Published online Sep 15, 2018. doi: 10.4239/wjd.v9.i9.141
Peer-review started: April 21, 2018
First decision: June 8, 2018
Revised: June 19, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: September 15, 2018
Epicardial adipose tissue is defined as a deposit of adipocytes with pathophysiological properties similar to those of visceral fat, located in the space between the myocardial muscle and the pericardial sac. When compared with subcutaneous adipose tissue, visceral adipocytes show higher metabolic activity, lipolysis rates, increased insulin resistance along with more steroid hormone receptors. The epicardial adipose tissue interacts with numerous cardiovascular pathways via vasocrine and paracrine signalling comprised of pro- and anti-inflammatory cytokines excretion. Both the physiological differences between the two tissue types, as well as the fact that fat distribution and phenotype, rather than quantity, affect cardiovascular function and metabolic processes, establish epicardial fat as a biomarker for cardiovascular and metabolic syndrome. Numerous studies have underlined an association of altered epicardial fat morphology, type 2 diabetes mellitus (T2DM) and adverse cardiovascular events. In this review, we explore the prospect of using the epicardial adipose tissue as a therapeutic target in T2DM and describe the underlying mechanisms by which the antidiabetic drugs affect the pathophysiological processes induced from adipose tissue accumulation and possibly allow for more favourable cardiovascular outcomes though epicardial fat manipulation.
Core tip: In this review, we aim to create a concise overview of the pathophysiology concerning the epicardial fat deposits on a type 2 diabetic individual, while, delving into the intricacies of each antidiabetic drug and exploring the manner by which it interacts with visceral fat accumulation in the sub-pericardial space.
- Citation: Xourgia E, Papazafiropoulou A, Melidonis A. Effects of antidiabetic drugs on epicardial fat. World J Diabetes 2018; 9(9): 141-148
- URL: https://www.wjgnet.com/1948-9358/full/v9/i9/141.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i9.141
Subcutaneous (SCAT) and visceral adipose tissue (VAT) are two extremely heterogenous tissue types, differentiated by anatomical, molecular, cellular, physiological and clinical characteristics[1]. Researchers have suggested that the variation of composition and function of the two tissue types is induced very early in the tissue developmental pathway, as a result of adipose stem cell distinction[2]. VAT has an anatomically distinct distribution in the mesentery and omentum, when compared to SCAT that is mainly located in the femerogluteal area, back and abdominal wall[1]. As a result of the anatomical differences, vascularization and innervation vary between the tissues, with VAT having superior nerve and vascular networks, as well as draining into the portal system of veins. Based on the aforementioned anatomical link, the “portal theory” of metabolic inflammation states that free fatty acids and pro-inflammatory molecules from VAT, interact with the liver, promoting hepatocellular dysfunction in the form of insulin resistance and steatosis[3]. The dissimilarity in cellular composition between SCAT and VAT is a result of divergent ratio of large to small adipocytes between the two tissues. Large, metabolically dysfunctional, adipocytes, predominate in VAT, while SCAT is mainly composed by small adipocytes with higher free fatty acids and triglycerides capacity and increased insulin sensitivity[4,5] . The signaling pathways activated in the two tissue types vary due to a shift in receptor distribution and adipokine synthesis[1]. Glucocorticoid and androgen receptors present with a higher density in VAT while oestrogen receptors are more active in SCAT. Adrenergic signaling patterns are distinct for the two cell populations, with VAT being more β3- and α2- adrenoreceptor sensitive[6]. The biologically active molecules produced by the adipose tissue, referred to as adipokines, are formed and released at different rates between VAT and SCAT. Adipokines are the basis of adipose tissue participating in and regulating endocrine and paracrine funtions[7]. The diversity of adipokines is directly linked to sympathetic excitation, metabolic regulation, including insulin sensitivity and appetite, inflammatory response and other homeostatic mechanisms. Some of the most prominent members of this family, as far as metabolic processes and cardiovascular function are examined, are: leptin, adiponectin, interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1) and tumor necrosis factor alpha (TNF-α)[1,7] . Leptin levels are elevated in obese subjects, along with TNF-α, IL-6 and PAI-1 that are proatherogenic and prodiabetic, in contrast to plasma adiponectin that protects against vascular damage and metabolic syndrome and is reduced, as it would be expected[8]. The variety in cytokine profile, along with the anatomic and cellular diversity that differentiate SCAT and VAT clarify and support the physiological and metabolic properties excreted by each adipocyte group. VAT cells allow for increased insulin-mediated glucose uptake and are more insulin-resistant and lipolysis-prone that those of SCAT. In contrast, the latter, exhibit a greater capacity for postprandial free fatty acid and triglyceride uptake and storage[1]. Taking into consideration the pivotal role of VAT in metabolic impairment, as such is supported by its aforementioned properties, it is comprehensible that studying the metabolic properties of visceral adiposity and mainly, organ-specific depositions, such as epicardial fat, has been incremental in the process of stratification of cardiometabolic risk factors. In this review, we aim to compare the morphology of epicardial fat deposits between non-diabetic individuals and subjects with type 2 diabetes mellitus (T2DM). Moreover, we will discuss the affect excreted by the antidiabetic substances in epicardial VAT, while contemplating on its clinical utility, as estimated by means of cardiovascular risk reduction.
Epicardial adipose tissue (EAT) is an adipocyte depot of VAT with anatomical continuity to the myocardial tissue, located under the visceral layer of the pericardium[9]. It has been suggested that it can serve as a quantifiable and modifiable therapeutic target for cardiovascular adverse events, as it can be measured with non-invasive imaging techniques such as two-dimensional echocardiography, computed tomography (CT) and magnetic resonance imaging (MRI)[10]. Spatial imaging, as such is provided by MRI and CT scans, is preferable to that of two-dimensional echocardiography technique, in order to accurately measure the thickness of EAT. Along with technical shortcomings, operator- and subject- related variability deem echocardiographic imaging a formidable solution solely because of the rapid and cost-effective patient assessment it facilitates. Otherwise, MRI is considered to be the gold-standard method for EAT quantification and area placement, even though three dimensional image reconstruction by utilizing multidetector-row CT is slightly superior in achieving the latter[10,11].
On a cellular level, the epicardial adipocytes are embryologically derived from the splachnopleuric mesoderm, similarly to the mesenteric and omental adipocytes. EAT is characterized by high cellularity, defined by the concentration of adipocytes in this tissue being notably higher than that of other depots of adipose tissue[12]. EAT is a depot of white adipocytes, cells that specialize in energy storage, as opposed to brown adipocytes that are involved in energy expenditure[13].
EAT extends on an area exceeding 80% of the myocardial total surface in an otherwise healthy individual, spreading heterogeneously, mostly accumulating on the lateral and anterior walls of the right atrium[14]. The physiological structure and composition of EAT variates depending on age, gender, body weight and ethnicity[14]. The properties of EAT and its contribution in physiological and pathophysiological pathways have been extensively described. Due to its spatial distribution, EAT acts as a mechanical and thermoprotective layer for the myocardial tissue and coronary arteries. Through endocrine and paracrine function, epicardial adipocytes ameliorate endothelial response of the coronaries and insulin sensitivity, while reducing oxidative stress of the cardiac tissue. Additionally, the small adipocytes of EAT are characterized by high rates of free fatty acids turnover, allowing for both energy supply and storage as demand shifts[14].
The prevalence of type 2 diabetes mellitus (T2DM) has quadrupled in the last three decades according to International Diabetes Federation (IDF) reports. The epidemic escalation has been attributed to numerous factors including population aging as a result of improved healthcare, socioeconomic development, unhealthy diet regimes and sedentary lifestyle[15].
EAT has been associated with numerous pathophysiological processes, such as coronary artery disease[16-18], even though the significance of such association has not been adequately supported by all relevant studies[19-20], electrophysiological abnormalities of the heart[21,22] , cardiovascular disease in human immunodeficiency virus treated with antiretroviral therapy[23], amplified severity of non-alcoholic fatty liver disease[24,25], metabolic syndrome[26-29] and increased cardiovascular risk along with decline of renal function in individuals with T2DM[30-34].
The pathophysiological pathways linking T2DM and EAT, support a multifactorial causative relationship between EAT attributes and structure such as volume and endocrine function and cardiovascular disease severity in the diabetic individual.
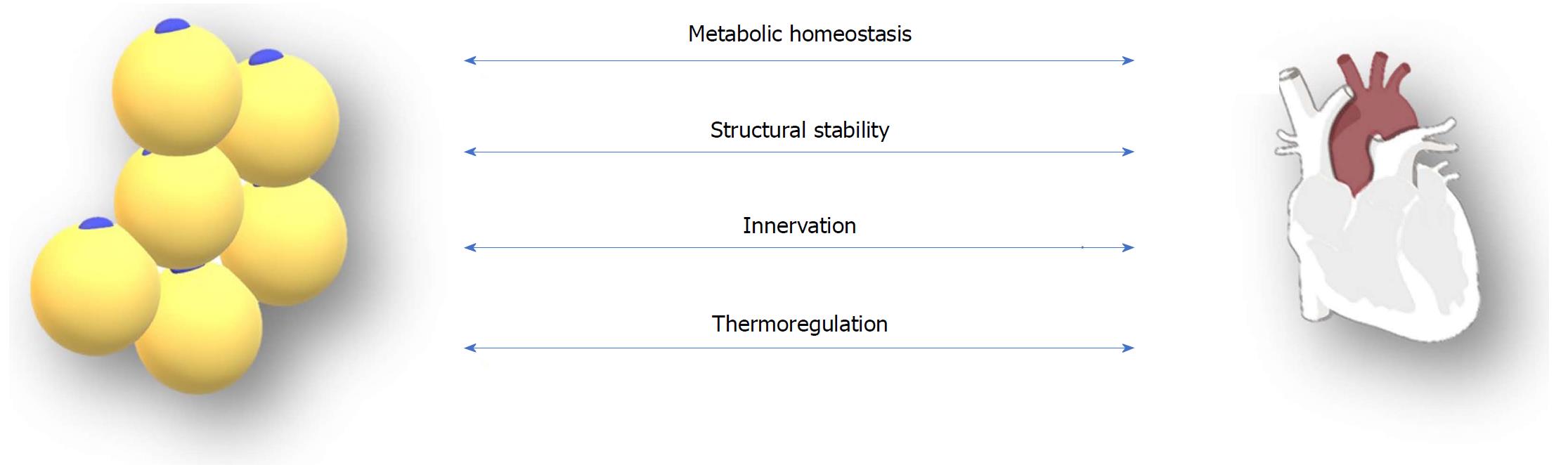
EAT deposition can be associated with coronary vascular disease pathogenesis mainly by the dysregulation of cardiac metabolic processes and the disruption of the epicardial and myocardial structural integrity. Other mechanisms that could be involved in the interaction between EAT and coronary vasculature are nerve damage and impaired cryoprotection of the heart[35,36]. Furthermore, the epicardial adipocytes exhibit and arrhythmogenic potential, a theory suggested by many clinical trials exploring the causative relationship between EAT and atrial fibrillation[21] (Figure 1).
Metformin is the most common first-line treatment choice for T2DM and a member of the biguanides drug class. Oral administration of the substance affects the liver and gut metabolic pathways in order for its hypoglycemic attributes to be put into effect[37]. Hepatic gluconeogenesis, glucose uptake, glycolysis and glucogen synthesis are some of the processes altered by metformin via AMP-activated protein kinase (AMPK)-dependent and -independent pathways[38].
At this point in time, there seem to be no randomized controlled trials designed for clarification of the effects excreted by metformin on the volume or function of EAT. Despite the fact that metformin has not been compared with placebo, as of yet, studies conducted on sitagliptin and liraglutide as add-on therapy to metformin monotherapy, combined with epicardial fat measurement, can be used as a preliminary source of data[39,40] .
Results from these trials confirm the inferiority of metformin monotherapy when compared to metformin/sitagliptin and metformin/liraglutide for reduction of EAT volume. The findings can be either attributed to the synergy of two antidiabetic substances, affecting the EAT in a more effective manner than metformin alone, or to the complete lack of action of the biguanide class on the cardiac VAT deposits. The latter is supported by the results of the study performed by Iacobellis et al[40], that noted no EAT reduction in the metformin group during the 6-mo follow up period. Conversely, metformin has been previously shown to have positive effects on VAT, inducing its reduction on diabetic subjects[41]. Furthermore, studies have confirmed a metformin-induced increase of plasma omentin-1 levels, an adipokine produced by epicardial fat that ameliorates insulin sensitivity, inflammatory response and cardiovascular function[42]. Given the contradicting evidence concerning metformin, there is need for further research, as a definite conclusion on the manner by which biguanides interact with epicardial fat can only be provided by a randomized controlled trial with EAT measurement.
Alpha-glucosidase inhibitors (α-GIs) are a class of antidiabetic drugs acting in the epithelium of the small intestine mainly by delaying the digestion of carbohydrates through reversible and competitive inhibition of intestinal alpha-glucosidases, consequently reducing glucose absorption and attenuating postprandial hyperglycemia[43]. Some α-GIs are acarbose, miglitol and voglibose. Similarly to the biguanide class of antidiabetic medication, there is a lack of data concerning the administration of α-GIs and their effect on EAT mass, volume or metabolic activity.
Thiazolidinediones (TZDs), also known as glitazones, are peroxisome proliferator-activated receptor (PPAR) agonists with numerous actions, spanning from glycemic and lipid control to inflammatory signaling and cell cycle mediation[44]. The phenomenon of glitazone treatment and subsequent increase in body weight that has been supported by the results of numerous studies appears to be tissue-specific, since the VAT depot of the subjects remains unaffected while there is a shift of excess energy storage towards the SCAT[45-47].
Furthermore, pioglitazone treatment in T2DM or metabolic syndrome has been shown to attenuate the inflammatory signature of EAT by means of decreased expression of proinflammatory interleukins (IL) such as IL-1β, IL-1Ra and IL-10[48]. In addition to the positive effect on the metabolic profile of EAT, pioglitazone can affect the epicardial fat depot directly. Nagai et al[49] recruited 97 T2DM individuals that were divided into two groups according to baseline EAT thickness and underwent therapy with pioglitazone, along with EAT thickness measurement, at the beginning and after a nine-month follow-up period. Pioglitazone reduced the EAT thickness in both groups, with more prominent results in the subjects that had a greater EAT depot at baseline.
A different TZD, rosiglitazone, when administered to mice, induced the expression of brown adipose tissue-specific proteins by the EAT, a tissue type normally presenting having a hormonal profile consistent with that of white adipose tissue[50]. Brown adipose tissue has been linked to high rates of lipid turnover and reduced body weight, while it is essential for thermogenesis and homeostasis, in contrast to white adipose tissue that serves as an energy reservoir for the body[51].
The data derived from the studies examining the effect of glitazones and EAT correlates with the established theory that TZD-induced weight gain is not concurrent with VAT deposition. Moreover, TZDs appear to have a favorable effect on EAT both by regulation of endocrine functions and mass reduction.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that delays gastric motility, supresses appetite, stimulates glucose-dependent insulin and decreases glucagon secretion[52]. The enzyme dipeptidyl peptidase-4 (DPP-4) deactivates GLP-1 interrupting all incretin-stimulated signalling. DPP-4 inhibitors (DPP-4i) are one of the two categories of antidiabetic drugs acting on the incretin pathway, the other being GLP-1 receptor agonists (GLP-1 RA)[52]. DPP-4i inhibit both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) degradation, thereby increasing plasma concentrations and stimulating the pancreatic β-cell in order to better regulate glucose homeostasis.
The class of DPP-4is includes sitagliptin, vildagliptin, saxagliptin, linagliptin and alogliptin[53]. Sitagliptin is the only DPP-4i whose effect on epicardial fat has been studied at this point in time. Lima-Martínez et al[39] formed a 24-wk interventional plan for 26 obese subjects with T2DM inadequately controlled on metformin monotherapy. Subjects meeting the inclusion criteria were introduced to a new regimen, receiving sitagliptin/metformin at a dosage of 50 mg/1000 mg respectively, twice a day. EAT deposits were reduced in size by approximately 15% (from 9.98 ± 2.63 to 8.10 ± 2.11 mm, P = 0.001) while the percentage of reduction in EAT was analogous to that of VAT (r = 0.456, P = 0.01).
While the aforementioned study establishes a favourable effect of sitagliptin on the mass of epicardial VAT, there is definite need for further research, in order to establish the reduction of EAT as a class effect of DPP-4is[39].
GLP-1 RAs utilize the “incretin effect”, similarly to DPP-4 inhibitors, so as to attenuate the diabetes-induced hyperglycemia. GLP-1 RAs are divided into short- and long-acting compounds that activate the GLP-1 receptor in a manner similar to that of the endogenous GLP-1[54]. Epicardial adipocytes have been shown to express GLP-1 and 2 receptor genes by use of RNA sequencing, while the possible quantity and dispersion pattern of the receptors in vivo has not been described[55]. Furthermore, GLP-1 and GLP-1 receptor signaling affect the differentiation and growth of adipocytes by regulation of fatty acid synthase activity[56]. Even though, the effects of numerous GLP-1 RAs have been studied in correlation to the metabolic regulation or mass reduction of visceral adipose tissue, the clinical trials concerning organ-specific deposits are few[40,57-60]. Current data on EAT remodeling by GLP-1 RAs is derived by two studies, conducted with liraglutide and exenatide[40,60] .
A trial designed by Iacobellis et al[40] included 95 T2DM obese subjects with hemoglobin A1c ≤ 8% while being treated with metformin. The patients were randomized into two groups to either receive a combination of metformin/liraglutide, with the latter being administered once daily, in doses up to 1.8 mg, or stay on metformin monotherapy, up to 1000 mg administered twice daily for 6 mo. EAT thickness measurements were acquired by ultrasound imaging at baseline and at 3 and 6 mo. Subjects in the liraglutide group presented with a decline in EAT thickness, 29% and 36% reduction from baseline at 3 and 6 mo respectively. Given that there were no similar changes in the metformin group, the EAT mass reduction is considered to be an effect of the liraglutide treatment, or possibly a result of the synergy between the two antidiabetic substances.
The study involving exenatide had a broader spectrum than that of liraglutide, examining the effect of the GLP-1RA on numerous VAT depots including epicardial, myocardial, hepatic and pancreatic adipose pads. Measurements of EAT thickness were performed by magnetic resonance imaging and spectroscopy at baseline and at 26 wk. A total of 44 obese individuals with uncontrolled T2DM, originally receiving oral therapy, were randomized to two groups, either receiving exenatide or other treatment chosen according to the local guidelines. EAT was reduced by approximately 8.8% after treatment with exenatide and by 1.2% on the patients receiving oral therapy, with the difference between the two being statistically significant (P = 0.003)[60].
Current research conducted on incretin treatment and ectopic adipose tissue deposition supports the theory that EAT reduction could be a class effect of GLP-1RAs and possibly a mediator of their beneficial actions on cardiovascular disease in the diabetic and obese subjects.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of antidiabetic substances that bind on the SGLT2 transporter in the proximal tubule of the kidney, facilitating glucose excretion via hindering reabsorption. SGLT2-mediated reabsorption constitutes the main pathway by which the renal system maintains glucose homeostasis[61]. Administration of SGLT2 inhibitors in obese individuals with T2DM has been linked with abdominal VAT size reduction[62]. Additionally, the effects of SGLT2 inhibition on tissue-specific depots such as EAT have been clarified by studies performed on luseogliflozin, ipragliflozin, canagliflozin and dapagliflozin[63-67].
EAT measurements following a 12-wk period of luseogliflozin administration demonstrate that treatment with luseogliflozin can reduce EAT volume in combination with adipocyte-related inflammation and metabolic dysregulation on type 2 diabetic patients. Along with EAT, numerous parameters were modified after luseogliflozin therapy including body weight, fasting plasma glucose, insulin resistance and C-reactive protein (CRP) levels. A positive correlation was established between CRP and EAT reduction (r = 0.493, P = 0.019), suggesting a concurrent effect of the SGLT2 inhibitor on both the adipose tissue mass and metabolic activity[63].
Similar results concerning both EAT and biomarkers reduction were acquired after ipragliflozin administration, in a study designed similarly to that conducted for luseogliflozin. The two models differed in the selection of the study population, with luseogliflozin treatment being applied to obese subjects while ipragliflozin was administered to non-obese T2DM individuals[64].
Yagi et al[65] studied the interaction of canagliflozin and EAT during a 6-mo period of treatment. The sample consisted of type 2 diabetic individuals, each of which was administered 100 mg of canagliflozin once daily. During the follow-up period EAT was evaluated by echocardiographic imaging while VAT and SCAT size fluctuation was monitored by use of impedance methods. The mean EAT thickness values were 9.3 mm and 7.3 mm at baseline and at 6 mo, respectively, with the change observed being statistically significant (P < 0.001) while there was only a trend for VAT and SCAT reduction.
Dapagliflozin and epicardial adiposity were examined through two different clinical trials, studying both the shift in metabolic activity and size of the adipocytes after treatment[66,67]. The metabolic profile of adipocytes promoted by dapagliflozin was assessed ex vivo on fat explants obtained from patients undergoing cardiac surgery on a trial designed by Díaz-Rodríguez et al[66]. Glucose uptake, transporter expression and adipokine secretion patterns were altered as a result of dapagliflozin application, a change indicative of a positive metabolic reform of the tissue induced by SGLT2 inhibition. Simultaneously, Sato et al[67] followed a more conventional approach, estimating the dapagliflozin-induced EAT volume reduction, by means of computed tomography imaging. Individuals receiving both dapagliflozin and other regimens for T2DM control were observed for 6 mo, with biomarker and EAT measurement at baseline and following completion of the study. While the two groups had similar EAT size measurement before the initiation of dapagliflozin therapy, the patients receiving the SGLT2 inhibitor presented with a greater reduction of epicardial VAT volume after treatment (-16.4 ± 8.3 for the dapagliflozin vs 4.7 ± 8.8 cm3 for the control group, P = 0.01), combined with lowered plasma levels of inflammatory adipokines.
Numerous studies conducted on many members of the SGLT2 inhibitor class of antidiabetic substances support the conclusion that EAT undergoes a multifaceted remodelling after SGLT2 inhibition, a trend that could be considered a class effect. The interconnection established between SGLT2 inhibitors and a known factor of cardiovascular risk such as epicardial adiposity could elucidate the manner by which the members of this class are cardioprotective, while, providing grounds for further therapeutic targeting of EAT (Table 1).
| Antidiabetic drug | Effect on epicardial adipose tissue |
| Biguanides | No effect/Possible synergistic effect with DPP-4 and/or GLP-1[39,40] |
| Alpha-Glucosidase Inhibitors | Lack of data concerning the effect of this class |
| Thiazolidinediones | Decreased inflammatory cytokine release and thickness of EAT (pioglitazone) modulation of cellular hormonal profile (rosiglitazone)[49,50] |
| Dipeptidyl peptidase-4 inhibitors | Reduction of EAT thickness (sitagliptin)[39] |
| Glucagon-like peptide-1 receptor agonists | Reduction of EAT thickness (liraglutide and exenatide)[40,60] |
| Sodium-glucose cotransporter 2 inhibitors | Reduction of EAT thickness (luseogliflozin, ipragliflozin, canagliflozin, dapagliflozin) and inflammation (luseogliflozin, ipragliflozin, dapagliflozin)[63-67] |
Epicardial adipose tissue exhibits a unique metabolic and pathophysiologic profile, as a result of its anatomical location and its cellular composition, rendering it an appealing therapeutic target for reducing cardiovascular risk and enabling endocrine homeostasis in the dysmetabolic individual. The recent studies concerning the effect of the antidiabetic substances on the multifactorial cardiomyopathy of the diabetic patient and, by extension, on epicardial adiposity, have yielded interesting results that support the use of treatment for a targeted approach, in order to reduce the size and metabolic activity of ectopic adipose tissue clusters. Despite the capacity of certain treatment regimens, mostly newer agents like GLP-1 agonists and SGLT-2 inhibitors, in the manipulation of both structural and functional parameters of the epicardial adipose tissue, the clinical efficacy of this approach remains unsubstantiated for the time being. There is definite need for further research, in order to elucidate whether the targeting of epicardial adiposity facilitates the procurement of better outcomes for individuals with diabetes and cardiovascular disease, while, additionally, clarify the manner by which the antidiabetic substances can attain such results.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Baptista LS, Nakhoul FM, Psychari SN S- Editor: Cui LJ L- Editor: A E- Editor: Yin SY
| 1. | Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1159] [Cited by in F6Publishing: 1274] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 2. | Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. 2012;13 Suppl 2:30-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Fang L, Guo F, Zhou L, Stahl R, Grams J. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte. 2015;4:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Poret JM, Souza-Smith F, Marcell SJ, Gaudet DA, Tzeng TH, Braymer HD, Harrison-Bernard LM, Primeaux SD. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int J Obes (Lond). 2018;42:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | La Fountaine MF, Cirnigliaro CM, Kirshblum SC, McKenna C, Bauman WA. Effect of functional sympathetic nervous system impairment of the liver and abdominal visceral adipose tissue on circulating triglyceride-rich lipoproteins. PLoS One. 2017;12:e0173934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Raucci R, Rusolo F, Sharma A, Colonna G, Castello G, Costantini S. Functional and structural features of adipokine family. Cytokine. 2013;61:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015;96:124-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol. 2016;27:13-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101:e18-e28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Gulgun M, Genç FA. Measurement of Epicardial Fat Thickness by Echocardiography Presents Challenges. Arq Bras Cardiol. 2016;107:497-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992-3000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Nagy E, Jermendy AL, Merkely B, Maurovich-Horvat P. Clinical importance of epicardial adipose tissue. Arch Med Sci. 2017;13:864-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2249] [Cited by in F6Publishing: 2633] [Article Influence: 438.8] [Reference Citation Analysis (0)] |
| 16. | Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 768] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 17. | Sato F, Maeda N, Yamada T, Namazui H, Fukuda S, Natsukawa T, Nagao H, Murai J, Masuda S, Tanaka Y. Association of Epicardial, Visceral, and Subcutaneous Fat With Cardiometabolic Diseases. Circ J. 2018;82:502-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | McKenney-Drake ML, Rodenbeck SD, Bruning RS, Kole A, Yancey KW, Alloosh M, Sacks HS, Sturek M. Epicardial Adipose Tissue Removal Potentiates Outward Remodeling and Arrests Coronary Atherogenesis. Ann Thorac Surg. 2017;103:1622-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Zhang A, Hamilton DJ, Deng T. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist Debakey Cardiovasc J. 2017;13:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, Cha S, Stepanek J, Hurst RT. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 22. | Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 23. | Guaraldi G, Scaglioni R, Zona S, Orlando G, Carli F, Ligabue G, Besutti G, Bagni P, Rossi R, Modena MG. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25:1199-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Petta S, Argano C, Colomba D, Cammà C, Di Marco V, Cabibi D, Tuttolomondo A, Marchesini G, Pinto A, Licata G. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Brouha SS, Nguyen P, Bettencourt R, Sirlin CB, Loomba R. Increased severity of liver fat content and liver fibrosis in non-alcoholic fatty liver disease correlate with epicardial fat volume in type 2 diabetes: A prospective study. Eur Radiol. 2018;28:1345-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Calabuig Á, Barba J, Guembe MJ, Díez J, Berjón J, Martínez-Vila E, Irimia P, Toledo E. Epicardial Adipose Tissue in the General Middle-aged Population and Its Association With Metabolic Syndrome. Rev Esp Cardiol (Engl Ed). 2017;70:254-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Fernández Muñoz MJ, Basurto Acevedo L, Córdova Pérez N, Vázquez Martínez AL, Tepach Gutiérrez N, Vega García S, Rocha Cruz A, Díaz Martínez A, Saucedo García R, Zárate Treviño A. Epicardial adipose tissue is associated with visceral fat, metabolic syndrome, and insulin resistance in menopausal women. Rev Esp Cardiol (Engl Ed). 2014;67:436-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12:31-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 30. | Blumensatt M, Fahlbusch P, Hilgers R, Bekaert M, Herzfeld de Wiza D, Akhyari P, Ruige JB, Ouwens DM. Secretory products from epicardial adipose tissue from patients with type 2 diabetes impair mitochondrial β-oxidation in cardiomyocytes via activation of the cardiac renin-angiotensin system and induction of miR-208a. Basic Res Cardiol. 2017;112:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Homsi R, Sprinkart AM, Gieseke J, Meier-Schroers M, Yuecel S, Fischer S, Nadal J, Dabir D, Luetkens JA, Kuetting DL. Cardiac magnetic resonance based evaluation of aortic stiffness and epicardial fat volume in patients with hypertension, diabetes mellitus, and myocardial infarction. Acta Radiol. 2018;59:65-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Cernea S, Blendea C, Roiban AL, Benedek T. Cardio-renal Correlations and Epicardial Adipose Tissue in Patients with Type 2 Diabetes. J Interdiscip Med. 2017;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Uygur B, Celik O, Ozturk D, Erturk M, Otcu H, Ustabasıoglu FE, Yıldırım A. The relationship between location-specific epicardial adipose tissue volume and coronary atherosclerotic plaque burden in type 2 diabetic patients. Kardiol Pol. 2017;75:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Wang Z, Zhang Y, Liu W, Su B. Evaluation of Epicardial Adipose Tissue in Patients of Type 2 Diabetes Mellitus by Echocardiography and its Correlation with Intimal Medial Thickness of Carotid Artery. Exp Clin Endocrinol Diabetes. 2017;125:598-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol. 2017;595:3907-3917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 36. | Psychari SN, Rekleiti N, Papaioannou N, Varhalama E, Drakoulis C, Apostolou TS, Iliodromitis EK. Epicardial Fat in Nonalcoholic Fatty Liver Disease: Properties and Relationships With Metabolic Factors, Cardiac Structure, and Cardiac Function. Angiology. 2016;67:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Song R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care. 2016;39:187-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1214] [Article Influence: 173.4] [Reference Citation Analysis (0)] |
| 39. | Lima-Martínez MM, Paoli M, Rodney M, Balladares N, Contreras M, D’Marco L, Iacobellis G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: a pilot study. Endocrine. 2016;51:448-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring). 2017;25:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 41. | Tokubuchi I, Tajiri Y, Iwata S, Hara K, Wada N, Hashinaga T, Nakayama H, Mifune H, Yamada K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS One. 2017;12:e0171293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Watanabe T, Watanabe-Kominato K, Takahashi Y, Kojima M, Watanabe R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr Physiol. 2017;7:765-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 43. | Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995;18:303-311. [PubMed] [Cited in This Article: ] |
| 44. | Davidson MA, Mattison DR, Azoulay L, Krewski D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol. 2018;48:52-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Blanchard PG, Turcotte V, Côté M, Gélinas Y, Nilsson S, Olivecrona G, Deshaies Y, Festuccia WT. Peroxisome proliferator-activated receptor γ activation favours selective subcutaneous lipid deposition by coordinately regulating lipoprotein lipase modulators, fatty acid transporters and lipogenic enzymes. Acta Physiol (Oxf). 2016;217:227-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Aghamohammadzadeh N, Niafar M, Dalir Abdolahinia E, Najafipour F, Mohamadzadeh Gharebaghi S, Adabi K, Dalir Abdolahinia E, Ahadi H. The effect of pioglitazone on weight, lipid profile and liver enzymes in type 2 diabetic patients. Ther Adv Endocrinol Metab. 2015;6:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115 Suppl 8A:42S-48S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care. 2011;34:730-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Nagai H, Ito H, Iwakura K. Abstract 710: Pioglitazone Treatment Reduces Epicardial Fat in Patients with Type 2 Diabetes Mellitus and Improves Left Ventricular Diastolic Function. Circulation. 2008;118. [Cited in This Article: ] |
| 50. | Distel E, Penot G, Cadoudal T, Balguy I, Durant S, Benelli C. Early induction of a brown-like phenotype by rosiglitazone in the epicardial adipose tissue of fatty Zucker rats. Biochimie. 2012;94:1660-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Salazar J, Luzardo E, Mejías JC, Rojas J, Ferreira A, Rivas-Ríos JR, Bermúdez V. Epicardial Fat: Physiological, Pathological, and Therapeutic Implications. Cardiol Res Pract. 2016;2016:1291537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Vella A. Mechanism of action of DPP-4 inhibitors--new insights. J Clin Endocrinol Metab. 2012;97:2626-2628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care. 2011;34 Suppl 2:S276-S278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 811] [Cited by in F6Publishing: 829] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 55. | Iacobellis G, Camarena V, Sant DW, Wang G. Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Horm Metab Res. 2017;49:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y, Zong C, Qin D, Wang Y, Yingfeng Yang Y. GLP-1/GLP-1R Signaling in Regulation of Adipocyte Differentiation and Lipogenesis. Cell Physiol Biochem. 2017;42:1165-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Pastel E, Joshi S, Knight B, Liversedge N, Ward R, Kos K. Effects of Exendin-4 on human adipose tissue inflammation and ECM remodelling. Nutr Diabetes. 2016;6:e235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H, Liang H, Weng J. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia. 2016;59:1059-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Wang XC, Gusdon AM, Liu H, Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol. 2014;20:14821-14830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, Ronsin O, Pradel V, Lesavre N, Martin JC. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 61. | Jabbour SA. SGLT2 inhibitors to control glycemia in type 2 diabetes mellitus: a new approach to an old problem. Postgrad Med. 2014;126:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Tosaki T, Kamiya H, Himeno T, Kato Y, Kondo M, Toyota K, Nishida T, Shiroma M, Tsubonaka K, Asai H. Sodium-glucose Co-transporter 2 Inhibitors Reduce the Abdominal Visceral Fat Area and May Influence the Renal Function in Patients with Type 2 Diabetes. Intern Med. 2017;56:597-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 64. | Fukuda T, Bouchi R, Terashima M, Sasahara Y, Asakawa M, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H. Ipragliflozin Reduces Epicardial Fat Accumulation in Non-Obese Type 2 Diabetic Patients with Visceral Obesity: A Pilot Study. Diabetes Ther. 2017;8:851-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, Salim HM, Maimaituxun G, Nishio S, Takagawa Y. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 66. | Díaz-Rodríguez E, Agra RM, Fernández ÁL, Adrio B, García-Caballero T, González-Juanatey JR, Eiras S. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res. 2018;114:336-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 67. | Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |