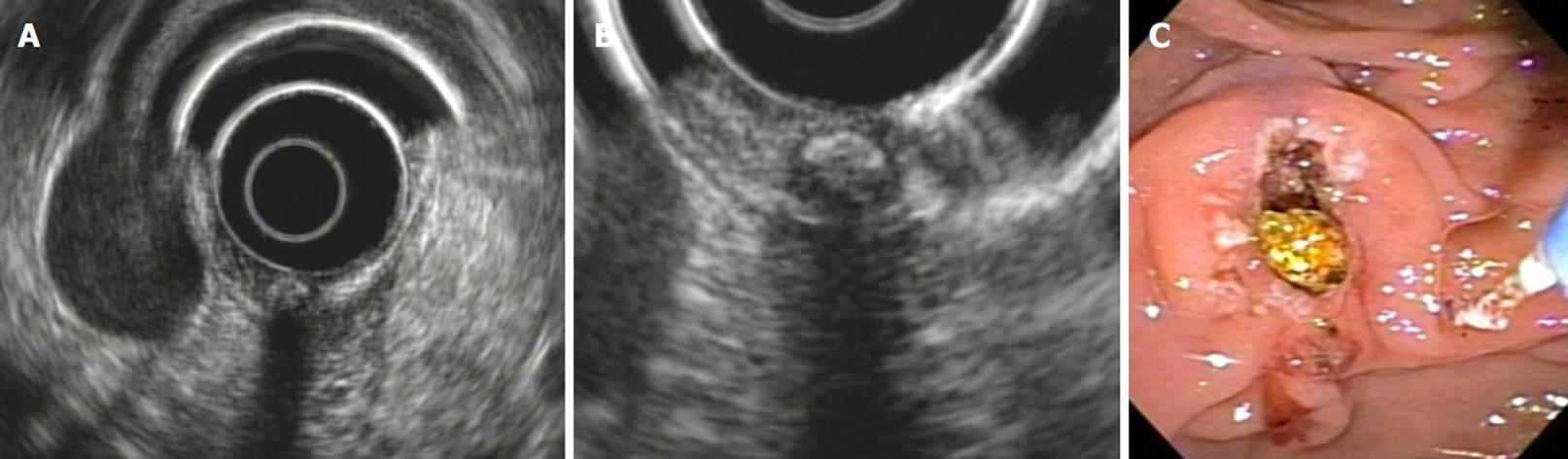
The malignancy “N” criteria for the finding of lymph nodes are common to other neoplastic processes: (1) size greater than 10 mm; (2) round shape; (3) distinct margins; and (4) hypoechoic echogenicity.
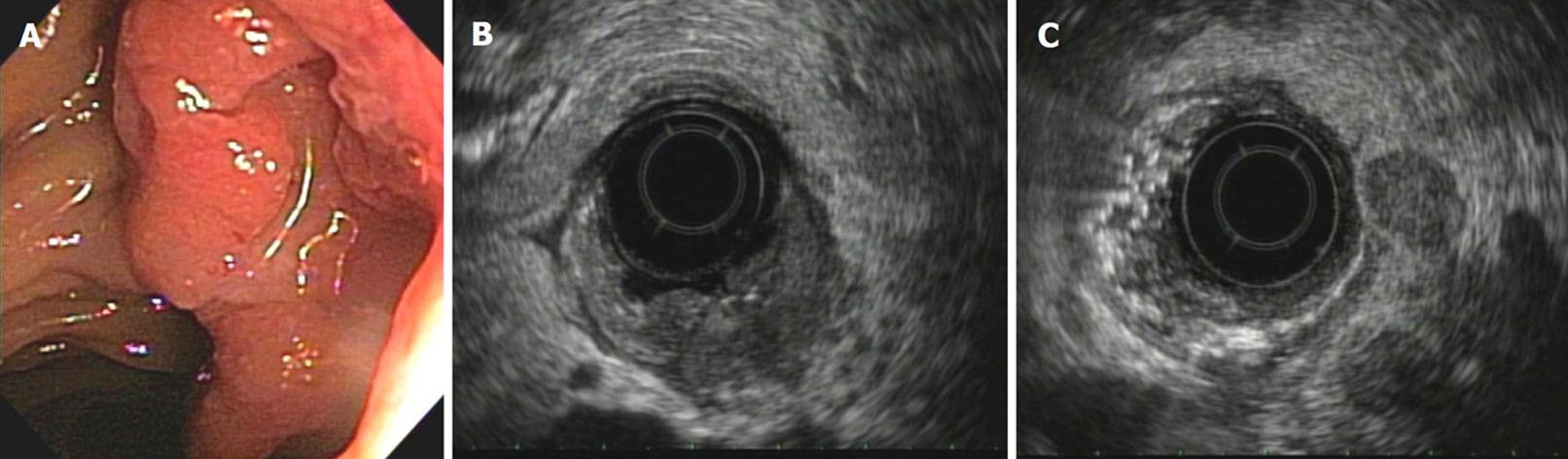
If these 4 criteria are present in a lymph node, there is a positive predictive value of 100% for malignancy. However, only 25% of the infiltrated lymph nodes have these 4 characteristics. The presence of only one of these findings can predict neoplastic infiltration. Up to two of these criteria may be found in normal lymph nodes. The FNA enables this to be confirmed[24] (Figure 6C).
We have a variety of images and procedures that can be used in evaluating an ampullary or periampullary tumor: US, CT, Magnetic Resonance Cholangiopancreatography (MRCP), Positron Emission Tomography (PET), EUS, IDUS, Endoscopic Retrograde Cholangiopancreatography (ERCP), Percutaneous Transhepatic Cholangiography (THC), laparoscopy or exploratory laparoscopy. Each method has an advantage over the others and all of them are constantly undergoing development. Each one requires special skills in order to acquire and interpret the images, or to carry out the procedures[25]. The results of each exam in detection, and accurate TNM staging of ampullary and periampullary lesions depends on the technology and the operator’s skills, which makes it difficult to compare studies[26,27]. Staging accuracy is highest for IDUS, followed by EUS, MRI, CT and finally abdominal US[10]. Each institution should adapt the study algorithm, depending on the availability, access, reliability, cost-benefit analysis and patient invasion[28].
Abdominal US is widely used and can evaluate the intra- and extrahepatic bile duct and the presence of lithiasis and vesicular pathology. It is useful for initiating the evaluation of patients with obstructive jaundice. It is not adequate for examining the distal bile duct, the papilla or the head of the pancreas, due to frequent interposition of intestinal air.
CT is an exam that is widely available and which provides a very complete representation of the pathology of the pancreas. It is valuable in detecting neoplasias and also distant metastasis, above all in relation to CT/PET. Vascular compromise, especially arterial, is well defined by the multisection spiral CT[29,30]. MRI enables the ruling out of the presence of choledocholithiasis. It provides guidance toward the etiology of periampullary tumors, with detection of lesions or with indirect signs in the MRCP. The compromise of the venous system is also adequately defined by MRI[31,32].
ERCP is not used for diagnosis, but to decompress the bile duct through an endoprosthesis in the event of cholangitis or in palliative therapy. It is also indicated for taking a biopsy directly from the papilla or the ducts[33]. THC is indicated for complementing therapeutic procedures, often in combination with endoscopic drainage techniques, ERCP or therapeutic enteroscopy. Bile duct drainage guided by EUS from the duodenum or the stomach is an endoscopic procedure that is increasingly used as an alternative to percutaneous procedures[34,35].
It would seem reasonable to combine the technologies to arrive at a better diagnosis.
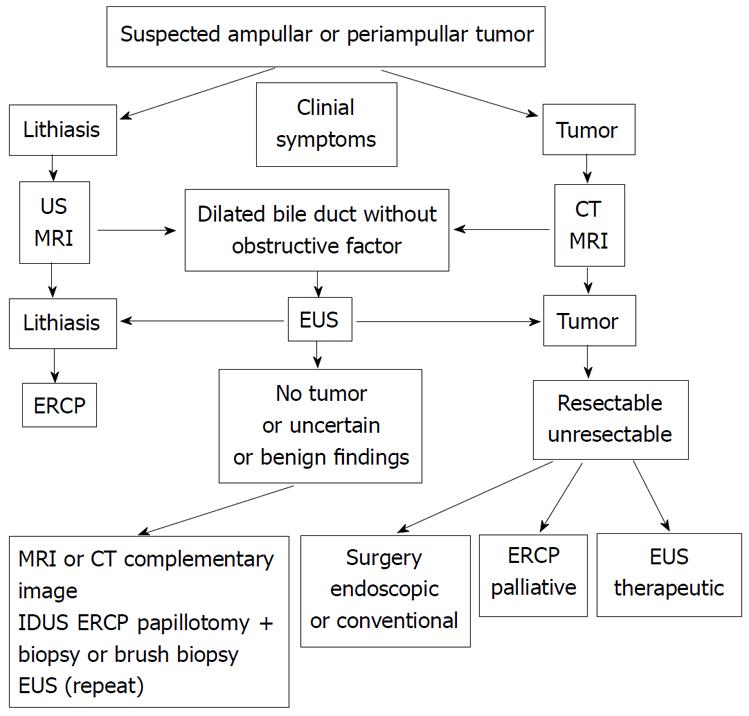
In Figure 7, an algorithm for a suspected ampullary or periampullary tumor is proposed. This procedural algorithm summarizes the most common clinical situations.
The most frequent cause of suspicion is obstructive jaundice, as a clinical or laboratory finding. The other causes correspond to the discovery of bile duct dilatation in some images, with or without dilatation of the pancreatic duct, and also the endoscopic discovery of an ampullary growth.
Patients with suspected lithiasic etiology are usually studied first with an abdominal US and/or an MRI. In those where there is a suspected tumor etiology, often the study will begin with a CT and/or an MRI.
EUS plays an important role (Figure 8) in those situations in which the findings from conventional images, US, CT or MRI (and even ERCP) are not concordant with the clinical symptoms, or are not sufficient to confirm or rule out the presence of a tumor[36].
EUS in tumoral pathology: The principal role of EUS in a suspected periampullary tumor is detection, and if there is indeed a tumor, in the staging. In the ampullary tumor, the diagnosis is done through endoscopy, and EUS is indicated for staging and to evaluate its endoscopic or surgical resectability[4,37]. The depth of the tumor “T” compromise, the presence of lymph nodes “N” and vascular compromise is evaluated. The FNA of the papilla, the periampullary region, or regional lymph nodes for cytology, is occasionally required to confirm the findings or to plan a neoadjuvant or palliative therapy (Figures 9 and 10).
The indication for endoscopic ampullectomy with curative option requires that the EUS exclude invasion of the duodenal muscularis propria layer and that there is also no tumor growth beyond 1 cm inside the bile duct or pancreatic duct. The outcome of the final procedure depends on the histological evaluation of the tissue sample: free lateral and in-depth margins and the absence of lymphovascular compromise are required. The histology should correspond to benign adenomas, in situ tumors (Tis) or early well or moderately differentiated type cancer T1N0M0.
If one of these requisites is not fulfilled, it is considered an inadequate endoscopic resection and surgery should then be considered, with or without adjuvant therapy[38,39].
Surgical resectability of an ampullary or periampullary tumor is a topic of debate on which there is no consensus. The most important prognosis factors are the tissue of origin and the presence of compromised lymph nodes. Vascular invasion is not an unresectability factor for all surgical teams; however, it has been demonstrated that survival does not improve with more radical surgery[40].
Correct staging will permit comparative studies that help to ensure rational procedures. The combination of EUS and CT currently allow for improved evaluation of local and distant staging[19].
In cases of advanced tumor pathology with a palliative therapy indication, the EUS can be used as a guide to obtain access to the bile duct from the duodenum, or to the intrahepatic bile duct from the stomach. In expert hands, it is not very invasive and yields good results. However, there are only case series reports, and the most frequent complication is bile leakage and migration of the prosthesis. It should be reserved for highly specialized centers that manage biliopancreatic patients, with the requirement that access failed or was not possible through ERCP[34,35].
Another objective for the EUS is monitoring the response to neoadjuvant radiochemotherapy, where it can have a positive influence through improved outcomes for patients with locally invasive pancreatic adenocarcinoma[19].
In the case of pain management, neurolysis of the celiac plexus can be performed through EUS[41]. The efficacy and risk of hypotension, increased pain and diarrhea are all transient and similar to those of other access routes. The risk of neurological damage diminishes in procedures that address the celiac plexus through the anterior route and severe complications have not been reported for EUS[42].
EUS in benign pathology: EUS provides endoscopic information, with the visualization of the papilla and its surroundings, and the eventual performance of a biopsy with forceps. A half-open pore can be indicative of the migration of a stone. The intradiverticular location of a papilla can be the only cause that explains a bile duct dilatation.
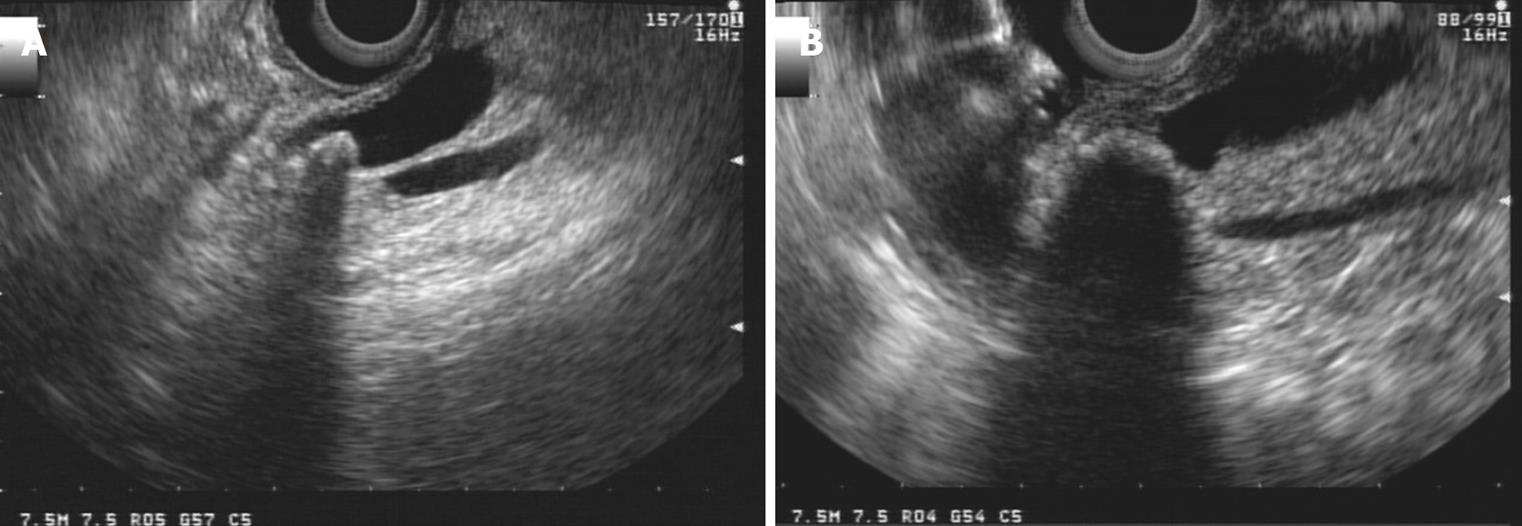
The presence of lithiasis will guide the procedure toward a therapeutic ERCP. It is a simple diagnosis for EUS with a better outcome than MRI when the bile duct is not dilated, when the stones are smaller than 3 mm, or when the stones are impacted[43] (Figures 11 and 12). We should bear in mind the association of lithiasis in obstructed bile ducts, which can be as high as 25%.
EUS is of great value in differential diagnosis with other benign pathologies in addition to lithiasis, such as chronic pancreatitis, the presence of a periampullary diverticulum, choledochocele or pancreas divisum.
In the diagnosis of chronic pancreatitis, EUS together with MRI achieves an accuracy close to 100%. FNA is useful, in these cases, for differentiating neoplasias from inflammatory pseudotumors[44,45].
Perhaps the most challenging aspect of evaluation by endosonography is confirming that there is no obstructive tumor lesion or that there are only benign findings (Figure 13). In these cases it is essential to look for agreement between the clinical symptoms, the radiological images, the laboratory and endoscopic findings and the EUS[46].
We already emphasized the importance of having access to a complete clinical history to guide our search. The value we give to our findings is related to our own clinical assessment.
In the event that suspicion of the presence of a neoplasia persists, there are different actions we can take, which will depend on the clinical suspicion: repeat the EUS with another, more experienced operator[47], repeat or perform another complementary image: MRI or CT, perform an ERCP with a papillotomy and biopsy or brush biopsy for cytology, depending on the morphology of the papilla. Another option in cases where there is doubt is to repeat the EUS in 2 mo.
We should keep in mind the multicenter study by Bhutani et al[48], which sets forth that a recent acute pancreatitis (prior to 4 wk), changes in a chronic pancreatitis, a prominent ventral/dorsal fissure, and the diffuse infiltrative carcinoma of the pancreas are all conditions that can lead endosonography experts to err in their interpretation and fail to detect a neoplasia through EUS. Repetition of the EUS within two months enabled them to identify the lesions that had not previously been seen[48].
Recourse to surgical exploration is always present and is indicated in selected circumstances.
Ampullary and periampullary pathology is rich and diverse, more frequently tumoral and among these, malignant. Although there is not a higher prevalence, its clinical presentation tends to be early, enabling the initiation of curative therapies. This requires a previous study that addresses questions of resectability and the best way to achieve it.
Clinical presentation and radiological images are shared for ampullary and periampullary pathology, as are also surgical treatment and palliative treatment. The greatest differences are in the endoscopic therapy which is possible in some ampullary tumors, and in the long-term prognosis. The prognosis is better for duodenal or ampullary tumors than for those of bile duct or pancreatic origin.
Several images contribute to diagnosis and evaluation, in order to determine the most appropriate therapeutic procedure.
We present a study algorithm that includes EUS as an important diagnostic imaging tool, supported by FNA and also as a less invasive therapeutic alternative for pain management and bile duct drainage.
The best available study and treatment options should be offered to patients with pathology of the papilla or periampullary region, taking into consideration each individual situation and also that of each institution.
Peer reviewer: Viktor E Eysselein, MD, Professor of Medicine, Division of Gastroenterology, Harbor-UCLA Medical Center, 1000 W. Carson Street, Box 483, Torrance, CA 90509, United States
| 1. | Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, Davila RE, Dominitz JA, Harrison ME 3rd, Ikenberry SO. Role of EUS. Gastrointest Endosc. 2007;66:425-434. [Cited in This Article: ] |
| 2. | Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849-854. [Cited in This Article: ] |
| 3. | Trede M, Richter A, Wendl K. Personal observations, opinions, and approaches to cancer of the pancreas and the periampullary area. Surg Clin North Am. 2001;81:595-610. [Cited in This Article: ] |
| 4. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598. [Cited in This Article: ] |
| 5. | Sarmiento JM, Nagomey DM, Sarr MG, Farnell MB. Periampullary cancers: are there differences? Surg Clin North Am. 2001;81:543-555. [Cited in This Article: ] |
| 6. | O’Connell JB, Maggard MA, Manunga J Jr, Tomlinson JS, Reber HA, Ko CY, Hines OJ. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820-1817. [Cited in This Article: ] |
| 7. | Burgos L. Cholangiocarcinoma. Rev Med Chil. 2008;136:240-248. [Cited in This Article: ] |
| 8. | Binmoeller , KF , Boaventura , S , Ramsperger , K , Soehendra , N . Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127. [Cited in This Article: ] |
| 9. | Rösch T, Dittler H, Strobel K. Endoscopic Ultrasound Criteria for Vascular Invasion in the Staging of Cancer of the Head of the Pancreas: a Blind Reevaluation of Videotapes. Gastrointest Endosc. 2000;52:469-477. [Cited in This Article: ] |
| 10. | DeWitt J. EUS in Pancreatic Neoplasms. In Hawes R, Fockens P, Editors. Endosonography. Philadelphia: Saunders Elsevier; 2006; . [Cited in This Article: ] |
| 11. | Vazquez-Sequeiros E, Baron TH, Clain JE, Gostout CJ, Norton ID, Petersen BT, Levy MJ, Jondal ML, Wiersema MJ. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372. [Cited in This Article: ] |
| 12. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [Cited in This Article: ] |
| 13. | Fusaroli P, Manta R, Fedeli P, Maltoni S, Grillo A, Giovannini E, Bucchi L, Caletti G. The influence of endoscopic biliary stents on the accuracy of endoscopic ultrasound for pancreatic head cancer staging. Endoscopy. 2007;39:813-817. [Cited in This Article: ] |
| 14. | Chen CH, Tseng LJ, Yang CC, Yeh YH. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. J Clin Ultrasound. 2001;29:313-321. [Cited in This Article: ] |
| 15. | Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33. [Cited in This Article: ] |
| 16. | International Union Against Cancer’s TNM. In Classification of Malignant Tumours. Sixth edition. Wiley-Liss, 2002, American Joint Committee on Cancer. AJCC Cancer Staging Manual. Sixth edition. Springer;. Philadelphia: Saunders Elsevier; 2002; . [Cited in This Article: ] |
| 17. | Brugge WR. Pancreatic cancer staging. Endoscopic ultrasonography criteria for vascular invasion. Gastrointest Endosc Clin N Am. 1995;5:741-753. [Cited in This Article: ] |
| 18. | Fritscher-Ravens A, Knoefel WT, Krause C, Swain CP, Brandt L, Patel K. Three-dimensional linear endoscopic ultrasound-feasibility of a novel technique applied for the detection of vessel involvement of pancreatic masses. Am J Gastroenterol. 2005;100:1296-1302. [Cited in This Article: ] |
| 19. | Snady H. EUS criteria for vascular invasion: analyzing the meta-analysis. Gastrointest Endosc. 2007;65:798-807. [Cited in This Article: ] |
| 20. | Buscail L, Pagès P, Berthélemy P, Fourtanier G, Frexinos J, Escourrou J. Role of EUS in the management of pancreatic and ampullary carcinoma: a prospective study assessing resectability and prognosis. Gastrointest Endosc. 1999;50:34-40. [Cited in This Article: ] |
| 21. | Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788-797. [Cited in This Article: ] |
| 22. | Brugge WR, Lee MJ, Kelsey PB, Schapiro RH, Warshaw AL. The use of EUS to diagnose malignant portal venous system invasion by pancreatic cancer. Gastrointest Endosc. 1996;43:561-567. [Cited in This Article: ] |
| 23. | Hurtuk MG, Hughes C, Shoup M, Aranha GV. Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg. 2009;197:348-352. [Cited in This Article: ] |
| 24. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [Cited in This Article: ] |
| 25. | Maluf-Filho F, Sakai P, Cunha JE, Garrido T, Rocha M, Machado MC, Ishioka S. Radial endoscopic ultrasound and spiral computed tomography in the diagnosis and staging of periampullary tumors. Pancreatology. 2004;4:122-128. [Cited in This Article: ] |
| 26. | Ho JM, Eysselein VE, Stabile BE. The value of endoscopic ultrasonography in predicting resectability and margins of resection for periampullary tumors. Am Surg. 2008;74:1026-1029. [Cited in This Article: ] |
| 27. | Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18-25. [Cited in This Article: ] |
| 28. | Schwarz M, Pauls S, Sokiranski R, Brambs HJ, Glasbrenner B, Adler G, Diederichs CG, Reske SN, Möller P, Beger HG. Is a preoperative multidiagnostic approach to predict surgical resectability of periampullary tumors still effective? Am J Surg. 2001;182:243-249. [Cited in This Article: ] |
| 29. | Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717-725; quiz 664. [Cited in This Article: ] |
| 30. | Mansfield SD, Scott J, Oppong K, Richardson DL, Sen G, Jaques BC, Manas DM, Charnley RM. Comparison of multislice computed tomography and endoscopic ultrasonography with operative and histological findings in suspected pancreatic and periampullary malignancy. Br J Surg. 2008;95:1512-1520. [Cited in This Article: ] |
| 31. | Kan SJ, Suyama M, Kubokawa Y. Early Detection of Extrahepatic Bile-Duct Carcinomas in the Non Icteric Stage by Using MRCP Followed by EUS. Gastrointest Endosc. 2009;70:29-36. [Cited in This Article: ] |
| 32. | Kim JH, Kim MJ, Chung JJ, Lee WJ, Yoo HS, Lee JT. Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics. 2002;22:1335-1352. [Cited in This Article: ] |
| 33. | Escalante-Glorsky S, Raijman I, Angulo P. Endoscopic Methods for the Diagnosis of Pancreaticobiliary Neoplasms. 2008;in press. [Cited in This Article: ] |
| 34. | Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-S7. [Cited in This Article: ] |
| 35. | Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video). Gastrointest Endosc. 2009;69:S8-S12. [Cited in This Article: ] |
| 36. | Will U, Bosseckert H, Meyer F. Correlation of endoscopic ultrasonography (EUS) for differential diagnostics between inflammatory and neoplastic lesions of the papilla of Vater and the peripapillary region with results of histologic investigation. Ultraschall Med. 2008;29:275-280. [Cited in This Article: ] |
| 37. | Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Cummings O, Kopecky K, Sherman S, Wiersema M, Lehman GA. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50:786. [Cited in This Article: ] |
| 38. | Seewald S, Omar S, Soehendra N. Endoscopic resection of tumors of the ampulla of Vater: how far up and how deep down can we go? Gastrointest Endosc. 2006;63:789-791. [Cited in This Article: ] |
| 39. | Baillie J. Endoscopic ampullectomy. Am J Gastroenterol. 2005;100:2379-2381. [Cited in This Article: ] |
| 40. | Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355-366;. discussion 366-368. [Cited in This Article: ] |
| 41. | Brugge WR, Van Dam J. Pancreatic and biliary endoscopy. N Engl J Med. 1999;341:1808-1816. [Cited in This Article: ] |
| 42. | Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923-930. [Cited in This Article: ] |
| 43. | Kondo S, Isayama H, Akahane M, Toda N, Sasahira N, Nakai Y, Yamamoto N, Hirano K, Komatsu Y, Tada M. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol. 2005;54:271-275. [Cited in This Article: ] |
| 44. | Pungpapong S, Wallace MB, Woodward TA, Noh KW, Raimondo M. Accuracy of endoscopic ultrasonography and magnetic resonance cholangiopancreatography for the diagnosis of chronic pancreatitis: a prospective comparison study. J Clin Gastroenterol. 2007;41:88-93. [Cited in This Article: ] |
| 45. | Pungpapong S, Noh KW, Woodward TA, Wallace MB, Al-Haddad M, Raimondo M. Endoscopic ultrasound and IL-8 in pancreatic juice to diagnose chronic pancreatitis. Pancreatology. 2007;7:491-496. [Cited in This Article: ] |
| 46. | Malik S, Kaushik N, Khalid A, Bauer K, Brody D, Slivka A, McGrath K. EUS yield in evaluating biliary dilatation in patients with normal serum liver enzymes. Dig Dis Sci. 2007;52:508-512. [Cited in This Article: ] |
| 47. | DeWitt J, McGreevy K, Sherman S, LeBlanc J. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610-619. [Cited in This Article: ] |