Published online Mar 15, 2016. doi: 10.4251/wjgo.v8.i3.305
Peer-review started: June 19, 2015
First decision: October 21, 2015
Revised: November 14, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: March 15, 2016
AIM: To investigate the dynamic expression of p-signal transducer and activator of transcription 3 (STAT3) and vascular endothelial growth factor (VEGF) in the formation of gastric tumors induced by drinking water containing N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) in Wistar rats.
METHODS: One hundred and twenty Wistar rats were randomly divided into two groups (60 in each group): Control group and Model group. The rats in each group were then randomly divided into three groups (20 in each group): C/M15, C/M25 and C/M40 (15, 25 and 40 represent the number of feeding weeks from termination). Rats in the control group received normal drinking water and rats in the model group received drinking water containing 100 μg/mL MNNG. Stomach tissues were collected at the end of the 15th, 25th and 40th week, respectively, for microscopic measurement using hematoxylin and eosin staining. The expression of p-STAT3 and VEGF in different pathological types of gastric tissue, including normal, inflammation, atrophy, hyperplasia and gastric stromal tumor, was observed by immunohistochemistry and Western blot, and the corelation between p-STAT3 and VEGF was analyzed.
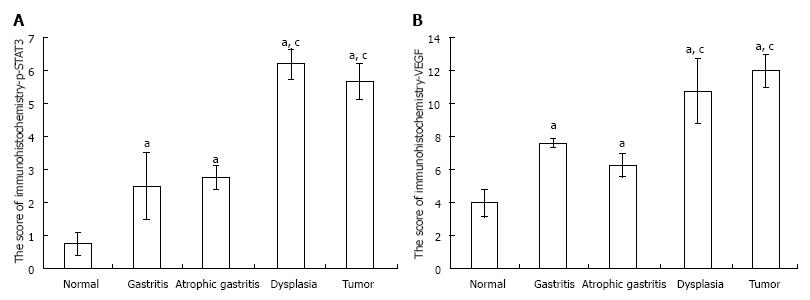
RESULTS: (1) The expression of p-STAT3 in tissue with gastritis, atrophy, dysplasia and gastric stromal tumor were significantly increased in the model group compared with the control group (2.5 ± 1.0, 2.75 ± 0.36, 6.2 ± 0.45, 5.67 ± 0.55 vs 0.75 ± 0.36, P = 0.026, 0.035, 0.001, 0.002, respectively); the expression of p-STAT3 in tissue with dysplasia was higher than that in samples with gastritis or atrophy (6.2 ± 0.45 vs 2.5 ± 1.0, P = 0.006; 6.2 ± 0.45 vs 2.75 ± 0.36, P = 0.005, respectively); however, the expression of p-STAT3 in gastritis and atrophy was not significantly different (P > 0.05); (2) the expression of VEGF in tissue with gastritis, atrophy, dysplasia and gastric stromal tumor was significantly increased in the model group compared with normal gastric mucosa; and the expression of VEGF in tissue with dysplasia was higher than that in tissue with inflammation and atrophy (10.8 ± 1.96 vs 7.62 ± 0.25, P = 0.029; 10.8 ± 1.96 vs 6.26 ± 0.76, P = 0.033, respectively); similarly, the expression of VEGF in tissue with gastritis and atrophy was not significantly different (P > 0.05); and (3) the expression of VEGF was positively correlated with p-STAT3.
CONCLUSION: p-STAT3 plays an important role in gastric cancer formation by regulating the expression of VEGF to promote the progression of gastric tumor from gastritis.
Core tip: The results show that signal transducer and activator of transcription 3 (STAT3) is partially responsible for the progression from chronic gastritis to gastric carcinoma induced by N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) and is significantly related to the expression of vascular endothelial growth factor (VEGF) during this process. It is considered that STAT3 induces an abnormal level of VEGF expression to promote the formation of gastric carcinoma. To the best of our knowledge, this is the first report to show that p-STAT3 is persistently activated during the progression of chronic gastritis to gastritis carcinoma induced by the administration of MNNG in rats, and was positively associated with the expression of VEGF.
- Citation: Wang XY, Wang LL, Zheng X, Meng LN, Lyu B, Jin HF. Expression of p-STAT3 and vascular endothelial growth factor in MNNG-induced precancerous lesions and gastric tumors in rats. World J Gastrointest Oncol 2016; 8(3): 305-313
- URL: https://www.wjgnet.com/1948-5204/full/v8/i3/305.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i3.305
Gastric cancer is the second leading cause of cancer-related death worldwide, its prevalence rate and mortality are very high, especially in some developing countries, and the high incidence of gastric cancer is a major public health problem worldwide[1]. The formation and development of gastric cancer are complex and are associated with a multifactorial etiology[2]. Helicobacter pylori, high-salt diet, smoking, obesity and Epstein-Barr virus are factors which increase gastric cancer risk[3-7]. In addition, environmental factors significantly contribute to the etiology of cancer[2]. N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) is commonly used to induce gastric tumors in basic research[8,9], therefore we chose MNNG to induce gastric precancerous leisions and gastric tumors in this study. Consistent with other tumors, the pathogenesis of gastric tumors is still unclear.
Signal transducer and activator of transcription 3 (STAT3), a key transcription factor[10] was shown to play a vital role in human gastric cancer angiogenesis[11]. It was reported that activation of STAT3 and the microbial environment were important for gastric tumor initiation and development in the gp130 (757F/F) mouse[11], and it is believed by some investigators that STAT is directly involved in gastric tumorigenesis[12].
Vascular endothelial growth factor (VEGF) plays a very critical role in cancer metastasis due to its function in the generation of vascular tissue[13,14]. Recently, VEGF was reported to be closely associated with gastric cancer, which may provide additional prognostic information for the preoperative evaluation of gastric cancer invasion and tumor type[15], thus anti-VEGF may be a targeted therapy for the treatment of gastric cancer[16]. Several researchers demonstrated that the expression of STAT3 was potentially associated with the expression of VEGF-C in various malignant diseases[17,18], and Judd et al[11] reported that constitutive STAT3 activation promoted VEGF expression and stimulated tumor angiogenesis. However, to our knowledge, the roles of STAT3 and VEGF in the formation of gastric precancerous lesions and gastric cancer induced by MNNG are still unknown.
The aim of this study was to investigate the dynamic expression of p-STAT3 and VEGF as well as the relationship between them in the formation of gastric tumors from gastritis induced by MNNG in male Wistar rats.
One hundred and twenty male Sprague-Dawley (SD) rats aged 4 wk and weighing 125-150 g were provided by the Animal Center of Zhejiang Chinese Medical University. The animals were housed in groups of five per cage under controlled illumination (12:12 h light/dark cycle, lights on/off: 6 h/18 h), humidity (60%) and temperature (22 °C ± 2 °C) for one week to acclimatize to the environment. The animals were then randomly divided into two groups (60 in each group): Control group (the rats were able to eat regular food and drinking water) and the model group (the rats were able to eat regular food and drinking water containing 100 μg/mL MNNG); each group was then randomly divided into three groups (20 rats in each group): C/M15, C/M25 and C/M40 (15, 25 and 40 represent the number of feeding weeks). The Animal Care and Use Committee of the Zhejiang Chinese Medical University approved the protocol.
Rats were fed normal food and drinking water or drinking water with MNNG. Rats in C/M15 were sacrified at the end of the 15th week in both the control group (C15) and the model group (M15) and the gastric tissue was collected immediately and stored at -80 °C or was perfused with cold PBS on ice and then placed in a tube with 4% paraformaldehyde to obtain various measurements as follows. Similarly, rats in C/M25 or C/M40 were sacrified at the end of the 25th week or 40th week and gastric tissue was collected. The experimental protocol is described in Figure 1.
The rats were killed at the end of the 15th week, 25th week and 40th week respectively. The stomachs were separated immediately and were opened along the long axis on ice to record the following conditions: Edema, hyperemia, erosion, ulcer or mass, and photographs were taken by two pathologists who were unaware of the treatment groups. The stomach tissue was then divided into two parts, one was stored at -80 °C and the other was placed in tubes with 4% paraformaldehyde (PFA).
Gastric tissue was perfused with PBS followed by 4% PFA in 0.1 mol/L phosphate buffer and cryoprotected in 30% sucrose in PBS overnight at 4 °C. Samples were embedded in paraffin wax, and 5 μm specimens were stained with hematoxylin and eosin. The results of histology were divided into four types according to the following histological features: Chronic superficial gastritis, chronic atrophic gastritis, dysplasia and tumor.
Gastritis was defined as inflammation of the stomach lining associated with mucosal injury. Atrophic gastritis was characterized by chronic inflammatory processes of gastric mucosa leading to the loss of appropriate glands[19] and gastric dysplasia was diagnosed according to the classification of gastrointestinal tract epithelial neoplasia by the World Congress of Gastroenterology in Vienna in 1998[20]; gastric tumor was diagnosed by the presence of tumor cells.
The expression of p-STAT3 and VEGF in gastric mucosa in the different groups was evaluated using immunohistochemistry. Specimens were cut into 1-cm blocks transversely oriented to the hippocampal long axis. Blocks were placed in buffered PFA (Sigma, St Louis, MO, United States). After 48 h, the specimens were paraffin-embedded for immunohistochemistry as described by Jung et al[21] and Meng et al[22]. Briefly, endogenous peroxidase activity was blocked using 3% H2O2 in methanol for 15 min. The sections were washed, and stained with a rabbit polyclonal antibody against p-STAT3 and VEGF (Santa Cruz Biotechnology, TX, United States) diluted 1:50. Primary antibody binding was detected using the Bond Polymer Refine Detection Kit (Leica, Wetzlar, Germany). The sections were counterstained with hematoxylin, dehydrated and mounted.
Scoring of immunohistochemical results was performed according to the semi-quantitative method as described by Meng et al[22]. Briefly, the percentage of immunopositive cells was scored using a four-point system: 0 points, < 5% of positive cells; 1 point, 5%-25% of positive cells; 2 points, 26%-50% of positive cells; 3 points, 51%-75% of positive cells; 4 points, > 75% of positive cells. The staining intensity was scored similarly, with 0 points for negative staining, 1 point for weak staining (light yellow), 2 points for moderate staining (brown), and 3 points for strong staining (dark brown). The immunoreactivity score for each lesion was calculated as (the score for the percentage of immunopositive cells + the score for the staining intensity)/2. Based on the immunoreactivity scores, immunohistochemical staining was considered negative (< 0.5), weakly positive (0.5-1.5), or strongly positive (> 1.5).
Frozen gastric tissue samples (whole layer) were homogenized in homogenization buffer (50 mmol/L Tris-HCl, pH 7.2) containing Na3VO4 and a protease inhibitor cocktail (Sigma-Aldrich) using an OmniTH homogenizer (Omni International, Marietta, GA, United States). After sonication, the homogenate was centrifuged at 2000 rpm for 5 min. The resulting supernatants were collected as total proteins and protein concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad Inc., Hercules, CA, United States)[23].
Western blots were performed as previously described by Li et al[24] with minor modifications. Briefly, the proteins were separated on 12.5% SDS polyacrylamide gels and blotted onto nitrocellulose membranes. These membranes were incubated in PBST-milk (0.1% Tween-20 and 5% milk), followed by primary antibodies (2 h) for p-STAT3 and VEGF and β-actin (Santa Cruz Biotechnology Inc., TX, United States). The blots were then washed with PBST 3 times, and subsequently incubated for 1 h with corresponding fluorescently labeled secondary antibodies (Rockland Immunochemicals, Inc., Gilbertsville, PA, United States) diluted in PBST-milk. The blots were washed 3 times and the fluorescence intensity of the detected protein bands was quantified using the Quantity One system (Bio-Rad Inc., Hercules, CA, United States).
Statistical analyses were performed using SigmaStat 17.0 (SPSS, Chicago, IL, United States). Data are reported as means ± SE. The Student’s t-test was used to analyze the difference in measurements between the ES group and sham-ES group. Analysis of variance (ANOVA) was used for multiple comparisons. A P value of < 0.05 was considered statistically significant.
Administration of MNNG induced significant changes in macroscopy and microscopy in the model group compared with the control group. Little hyperemia and sporadic erosion were observed in gastric tissue at the end of the 15th week (A15 group); and ulcer, reduction in mucosal folds, gray mucosa and even some mucosal hyperplasia in addition to hyperemia and erosion were observed at the end of the 25th week (A25 group); obvious masses were observed at the end of the 40th week (A40 group) (Figure 2A). Respective changes in microscopy were observed in these treatment groups. Inflammatory cells were observed in gastritis samples in the A15 group, loss of appropriate glands in some samples in the A25 group and A40 group and tumour cells were noted in some samples in the A40 group (Figure 2B). These changes were not observed in the control group.
Only 20% of rats had gastritis (4/20) in the C15 group, while 42.1% of rats had gastritis (8/19), 10.53% of rats had atrophic gastritis (2/19) and 5.3% of rats had dysplasia (1/19) in the M15 group (Table 1). In the C25 group, 30% of rats had gastritis (6/20), 10% of rats had atrophic gastritis (2/20) and none of the rats had dysplasia or tumour; however, in the M25 group, 31.58% of rats had gastritis (6/19), 26.32% of rats had atrophic gastritis (5/19) and 15.79% of rats had dysplasia (3/19) although no rats had tumours (Table 1). At the end of the study, 16.67% of rats had gastritis (3/18) in the M40 group (vs 35% in the C40 group, 7/20), 27.78% of rats had atrophic gastritis (5/18) (vs 20% in the C40 group, 4/20) and 27.78% of rats had dysplasia (5/18) (vs 5% in the C40 group, 1/20). It was observed that 27.78% of rats had tumours (5/18) in the M40 group (vs 0% in the C40 group, 0/20) (Table 1).
| Pathological type | ||||||
| Group | n | Normal | Gastritis | Atrophic gastritis | Dysplasia | Tumour |
| A15 group | ||||||
| Control | 20 | 80.00% (16/20) | 20.00% (4/20) | 0 | 0 | 0 |
| A15 group | 19 | 42.11% (8/19) | 42.11% (8/19) | 10.53% (2/19) | 5.26% (1/19) | 0 |
| A25 group | ||||||
| Control | 20 | 60.00% (12/20) | 30.00% (6/20) | 10.0% (2/20) | 0 | 0 |
| A25 group | 19 | 26.32% (5/19) | 31.58% (6/19) | 26.32% (5/19) | 15.79% (3/19) | 0 |
| A40 group | ||||||
| Control | 20 | 40.00% (8/20) | 35.00% (7/20) | 20.00% (4/20) | 5.00% (1/20) | 0 |
| A40 group | 18 | 0 | 16.67% (3/18) | 27.78% (5/18) | 27.78% (5/18) | 27.78% (5/18) |
The expression of p-STAT3 and VEGF in the model groups measured by immunohistochemistry increased significantly compared with the control groups. The expression of p-STAT3 in gastric tissue was mainly in the nucleus (Figure 3A), and the immunochemical score for p-STAT3 in tissues with gastritis, tissues with atrophy and tissues with dysplasia were significantly higher than those in the control groups (all P values < 0.05), and the expression of p-STAT3 in tissues with dysplasia was higher than that in tissues with gastritis and tissues with atrophy (P < 0.001, P < 0.001, respectively). The expression of p-STAT3 in tissues with tumor was substantially higher than that in tissues in the control group (P < 0.001); however, the difference in expression of p-STAT3 between tissues with gastritis and tissues with atrophy was not significant (P > 0.05) (Figure 4A).
Similarly, the expression of VEGF, mainly in the cytoplasm (Figure 3B), was higher in precancerous lesions than in the control group (P < 0.05) (Figure 4B). Consistent with p-STAT3, the expression of VEGF in tissue with dysplasia was higher than that in tissue with gastritis, tissue with atrophy and the control group (P < 0.001, P < 0.001, respectively); the expression of VEGF in tissue with stromal tumor was substantially higher than tissue in the control group (P < 0.001) (Figure 4B), however, the difference between the tissue with gastritis and tissue with atrophy was not significant (P > 0.05).
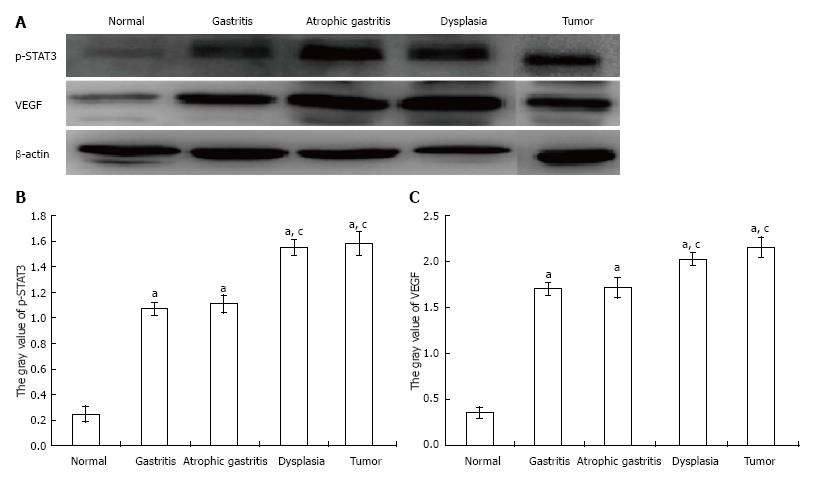
To further determine whether the observed p-STAT3 and VEGF changes were consistent with the changes shown by immunohistochemistry, proteins were extracted from scraped freshly excised stomach tissue, and the samples were analyzed by SDS-PAGE and Western-blot using primary antibodies for p-STAT3 and VEGF. As shown in Figure 5: (A) both p-STAT3 and VEGF protein expression were significantly higher in tissue with gastritis, atrophy, dysplasia or tumor than in the control group (P < 0.05); (B) both p-STAT3 and VEGF protein expression were significantly higher in tissue with dysplasia or tumor compared with that in tissue with gastritis or atrophy (P < 0.05); and (C) there was no significant difference in p-STAT3 and VEGF protein expression between tissue with dysplasia and tumor (P > 0.05).
A strong correlation was observed between the expression of p-STAT3 and VEGF both in the immunohistochemistry and Western Blot results. In order to further examine the relationship between the expression of p-STAT3 and VEGF, we performed a relativity analysis and found that there was a strong positive correlation in the immunohistochemical score between the expression of p-STAT3 and VEGF (R2 = 0.86, P < 0.001) as well as protein expression measured by Western Blot (R2 = 0.90, P < 0.001), suggesting that p-STAT3 may regulate VEGF to promote the formation and development of gastric cancer (Figure 6).
In this study, we examined the significance of p-STAT3 and VEGF expression in the development of gastric cancer from gastritis, atrophic gastritis and dysplasia induced by the administration of MNNG in rats. The results showed that STAT3 is partially responsible for the development of gastric carcinoma from chronic gastritis induced by MNNG and was significantly related to the expression of VEGF during this process. Although overexpression of p-STAT3 in primary tumor sites has been recognized as a predictor of poor survival in many malignancies, including gastric cancer[25,26], to the best of our knowledge, this is the first report to show that p-STAT3 is also persistently activated in the progression of chronic gastritis to gastric carcinoma induced by MNNG in rats, and was positively associated with the expression of VEGF.
STAT3, plays important roles in the development of inflammation and carcinogenesis as well as tumor metastasis[27,28]. Normally, STAT3 is activated for a short time and then is deactivated to maintain homeostasis; however, under abnormal conditions, STAT3 activation continues, which triggers oncogene transcription[29]. Only a few studies have reported the difference in expression of p-STAT3 between normal gastric mucosa and precancerous lesions. In a clinical study[30], it was reported that p-STAT3 expression was significantly higher in chronic atrophic gastritis (60.0%), intestinal metaplasia (77.1%), dysplasia (68%) and gastric cancer (60.1%) than in normal gastric mucosa (41.1%, P < 0.05). Consistent with these findings, we found that p-STAT3 expression in samples with chronic gastritis or atrophic gastritis was significantly higher than that in normal mucosa and was substantially increased in samples with dysplasia or tumor compared with normal mucosa, and even higher than that in samples of chronic gastritis or atrophic gastritis, further indicating the potential role of p-STAT3 not only in the invasion and metastasis of gastric cancer, but also in the development of gastric cancer from chronic gastritis. However, there was no significant difference in the expression of p-STAT3 between chronic gastritis and atrophic gastritis samples. Similar results were observed between dysplasia and tumor samples, indicating the important effect of p-STAT3 on the progression from atrophic gastritis to gastric carcinoma.
VEGFs are glycoproteins secreted by tumor cells and are the most important factors in angiogenesis and tumor metastasis[31]. The VEGF family includes VEGF-A to F and placental growth factor. It has been reported that VEGF-A and B play a key role in blood vessel growth, whereas VEGF-C and D are important for the growth of lymphatic vessels[32,33]. VEGFs, particularly VEGF-A, C, and D promote angiogenesis and metastasis of many cancers including gastric cancer[34].
Some clinical studies have shown the potential effects of VEGF inhibitors in the treatment of multiple cancers including gastric cancer[35]. Raica et al[36] investigated the immunohistochemical expression of VEGF on 80 patients with intestinal-type gastric carcinoma and found that VEGF was positive in 52 of 80 cases (70%), indicating that the expression of VEGF could signify an early angiogenic switch during tumorigenesis. Consistent with this finding, we also found that the expression of VEGF in dysplasia and tumor samples was higher than that in chronic gastritis and astrophic gastritis samples. However, consistent with the expression of p-STAT3, both the difference in expression of VEGF between chronic gastritis and atrophic gastritis samples and the difference between dysplasia and tumor samples were not significant. According to these results, we analyzed the correlation between the expression of p-STAT3 and VEGF and found that the expression of VEGF was significantly and positively related to p-STAT3. To the best of our knowledge, this is the first report on the positive relationship between p-STAT3 and VEGF in precancerous lesions of gastric cancer induced by MNNG.
Some published papers support the findings in our study regarding the relationship between the expression of p-STAT3 and VEGF in gastric cancer. Deng et al[37] reported that STAT3, p-STAT3, and VEGF-D expression in gastric cancer tissue was significantly higher than those in adjacent non-tumor tissue, and both STAT3 and VEGF-D mRNA expression was much higher in many gastric cancer cell lines than in the GES-1 cell line. Using STAT3 siRNA transfection, these authors found that VEGF-D expression significantly decreased in HGC-27 cells, representing the potential STAT3 regulation of VEGF-D expression[37]. Similar results were observed by Zhu et al[38] who found that (-)-epigallocatechin-3-gallate inhibited IL-6-induced VEGF expression and angiogenesis by suppressing STAT3 activity in human gastric cancer cells. However, the relationship between p-STAT3 and VEGF in precancerous lesions of gastric cancer is still unclear. In the current study, we found that even in chronic gastritis and atrophic gastritis samples, the expression of both p-STAT3 and VEGF were higher than that in normal mucosa and showed a positive relationship, suggesting that VEGF may be activiated on initial damage of gastric mucosa induced by MNNG via the p-STAT3 pathway and contributes to the development and progression of gastric cancer, highlighting the potential for anti-p-STAT3 as a therapeutic target for the prevention of gastric cancer.
In conclusion, STAT3 activation plays an important role in the changes in gastric mucosa from chronic gastritis to gastric tumor via the STAT/VEGF pathway, confirming the potential value of targeted therapy focusing on STAT/VEGF in protecting against gastric cancer in clinical applications.
Signal transducer and activator of transcription 3 (STAT3) has been shown to play a vital role in human gastric cancer angiogenesis. Vascular endothelial growth factor (VEGF) plays a very critical role in cancer metastasis due to its function in vascular formation. Several researchers have demonstrated that the expression of STAT3 is potentially associated with the expression of VEGF-C in various malignant diseases; Judd et al reported that constitutive STAT3 activation promoted VEGF expression and stimulated tumor angiogenesis. However, to the best of our knowledge, the role of STAT3 and VEGF in the formation of gastric precancerous lesions and gastric cancer induced by “N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) is still unknown.
The effects of STAT3 and VEGF on gastric cancer have been reported. The present study showed that STAT3 activation plays an important role in the changes in gastric mucosa from chronic gastritis to gastric tumor via the STAT/VEGF pathway.
To the best of our knowledge, this is the first report to show that p-STAT3 is also persistently activated during the progression from chronic gastritis to gastric carcinoma induced by the administration of MNNG in rats, and was positively associated with the expression of VEGF.
It was shown in this study that STAT3 is partially responsible for the progression from chronic gastritis to gastric carcinoma induced by MNNG and was significantly related to the expression of VEGF during this process. It is considered that STAT3 can induce an abnormal level of VEGF expression to promote the formation of gastric carcinoma.
Previous studies have established that STAT3 has close relationship with VEGF in cancers. However, this is the first time to report that p-STAT3 is also persistently activated from chronic gastritis to gastritis carcinoma induced by administration of MNNG in rats, which is positively associated with the expression of VEGF.
P- Reviewer: Slomiany BL S- Editor: Wang JL L- Editor: Webster JR E- Editor: Lu YJ
| 1. | Chan AO, Wong BC, Lam SK. Gastric cancer: past, present and future. Can J Gastroenterol. 2001;15:469-474. [PubMed] [Cited in This Article: ] |
| 2. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1193] [Cited by in F6Publishing: 1204] [Article Influence: 66.9] [Reference Citation Analysis (7)] |
| 3. | Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Abnet CC, Corley DA, Freedman ND, Kamangar F. Diet and upper gastrointestinal malignancies. Gastroenterology. 2015;148:1234-1243.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Hishida A, Matsuo K, Goto Y, Naito M, Wakai K, Tajima K, Hamajima N. Smoking behavior and risk of Helicobacter pylori infection, gastric atrophy and gastric cancer in Japanese. Asian Pac J Cancer Prev. 2010;11:669-673. [PubMed] [Cited in This Article: ] |
| 7. | Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, Wang HP, Lin JT. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000;118:1031-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Hu PJ, Yu J, Zeng ZR, Leung WK, Lin HL, Tang BD, Bai AH, Sung JJ. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Livingstone JI, Filipe MI, Wastell C. Expression of transforming growth factor alpha in experimental gastric carcinogenesis. Gut. 1994;35:604-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Giraud AS, Menheniott TR, Judd LM. Targeting STAT3 in gastric cancer. Expert Opin Ther Targets. 2012;16:889-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Denson LA. Adding fuel to the fire: STAT3 priming of gastric tumorigenesis. Gastroenterology. 2006;131:1342-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Wang CA, Tsai SJ. The non-canonical role of vascular endothelial growth factor-C axis in cancer progression. Exp Biol Med (Maywood). 2015;240:718-724. [PubMed] [Cited in This Article: ] |
| 14. | Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: Implications for cancer. Biochim Biophys Acta. 2015;1855:202-222. [PubMed] [Cited in This Article: ] |
| 15. | Bilgiç CI, Tez M. Serum VEGF levels in gastric cancer patients: correlation with clinicopathological parameters. Turk J Med Sci. 2015;45:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Thiel A, Ristimäki A. Targeted therapy in gastric cancer. APMIS. 2015;123:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Huang C, Huang R, Chang W, Jiang T, Huang K, Cao J, Sun X, Qiu Z. The expression and clinical significance of pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma. Neoplasma. 2012;59:52-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Jin C, Wang A, Chen J, Liu X, Wang G. Relationship between expression and prognostic ability of PTEN, STAT3 and VEGF-C in colorectal cancer. Exp Ther Med. 2012;4:633-639. [PubMed] [Cited in This Article: ] |
| 19. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3221] [Cited by in F6Publishing: 3376] [Article Influence: 120.6] [Reference Citation Analysis (2)] |
| 20. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1463] [Cited by in F6Publishing: 1470] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 21. | Jung HY, Cho H, Oh MH, Lee JH, Lee HJ, Jang SH, Lee MS. Loss of FAT Atypical Cadherin 4 Expression Is Associated with High Pathologic T Stage in Radically Resected Gastric Cancer. J Gastric Cancer. 2015;15:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Meng XM, Zhou Y, Dang T, Tian XY, Kong J. Magnifying chromoendoscopy combined with immunohistochemical staining for early diagnosis of gastric cancer. World J Gastroenterol. 2013;19:404-410. [PubMed] [Cited in This Article: ] |
| 23. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 567] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 24. | Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na -H exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537:537-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437-442. [PubMed] [Cited in This Article: ] |
| 27. | Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502-2512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 695] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 28. | Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 29. | Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1755] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 30. | Zhang JG, Zhao J, Xin Y. Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol. 2010;16:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004;10:512-520. [PubMed] [Cited in This Article: ] |
| 32. | Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1241] [Cited by in F6Publishing: 1304] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 33. | Yuanming L, Feng G, Lei T, Ying W. Quantitative analysis of lymphangiogenic markers in human gastroenteric tumor. Arch Med Res. 2007;38:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352-355. [PubMed] [Cited in This Article: ] |
| 35. | Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2:123. [PubMed] [Cited in This Article: ] |
| 36. | Raica M, Mogoantă L, Cîmpean AM, Alexa A, Ioanovici S, Mărgăritescu C, Lazăr D, Izvernariu D. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in intestinal type gastric carcinoma. Rom J Morphol Embryol. 2008;49:37-42. [PubMed] [Cited in This Article: ] |
| 37. | Deng J, Cui J, Jiang N, Zhang R, Zhang L, Hao X, Liang H. STAT3 regulation the expression of VEGF-D in HGC-27 gastric cancer cell. Am J Transl Res. 2014;6:756-767. [PubMed] [Cited in This Article: ] |
| 38. | Zhu BH, Chen HY, Zhan WH, Wang CY, Cai SR, Wang Z, Zhang CH, He YL. (-)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J Gastroenterol. 2011;17:2315-2325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |