Published online Apr 15, 2013. doi: 10.4239/wjd.v4.i2.27
Revised: March 26, 2013
Accepted: March 28, 2013
Published online: April 15, 2013
Apoptosis contributes to the development of diabetic nephropathy, but the mechanism by which high glucose induces apoptosis is not fully understood. Apoptosis of tubular epithelial cells is a major feature of diabetic kidney disease, and hyperglycemia triggers the generation of free radicals and oxidant stress in tubular cells. Hyperglycemia and high glucose in vitro also lead to apoptosis, a form of programmed cell death. High glucose similar to those seen with hyperglycemia in people with diabetes mellitus, lead to accelerated apoptosis, a form of programmed cell death characterized by cell shrinkage, chromatin condensation and DNA fragmentation, in variety of cell types, including renal proximal tubular epithelial cells.
Core tip: Apoptosis contributes to the development of diabetic nephropathy, but the mechanism by which high glucose induces apoptosis is not fully understood. High glucose similar to those seen with hyperglycemia in people with diabetes mellitus, lead to accelerated apoptosis, a form of programmed cell death characterized by cell shrinkage, chromatin condensation and DNA fragmentation, in variety of cell types, including renal proximal tubular epithelial cells.
- Citation: Habib SL. Diabetes and renal tubular cell apoptosis. World J Diabetes 2013; 4(2): 27-30
- URL: https://www.wjgnet.com/1948-9358/full/v4/i2/27.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i2.27
Diabetes is the leading cause of end-stage renal failure in most developed countries. Although vascular and glomerular injuries have been considered the main features of diabetic kidney diseases, tubular atrophy is also plays a major role in the disease[1]. Diabetes induces early signs of tubular dysfunction[2]. In addition, diabetic kidneys are particularly prone to acute tubular necrosis in diverse clinical situations, such as post-cardiac surgery[3]. Hyperglycemia, by itself, is an independent risk factor for acute tubular necrosis under these conditions[3]. Hyperglycemia triggers the generation of free radicals and oxidant stress in tubular cells[4,5]. Reactive oxygen species are considered to be important mediators for several biologic responses, including proliferation, extracellular matrix deposition and apoptosis[6]. Apoptosis, a form of programmed cell death characterized by cell shrinkage, chromatin condensation and DNA fragmentation, which, can be induced by various stimuli[7]. High glucose concentration promotes apoptosis in variety of cell types including proximal tubular epithelial cells[5,8]. The mechanism by which hyperglycemia leads to apoptosis is not completely understood.
A high glucose concentration of 30 mmol/L for 18-48 h has been shown to induce apoptotic changes in HK2 cells via an increase in oxidative stress[8]. Prolonged exposure (1-13 d) of proximal tubular epithelial cells to hyperglycemic environment has been shown to inhibit cell proliferation and induce growth arrest or cellular apoptosis[8-12]. These cellular effects are caused by the activation of a network of intracellular signaling pathways and include the phosphatidylinostiol 3 kinase (PI3 kinase)/adams kara taylor (AKT) signaling pathway[13]. Activation of PI3 kinase and phosphorylation of serine/threonine kinase AKT/protein kinase B (PKB) by insulin, insulin like growth factors in human embryonic 293 (HEK-293) and HeLa cells lead to inactivation of tuberin by phosphorylating at Ser939, Ser1086/1088 and Thr1422[14,15]. In addition, phosphorylation of tuberin at Ser939 and Thr1422 in response to PDGF and insulin stimulation in a PI3K-dependent manner has been reported in NIH-3T3 and HEK-293 transfected with flag-tuberin[16]. Moreover, high glucose has shown to phosphorylate tuberin in renal cells[13].
Tuberin, which is the product of tumor suppressor gene, TSC-2[17] normally, exists in an active state physically bound to hamartin, the product of TSC-1 gene to form a stable complex[18]. These two proteins function within the same mTOR signaling pathway. mTOR is a serine/threonine kinase involved in numerous cell processes linked to cell growth control, like cell cycle progression, transcription and translation control as well as nutrient uptake[19]. Loss of TSC-2 function either by TSC-2 or TSC-1 deficiency leads to constitutive activation of mTOR and downstream signaling pathways due to increased levels of GTP-bound Rheb[20-23]. Therefore tuberin, through its Rheb-GAP activity, is a critical negative regulator of mTOR under physiological conditions[24,25]. mTOR phosphorylates p70S6K (p70 ribosomal protein S6 kinase) on Thr389, which correlates with the activation of p70S6kinase[24-26], while over-expression of TSC-2 suppresses phosphorylation and activation of p70S6K on residue Thr389[14-16]. In addition, several studies have shown that Akt/mTOR pathway is activated in diabetes and this activation is redox dependent in different cell types[27-29] including renal cells[13].
Previous reports have shown that the serine/threonine kinase, mTOR to be involved in the phosphorylation/inactivation of Bcl-2 in microtubules treated with apoptotic agents[30]. Bcl-2 plays a central role in monitoring the genetic programs of the organism[31,32]. Bcl2 related proteins comprise a family of positive and negative regulators of apoptosis. Bcl-2 and its close homolog Bcl-XL are anti-apoptotic, whereas other members of the Bcl-2 family, such as BAD or BAX are proapoptotic[33]. Bcl-2 has been shown to prevent the release of cytochrmoe C from mitochondria and hence activation of caspase 9, the initiator caspase[32]. Several kinases like JNK, p38[33] and cdc2/cyclin B kinase[34] have been noticed to phosphorylate/inactivate Bcl-2 as a physiological process during normal cell cycle progression or as a defense mechanism following the activation by various stimuli and stress. Phosphorylation/inactivation of Bcl-2 inactivates the antiapoptotic effect, which triggers the release of cytochrome C from the mitochondria leading to the activation of downstream caspases[35-37].
Another important protein involved in apoptosis is poly (ADP-Ribose) polymerase (PARP), a DNA repair enzyme that is cleaved by the downstream caspases. The essential role of PARP activation in diabetes induced by streptozotocin in adult male BALB/c mice[38]. PARP catalyzes the poly(ADP-ribosyl)ation of a variety of nuclear proteins with NAD substrate. Because it is activated by binding to DNA ends or strand breaks, an important feature of the cell in apoptosis, PARP was suggested to contribute to apoptosis by depleting the cell of NAD and ATP[39]. When PARP is cleaved into 89- and 24-kDa fragments that contain the active site and the DNA binding domain of the enzyme, respectively during drug induced apoptosis in a variety of cells[39]. Such cleavage essentially inactivates the enzyme by destroying its ability to respond to DNA strand breaks/fragmentation.
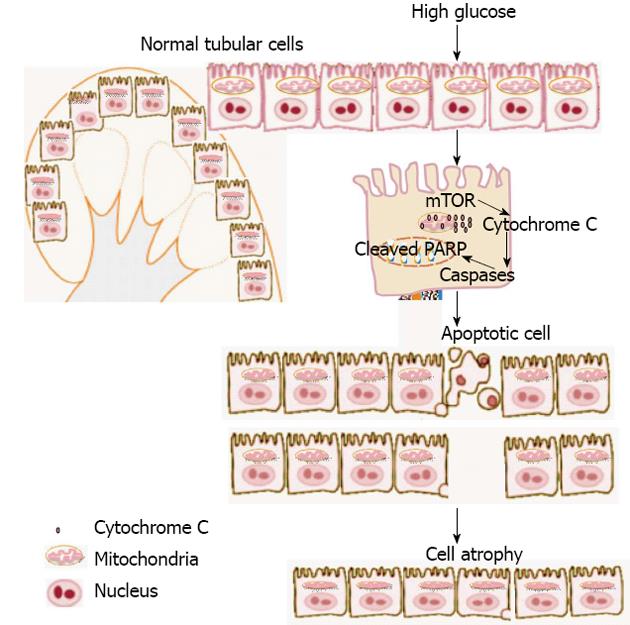
Proteases play a critical role in the initiation and execution of apoptosis. The caspases, a family of cysteine-dependent, aspartate-directed proteases, are prominent among apoptosis-associated molecules[40]. Activation of caspases cleaves a variety of intracellular polypeptides, including major structural elements of the cytoplasm and nucleus, components of DNA repair machinery and a number of protein kinases. Caspase 3, a member of the caspase family plays a central role in the execution of the apoptotic program[41-43]. Oxidative stress mediated activation of caspase 3 has been shown to be a principle mediator of hyperglycemia induced proximal tubular apoptosis[5]. Caspase 3 is primarily responsible for the cleavage of PARP during cell death[41-45]. Recent published data show that high glucose and hyperglycemia induced cell apoptosis mainly in proximal tubular cells through regulation Bcl2/caspase/PARP pathway[46-49]. The sequence at which caspase 3 cleave PARP is very well conserved in the PARP protein from very distant species, indicating the potential importance of PARP cleavage in apoptosis. Recent study from our lab showed the important role of tuberin/mTOR pathway in regulation of apoptosis[50]. We showed that induction of diabetes increased phosphorylation of tuberin in association with mTOR activation (measured by p70S6K phosphorylation), inactivation of Bcl-2, increased cytosolic cytochrome c expression, activation of caspase 3, and cleavage of PARP; insulin treatment prevented these changes. In addition, exposure of proximal tubular epithelial cells to high glucose increased phosphorylation of tuberin and p70S6K, phosphorylation of Bcl-2, expression of cytosolic cytochrome c, and caspase 3 activity. Moreover, high glucose induced translocation of the caspase substrate YY1 from the cytoplasm to the nucleus and enhanced cleavage of PARP. Cells treated with the mTOR inhibitor rapamycin resulted in reduce the number of apoptotic cells induced by high glucose[50]. This signaling cascade may play an important role in apoptosis induced by hyperglycemia during diabetic nephropathy. In summary, tubular apoptosis is one of the characteristic morphologic changes in human diabetic kidneys and tubular atrophy appears to be a better indicator of disease progression than glomerular pathology. A proposed model of induction of cell apoptosis and subsequent of cell atrophy by high glucose in kidney show in Figure 1. The mechanism by which hyperglycemia regulates apoptosis in renal tubular cells requires further study to provide the optimal management for diabetic complications.
P- Reviewers Li CJ, Pacifico L S- Editor Huang XZ L- Editor A E- Editor Li JY
| 1. | Barnes DJ, Pinto JR, Davison AM, Cameron JS, Grunfeld JP, Kerr DNS, Ritz E, Viberti GC. The patient with diabetes mellitus. 2nd ed. Oxford, United Kingdom: Oxford university Press 1998; 723-775. [Cited in This Article: ] |
| 2. | Ginevri F, Piccotti E, Alinovi R, DeToni T, Biagini C, Chiggeri GM, Gusmano R. Reversible tubular proteinuria precedes microalbuminuria and correlates with the metabolic status in diabetic children. Pediatr Nephrol. 1993;7:23-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194-203. [PubMed] [Cited in This Article: ] |
| 4. | Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Frumento G, Berruti V, Gandolfo MT, Garibotto G, Deferran G. Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med. 2002;50:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17:908-910. [PubMed] [Cited in This Article: ] |
| 6. | Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1728] [Cited by in F6Publishing: 1646] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 7. | Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 750] [Cited by in F6Publishing: 818] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 8. | Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT, Garibotto G, Deferrari G. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol. 2004;15 Suppl 1:S85-S87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Han HJ, Jeon YJ, Lee YJ. Involvement of NF-kappaB in high glucose-induced alteration of alpha-methyl-D-glucopyranoside (alpha-MG) uptake in renal proximal tubule cells. Cell Physiol Biochem. 2003;13:375-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yet SF, Kong CT, Peng H, Lever JE. Regulation of Na+/glucose cotransporter (SGLT1) mRNA in LLC-PK1 cells. J Cell Physiol. 1994;158:506-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Fujita H, Omori S, Ishikura K, Hida M, Awazu M. ERK and p38 mediate high-glucose-induced hypertrophy and TGF-beta expression in renal tubular cells. Am J Physiol Renal Physiol. 2004;286:F120-F126. [PubMed] [Cited in This Article: ] |
| 12. | Park SH, Choi HJ, Lee JH, Woo CH, Kim JH, Han HJ. High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-beta 1. Kidney Int. 2001;59:1695-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2’-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626-2636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364-35370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2234] [Cited by in F6Publishing: 2245] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 16. | Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1178] [Cited by in F6Publishing: 1182] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 17. | Habib SL, Riley DJ, Mahimainathan L, Bhandari B, Choudhury GG, Abboud HE. Tuberin regulates the DNA repair enzyme OGG1. Am J Physiol Renal Physiol. 2008;294:F281-F290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Plank TL, Yeung RS, Henske EP. Hamartin, the product of the tuberous sclerosis 1 (TSC1) gene, interacts with tuberin and appears to be localized to cytoplasmic vesicles. Cancer Res. 1998;58:4766-4770. [PubMed] [Cited in This Article: ] |
| 19. | Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible application in tuberous sclerosis treatment. Med Res Rev. 2006;26:160-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 768] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 21. | Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 874] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 680] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 24. | Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 25. | Freilinger A, Rosner M, Krupitza G, Nishino M, Lubec G, Korsmeyer SJ, Hengstschläger M. Tuberin activates the proapoptotic molecule BAD. Oncogene. 2006;25:6467-6479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 541] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699-22704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 436] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 28. | Calastretti A, Bevilacqua A, Ceriani C, Viganò S, Zancai P, Capaccioli S, Nicolin A. Damaged microtubules can inactivate BCL-2 by means of the mTOR kinase. Oncogene. 2001;20:6172-6180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 514] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 30. | Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2873] [Cited by in F6Publishing: 2863] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 31. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3451] [Cited by in F6Publishing: 3484] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 32. | Ling YH, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984-18991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 172] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 258] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Asnaghi L, Calastretti A, Bevilacqua A, D’Agnano I, Gatti G, Canti G, Delia D, Capaccioli S, Nicolin A. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene. 2004;23:5781-5791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Jeong BC, Kwak C, Cho KS, Kim BS, Hong SK, Kim JI, Lee C, Kim HH. Apoptosis induced by oxalate in human renal tubular epithelial HK-2 cells. Urol Res. 2005;33:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Garcia Soriano F L, Jagtap P, Szabó E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 482] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 37. | Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049-1057. [PubMed] [Cited in This Article: ] |
| 38. | Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3494] [Cited by in F6Publishing: 3370] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 39. | Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932-22940. [PubMed] [Cited in This Article: ] |
| 40. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2026] [Cited by in F6Publishing: 1983] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 41. | Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1-16. [PubMed] [Cited in This Article: ] |
| 42. | Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551-1570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 943] [Cited by in F6Publishing: 990] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 43. | Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3009] [Cited by in F6Publishing: 3104] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 44. | Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1846] [Cited by in F6Publishing: 1880] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 45. | Le Rhun Y, Kirkland JB, Shah GM. Cellular responses to DNA damage in the absence of Poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1998;245:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Chao LK, Chang WT, Shih YW, Huang JS. Cinnamaldehyde impairs high glucose-induced hypertrophy in renal interstitial fibroblasts. Toxicol Appl Pharmacol. 2010;244:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Lau GJ, Godin N, Maachi H, Lo CS, Wu SJ, Zhu JX, Brezniceanu ML, Chénier I, Fragasso-Marquis J, Lattouf JB. Bcl-2-modifying factor induces renal proximal tubular cell apoptosis in diabetic mice. Diabetes. 2012;61:474-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Covington MD, Schnellmann RG. Chronic high glucose downregulates mitochondrial calpain 10 and contributes to renal cell death and diabetes-induced renal injury. Kidney Int. 2012;81:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Velagapudi C, Bhandari BS, Abboud-Werner S, Simone S, Abboud HE, Habib SL. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J Am Soc Nephrol. 2011;22:262-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |