Review Article Open Access
Celiac Disease Review
Sandeep Nijhawan* and Gopal Goyal
Department of Gastroenterology, Sawai Man Singh Medical College, J L N Marg, Jaipur, 302 004, India
- *Corresponding Author:
- Sandeep Nijhawan
Department of Gastroenterology
Sawai Man Singh Medical College
J L N Marg, Jaipur
302 004, India
Tel: 9829272233
E-mail: dr_nijhawan@yahoo.com
Received date: September 29, 2015 Accepted date: October 26, 2015, Published date: November 04, 2015
Citation: Nijhawan S, Goyal G (2015) Celiac Disease Review. J Gastrointest Dig Syst 5:350. doi:10.4172/2161-069X.1000350
Copyright: © 2015 Nijhawan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Celiac disease (CD) is a systemic, immune-mediated disorder that primarily affect small intestine and triggered by dietary gluten in genetically susceptible individuals. Gluten is a water insoluble protein complex, which is found in wheat, rye and barley. A significant finding of celiac disease is villous atrophy of small intestine which leads to nutrient malabsorption and broad range of clinical manifestations.
Keywords
Celiac disease; Epidemiology
Introduction
Celiac disease (CD) is a systemic, immune-mediated disorder that primarily affect small intestine and triggered by dietary gluten in genetically susceptible individuals. Gluten is a water insoluble protein complex which is found in wheat, rye and barley. A significant finding of celiac disease is villous atrophy of small intestine which leads to nutrient malabsorption and broad range of clinical manifestations [1].
A recent International Consensus has defined three categories of adverse effects caused by wheat ingestion: (1) the autoimmune reaction classified as CD, dermatitis herpetiformis (DH) and gluten ataxia (2) allergic reactions and (3) non-celiac gluten sensitivity (NCGS) [2].
Epidemiology
Prevalence of celiac disease is approximately 0.6 to 1.0% worldwide, with wide regional differences [3]. The highest prevalence rate of the disease is noted in the Saharawi people of Africa in whom celiac disease prevalence is believed to be around 5% [4]. A recent multinational study in Europe found a big differences in CD prevalence with the lowest prevalence (0.3%) in Germany and the highest in Finland (2.4%) [5]. The frequency of celiac disease is increasing in many developing countries also. India has CD predominance in northern part of country where the prevalence is around 1.04% [6].
CD is prevalent in the first-degree relatives of patients with CD and it has been found to be 4.8% [7]. Book et al and Farre et al observed that the siblings (7.2%) are more affected than the parents (1.8%) [8,9].
Genetic background plays a key role in the predisposition to the disease. Majority (90%) of celiac disease patients express HLA-DQ2 haplotype (DQA1 0501/DQB1 0201). Another 5% of patients express HLA-DQ8 haplotype (DQA1 0301/DQB1 0302) [10]. Children with HLA haplotype DR3-DQ2 homozygote are at higher risk for celiac disease especially early in childhood by the age of 5 years [11].
The infants with family risk of developing CD with HLA-DQ2 haplotype influence the early gut microbiota composition and associated with intestinal dysbiosis and may contribute to determining disease risk [12]. Most patients are carriers of the HLA-DQ2/DQ8 genes but these genes are also present in about 40% of the general population, and only a small percentage (2-5%) develops CD [13,14] this indicate that the HLA-DQ genotype is necessary but not solely responsible for the development of the disease.
Clinical Presentation
Celiac disease is now being recognized as a systemic disease that may affect persons of any age, races and ethnic groups [15].
Traditionally the subtypes of the disease include typical, atypical, latent, silent and potential CD. The “typical” form is characterized by signs and symptoms caused by enteropathy and the consequent GI malabsorption, including diarrhea, weight loss, steatorrhoea and hypoalbuminemia. The “atypical” form does not present with weight loss and diarrhea but the clinical manifestation can nevertheless involve the GI, liver or organs other than the GI skin, nervous system, bones, metabolism, and reproductive system.
Oslo definitions discourage the term “silent” CD because it is equivalent to asymptomatic CD. They suggest instead, classifying as “subclinical CD” the forms that are below the threshold of clinical detection and whose signs and symptoms are not sufficient to fall within the CD diagnostic algorithm [16]. The Oslo definitions highlight the confusion regarding the term “latent” CD, and discourage its use. Instead, they recommend using the term “potential” CD for individuals without intestinal damage who are at increased risk of developing the disease as indicated by the presence of CD autoantibodies
Clinical presentation of celiac disease varies greatly according to age group. Paediatric patients of CD present more often with typical features than adult patients. A study has shown that diarrhoea is the presenting symptom in 74% of children as compared to 58.7% of adolescent/adults [17]. More than half of adult patients with celiac disease present with atypical manifestations [18]. Other manifestations of CD are chronic fatigue, aphthous stomatitis, short stature and reduced bone mineral density [1]. Celiac disease patients also have some nutritional deficiencies including iron, vitamin D, folate, vitamin B12, vitamin B6 and zinc [19]. Iron deficiency has been reported in up to half of newly diagnosed adults and in itself is an indication for screening [20].
CD patients who present with anaemia are more likely to have severe villous atrophy and a low bone mass density than patients who present with diarrhoea at the time of diagnosis [21]. Unusual manifestations of celiac disease include dermatitis herpetiformis, gluten ataxia and celiac crisis, a rare life-threatening syndrome mostly observed in children [1]. Neurological disorders like peripheral neuropathy, seizure disorders, ataxia and impaired cognitive function most often have been described in adults with CD at a lower incidence than in children [22,23]. In CD, hypertransaminasemia is often a subclinical finding. Rarely CD can be associated with severe liver disease also [24].
Increased Risk of other Diseases
Individuals with CD are at increased risk of non-alcoholic fatty liver disease (NAFLD) compared to the general population, excess risk is highest in the first year after CD diagnosis but persist throughout 15 years after the diagnosis of celiac disease [25]. Patients with celiac disease have an almost 3-fold increase in risk of developing pancreatitis [26].
Autoimmune thyroid disease and type I diabetes mellitus are the most common autoimmune diseases that occur with celiac disease. People with CD have 2.4-fold increased risk for type I diabetes mellitus before they are 20 years old [27]. CD is observed in approximately 7% of patients with autoimmune thyroid disorders [28]. Associations with Sjögren syndrome, Addison’s disease, parathyroid disorders, and growth hormone deficiency also have been reported [29].
CD affects approximately 2.5% of cirrhotic patients [30]. A study has shown that liver diseases are most commonly associated with CD [31]. Irritable bowel syndrome (IBS)-type symptoms occur frequently in patients with CD and are more common than general population. The pooled prevalence of IBS-type symptoms in all patients with CD is 38.0% [32]. CD is associated with small intestinal bacterial overgrowth (SIBO). In a study, it has been shown that large number of celiac patients from North India suffers from bacterial overgrowth (20.7%) which can be accordingly treated with antibiotics [33].
Celiac disease with pregnancy
Undiagnosed celiac disease during pregnancy has been associated with adverse outcomes. Many patients with celiac disease have malabsorption, weight loss, and anaemia. A nationwide study found that there is an increased risk for malformations among the offspring of mothers or fathers with celiac disease. However, the excess risk is small [34].
Non celiac gluten sensitivity (NCGS)
Present with symptoms which are often indistinguishable from CD. NCGS is characterized by intestinal and extra intestinal symptoms that occur soon after gluten ingestion, disappear during a gluten-free diet and relapse when the diet is interrupted. The main gastrointestinal symptoms are bloating, abdominal pain, diarrhea, and constipation. The extra intestinal symptoms include eczema, skin rashes, muscle and joint pains, leg or arm numbness, headache, “foggy mind” a sense of fatigue and depression. Several studies have suggested a link between gluten intakes and neuropsychiatric disorders particularly autism and schizophrenia [35].
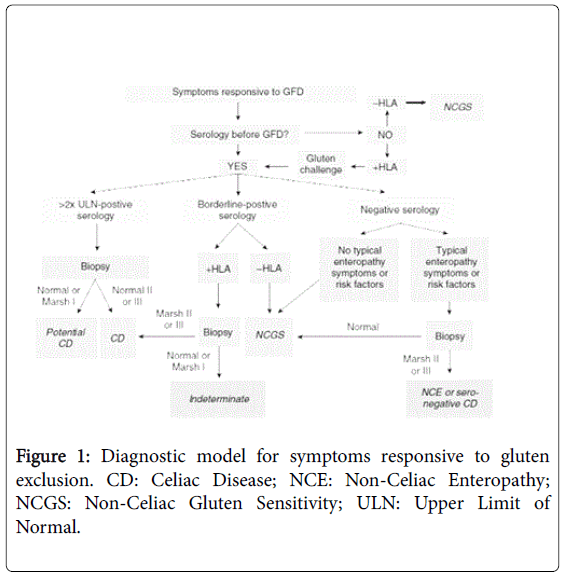
NCGS patients are distinguishable from CD by serology and biopsy as they are negative for more specific markers of CD such as tTG, EMA, and anti-DGP [36]. The prevalence of HLA-DQ2-DQ8 is about 50%, more than the 30% found in the general population but considerably below the prevalence among celiac patients. Intestinal biopsy is negative for CD in patients with NCGS. The mucosa appears without graded histological lesions, usually Marsh 0 or Marsh 1 type. A diagnosis of non-celiac gluten sensitivity should be considered only after CD has been excluded with appropriate testing [37]. An approach to diagnosis of NCGS and its differentiation from CD after appropriate testing has shown in Figure 1 [38].
Complication of Celiac Disease
Complications associated with untreated celiac disease include osteoporosis, impaired splenic function, neurologic disorders, infertility or recurrent abortion, ulcerative jejunoileitis and carcinomas [39].
Celiac disease with malignancy
Patients with CD have an increased risk of cancer in comparison to general population. Increased risk of malignancy is seen in oesophageal adenocarcinoma, small intestinal adenocarcinoma, non- Hodgkin's lymphoma and melanoma. Adenocarcinoma of the small intestine is rare but this risk is increased many folds in patients with celiac disease but overall risk is very low. The non-Hodgkin's lymphoma includes both T-cell and B-cell types and occur in both gastrointestinal and extraintestinal sites. The risk of non-Hodgkin's lymphoma persisted despite a gluten-free diet [40]. Most lymphomas complicating celiac disease are indeed related to the disease and are not of the enteropathy-type T-cell lymphoma (ETTL) [41].
The most common type is diffuse large B-cell lymphoma and carrying a better prognosis than T-cell lymphoma. Therapy is unsatisfactory for enteropathy-type T-cell lymphoma [42].
Diagnosis of celiac disease: The confirmation of a diagnosis of CD should be based on a combination of findings from the medical history, physical examination, serology and histological analysis of multiple biopsies of the duodenum.
Serology: Serological tests are fundamental for CD screening. Immunoglobulin A (IgA) anti-tissue transglutaminase (anti-tTG) antibody is the preferred single test in individuals over the age of 2 years who do not have concomitant IgA deficiency [43]. The sensitivity and specificity of various serological tests are shown in Table 1 [44,45].
| Test | Sensitivity (Range) Percent | Specificity (Range) | Comments |
|---|---|---|---|
| IgA anti-tTG antibodies | >95.0 (73.9-100) | >95.0 (77.8-100) | Recommended as first level screening test |
| IgG anti-tTG antibodies | Widely variable (12.6-99.3) | Widely variable (86.3-100) | Useful in patients with IgA deficiency |
| IgA antiendomysial antibodies | >90.0 (82.6-100) | 98.2 (94.7-100) | Useful in patients with an uncertain diagnosis |
| IgG DGP | >90.0 (80.1-98.6) | >90.0 (86.0-96.9) | Useful in patients with IgA deficiency and young children |
| HLA-DQ2 Or HLA-DQ8 |
91.0 (82.6-97.0) | 54.0 (12.0-68.0) | High negative predictive value |
Table 1: Various serum tests for diagnosis of celiac disease.
The sensitivity of serologic testing is markedly reduced in patients with a gluten-restricted diet, therefore patients should not restrict their diet before testing.
In persons with IgA deficiency, IgG anti-tTG antibodies can be measured. Measurement of deamidated gliadin peptide antibodies of the IgG class (IgG DGP), as an alternative test has better sensitivity and specificity than measurement of IgG anti-tTG antibodies [46].
Small intestinal biopsy: Upper GI endoscopy with multiple biopsies of the duodenum (one or two biopsies of the bulb and at least four biopsies of the distal duodenum) are ecommended to confirm the diagnosis [47]. In a study it has been shown that villous atrophy may be present only in the duodenal bulb [48-50]. The characteristic histologic changes include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts and partial to total villous atrophy [51].
However lymphocytic infiltration also known as lymphocytic duodenosis is common in the general population (prevalence of 5.4%) [52]. Diagnosis of CD requires the demonstration of histological changes associated with the disease which can be classified according to Marsh, Marsh modified (Oberhuber), or the more recent simplified Corazza classification which are shown in Table 2 [53-55].
| Marsh modified (Oberhuber) | Histologic criterion | Corazza | ||
|---|---|---|---|---|
| Increased intraepithelial lymphocytesa | Crypt hyperplasia | Villous atrophy | ||
| Type0 | No | No | No | None |
| Type1 | Yes | No | No | Grade A |
| Type2 | Yes | Yes | No | |
| Type3a | Yes | Yes | Yes(partial) | Grade B1 |
| Type3b | Yes | Yes | Yes(subtotal) | |
| Type3c | Yes | Yes | Yes(total) | Grade B2 |
Table 2: Summary of histologic classification frequently used for celiac disease, a >40 intraepithelial lymphocytes per 100 enterocytes for Marsh modified (Oberhuber); >25 intraepithelial lymphocytes per 100 enterocytes for Corazza.
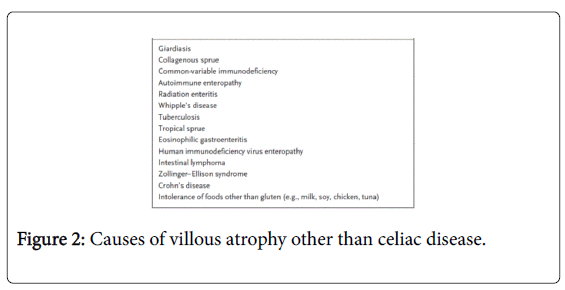
Lymphocytic infiltration of the intestinal epithelium in the absence of villous atrophy is not specific for CD and other causes should also be considered [51] (Figure 2).
In patients with an uncertain diagnosis such as patients with negative serology and positive results on biopsy, detection of sub epithelial anti-tTG IgA deposits by means of double immunofluorescencemay be useful [56].
Biopsy of the small intestine may not be required in children with typical symptoms, a high titre of anti-tTG IgA antibodies (higher than 10 times the upper limit of the normal range) and predisposing HLA genotypes [44].
Ancillary testing in CD: HLA-DQ2/DQ8 testing should not be used routinely in the initial diagnosis of CD. It should be used to effectively rule out the disease in selected clinical situations examples: Equivocal small-bowel histological finding (Marsh I-II) in seronegative patients, evaluation of patients on a GFD in whom no testing for CD was done before GFD, patients with discrepant celiac-specific serology and histology, patients with suspicion of refractory CD where the original diagnosis of celiac remains in question and in patients with Down’s syndrome [47,57]. Capsule endoscopy should be considered for the evaluation of small-bowel mucosa in patients with complicated CD [58,59].
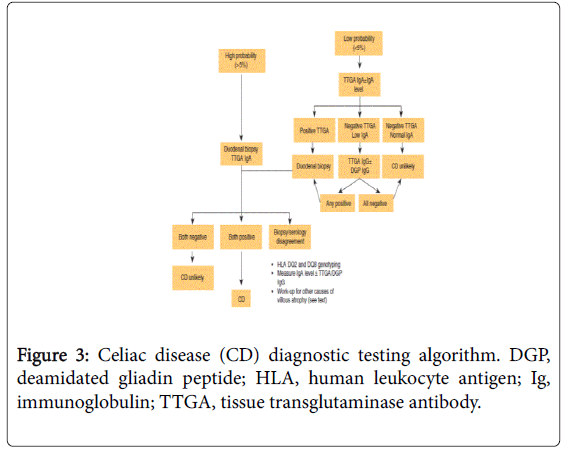
Intestinal permeability tests, D-xylose, and small-bowel followthrough are neither specific nor sensitive and are not recommended for CD diagnosis [47]. Considering the entire diagnostic test, following is the recommended algorithm for the diagnosis of celiac disease [47] (Figure 3).
Screening of celiac disease: Screening of CD Should be considered in patients with symptoms, signs or laboratory evidence suggestive of malabsorption. Patient with a first-degree family member who has a confirmed diagnosis of CD and showing possible signs or symptoms or laboratory evidence of CD. Celiac disease should be sought among the explanations for elevated serum aminotransferase levels when no other etiology is found. Patients with Type I diabetes mellitus (DM) should be tested for CD if they have any digestive symptoms and signs or laboratory evidence suggestive of CD [47].
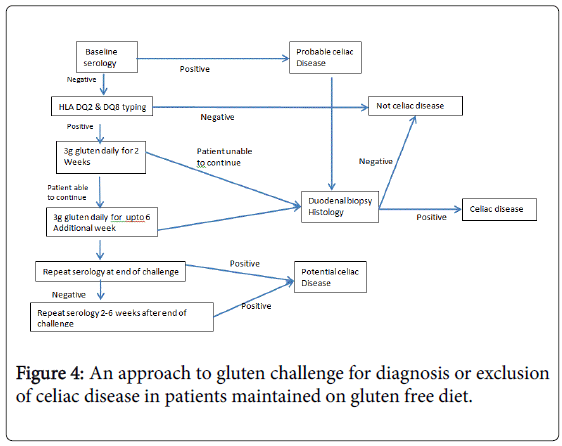
Diagnosis or exclusion of celiac disease in patients already on GFD: In this group of patients diagnosis begins with serologic tests and HLA typing. Positive results from serologic tests support a diagnosis of CD and indicate the need for duodenal biopsy analysis; conversely celiac disease is excluded for patients who are negative for HLA DQ2/ HLADQ8.
Gluten Challenge: Gluten challenge remains the gold standard for CD diagnosis in HLA-DQ2 or -DQ8-positive patients who have normal serologic and histologic findings when tested on a GFD. It must be noted that patients who develop severe symptoms following gluten ingestion are not suitable candidates for gluten challenge. Although gluten challenge with a diet containing at least 10 g of gluten per day for 6-8 weeks has long been the norm, there are few data to indicate the diagnostic efficacy of this approach or the optimum dose or duration of challenge.
A recent study found that even if a patient can only tolerate lower doses of gluten (3 g per day), diagnostic changes are seen in most CD patients after as little as 2 weeks of gluten ingestion [47]. An approach to gluten challenge for diagnosis or exclusion of celiac disease in patients maintained on gluten free diet has shown in Figure 4 [47].
Treatment of Celiac Disease
Treatment of CD involves a gluten free diet (i.e., a diet with no wheat, rye, or barley proteins) for lifelong. Although no gluten consumption is the ideal treatment for CD, a minimal degree of gluten contamination is difficult to avoid. The term “gluten free” indicates a diet that contains gluten at such a low level as to be considered harmless. The current international Codex Alimentarius defines gluten-free foods as having less than 20 ppm of gluten [1] the diet requires continuous education of patients and their families by both doctors and dieticians. A GFD will result in resolution of symptoms and repair of the intestinal damage over time in most people with CD. There is some evidence that people with untreated CD are more frequently deficient in a number of micronutrients compared to those without CD. Micronutrient deficiencies identified include iron, folic acid, and vitamin B12 and B6, copper, zinc and carnitine [60-64]. Low bone mineral density in people with untreated CD is believed to be partly due to vitamin D deficiency. Recommended gluten free diet has shown in Table 3 [51].
| Grains that should be avoided Wheat (includes spelt, kamut, semolina, triticale), rye, barley (including malt) |
| Safe grains (gluten-free) Rice, amaranth, buckwheat, corn, millet, quinoa, sorghum, teff (an Ethiopian cereal grain), oats |
| Sources of gluten-free starches that can be used as flour alternatives Cereal grains: amaranth, buckwheat, corn (polenta), millet, quinoa, sorghum, teff, rice (white, brown, wild, basmati, jasmine), montina (Indian rice grass) Tubers: arrow root, jicama, taro, potato, tapioca (cassava, manioc, yucca) Legumes: chickpeas, lentils, kidney beans, navy beans, pea beans, peanuts, Soybeans Nuts: almonds, walnuts, chestnuts, hazelnuts, cashews Seeds: sunflower, flax, pumpkin |
Table 3: Fundamentals of the Gluten free diet.
New Advances in Celiac Disease Treatment
Newer Strategies include developing agents to degrade or alter dietary gluten, prevent gluten peptides from crossing the epithelial barrier, inhibit tTG-induced potentiation of gliadin peptides or block gliadin binding to HLA-DQ2. Immune-based strategies involve preventing T-cell activation or innate and adaptive immune responses [65]. However only 2 agents are in late phase 2 clinical trials.
ALV003 (2 recombinant, orally administered, gluten-specific proteases) is given orally, reduce the small intestinal mucosal injury caused by 6 weeks of gluten challenge [66]. Larazotide acetate, an oral peptide that modulates intestinal tight junctions, reduce symptoms in patients who are symptomatic despite a GFD [67,68].
Additional studies are underway to determine if these agents are safe and effective for patients with persistent symptoms and mucosal injury despite a continued GFD. These or other effective therapies could reduce the burden of GFD by decreasing the effects of inadvertent gluten exposure.
Monitoring of celiac disease: CD is a lifelong inflammatory condition that affects multiple organ systems, so patients should be followed up routinely. Serologic testing should be done every 3-6 months until normal and then every 1-2 years. Nutritional evaluation for iron, vitamin B12, folate etc. should be done every 3-6 months until it becomes normal. Low bone mineral density is one of the more common extraintestinal manifestations of celiac disease, so dualenergy x-ray absorptiometry (DXA) evaluation generally is recommended in the first year after diagnosis. Thyroid functions monitoring should be done first at diagnosis and then in every 1-2 years [69].
The optimal timing of the follow-up biopsy is unclear as all celiac disease patients do not reach mucosal recovery in 1 year time despite strict gluten-free diet. In patients with high dietary adherence, incomplete villous recovery after 1 year does not affect the clinical response or long-term prognosis so personalized approach is required to decide the optimal timing of the follow-up biopsy [70].
Non responsive celiac disease (NRCD)
NRCD may be defined as persistent symptoms, signs or laboratory abnormalities typical of CD despite 6-12 months of dietary gluten avoidance. NRCD is common, affecting from 7 to 30% of patients treated with a GFD for CD [71-73]. There are many distinct etiologies including inadvertent gluten ingestion (the most common cause), other food intolerances (including lactose and fructose intolerance), small-intestinal bacterial overgrowth, microscopic colitis, pancreatic insufficiency, irritable bowel syndrome and refractory CD [71-75].
Thus careful evaluation is needed to identify and treat the specific source in any given patient.
The first step in evaluation is to re-confirm the initial diagnosis of CD by review of small-intestinal histology and serology obtained at the time of diagnosis [47].
Refractory Celiac Disease (RCD)
It may be defined as persistent or recurrent symptoms and signs of malabsorption with small-intestinal villous atrophy despite a strict GFD for more than 12 months and in the absence of other disorders including overt lymphoma. RCD is uncommon, affecting 1-2% of patients with CD [76]. Refractory celiac disease can be classified as type 1 (normal intraepithelial lymphocytes) or type 2 (abnormal intraepithelial lymphocytes; clonal intra epithelial lymphocytes lacking surface markers CD3, CD8, and T-cell receptors; or both). Type 2 RCD is associated with a higher risk of ulcerative jejunoileitis and lymphoma than type 1 and associated with a significantly less favourable prognosis as compared to Type I RCD [77].
Management of Type I RCD includes strict gluten free diet, nutritional support and in severe cases steroid therapy is required [78] in patients with an incomplete response to steroid treatment immunosuppressive agents such as azathioprine may be beneficial but should be used with caution, since they may promote the progression to lymphoma [79]. Management of Type II RCD is difficult because they are less likely respond to therapy. Treatment options include systemic corticosteroids, enteric-coated budesonide, azathioprine, alemtuzumab (anti-CD52 monoclonal antibody) infliximab or cladribine (2-chlorodeoxyadenosine) [80-82]. Transformation to enteropathy-associated T-cell lymphoma (EATCL) is a prominent risk and may require treatment by surgery, chemotherapy, or bone marrow transplantation. In some patients EATCL may run a prolonged, nonaggressive course but the overall prognosis remains poor [83,84].
Collagenous Sprue: It is characterized by deposition of collagen under the intestinal epithelial cells and associated with persistent diarrhea, progressive weight loss and severe malabsorption causing multiple nutrient deficiencies [85]. It has been noted in up to 36% of patients with classic celiac disease. Complications of celiac disease have been described in collagenous sprue, including small bowel ulceration and free perforation as well as both T cell and B cell lymphomas [86-89]. Collagenous sprue should be considered in the differential diagnosis of NRCD and must be distinguished from collagenous colitis (which rarely accompanies celiac disease).
References
- Fasano A, Catassi C (2012) Clinical practice. Celiac disease. N Engl J Med 367: 2419-2426.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, et al. (2012) Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 10: 13.
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, et al. (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 163: 286-292.
- Catassi C, Rätsch IM, Gandolfi L, Pratesi R, Fabiani E, et al. (1999) Why is coeliac disease endemic in the people of the Sahara? Lancet 354: 647-648.
- Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, et al. (2010) The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 42: 587-595.
- Makharia GK, Verma AK, Amarchand R, Bhatnagar S, Das P, et al. (2011) Prevalence of celiac disease in the northern part of India: a community based study. J Gastroenterol Hepatol 26: 894-900.
- DoÄŸan Y, Yildirmaz S, Ozercan IH (2012) Prevalence of celiac disease among first-degree relatives of patients with celiac disease. J Pediatr Gastroenterol Nutr 55: 205-208.
- Book L, Zone JJ, Neuhausen SL (2003) Prevalence of celiac disease among relatives of sib pairs with celiac disease in U.S. families. Am J Gastroenterol 98: 377-381.
- Farré C, Humbert P, Vilar P, Varea V, Aldeguer X, et al. (1999) Serological markers and HLA-DQ2 haplotype among first-degree relatives of celiac patients. Catalonian Coeliac Disease Study Group. Dig Dis Sci 44: 2344-2349.
- Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, et al. (2014) Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 371: 1295-1303.
- Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, et al. (2014) Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med 371: 42-49.
- Olivares M, Neef A, Castillejo G, Palma GD, Varea V, et al. (2015) The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 64: 406-417.
- Rossi M, Schwartz KB (2010) Editorial: Celiac disease and intestinal bacteria: not only gluten? J Leukoc Biol 87: 749-751.
- Romanos J, Rosén A, Kumar V, Trynka G, Franke L, et al. (2014) Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut 63: 415-422.
- (2004) NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements 21: 1-23.
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, et al. (2013) The Oslo definitions for coeliac disease and related terms. Gut 62: 43-52.
- Kochhar R, Jain K, Thapa BR, Rawal P, Khaliq A, et al. (2012) Clinical presentation of celiac disease among pediatric compared to adolescent and adult patients. Indian J Gastroenterol 31: 116-120.
- Makharia GK, Baba CS, Khadgawat R, Lal S, Tevatia MS, et al. (2007) Celiac disease: variations of presentations in adults. Indian J Gastroenterol 26: 162-166.
- Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA (2013) Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 5: 3975-3992.
- Harper JW, Holleran SF, Ramakrishnan R, Bhagat G, Green PH (2007) Anemia in celiac disease is multifactorial in etiology. Am J Hematol 82: 996-1000.
- Abu Daya H, Lebwohl B, Lewis SK, Green PH (2013) Celiac disease patients presenting with anemia have more severe disease than those presenting with diarrhea. Clin Gastroenterol Hepatol 11: 1472-1477.
- Freeman HJ (2008) Neurological disorders in adult celiac disease. Can J Gastroenterol 22: 909-911.
- Lionetti E, Francavilla R, Pavone P, Pavone L, Francavilla T, et al. (2010) The neurology of coeliac disease in childhood: what is the evidence? A systematic review and meta-analysis. Dev Med Child Neurol 52: 700-707.
- Kamath PS (1996) Clinical approach to the patient with abnormal liver test results. Mayo Clin Proc 71: 1089-1094.
- Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF (2015) Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol 62: 1405-1411.
- Sadr-Azodi O, Sanders DS, Murray JA, Ludvigsson JF (2012) Patients with celiac disease have an increased risk for pancreatitis. Clin Gastroenterol Hepatol 10: 1136-1142.
- Ludvigsson JF, Ludvigsson J, Ekbom A, Montgomery SM (2006) Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care 29: 2483-2488.
- Elfström P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF (2008) Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab 93: 3915-3921.
- Casella G, Antonelli E, Di Bella C, Villanacci V, Fanini L, et al. (2013) Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int 33: 1128-1131.
- Wakim-Fleming J, Pagadala MR, McCullough AJ, Lopez R, Bennett AE, et al. (2014) Prevalence of celiac disease in cirrhosis and outcome of cirrhosis on a gluten free diet: a prospective study. J Hepatol 61: 558-563.
- Nijhawan S, Katiyar P, Nagaich N, Saradava V, Nijhawan M, et al. (2013) Prevalence of associated disorders in Indian patients with celiac disease. Indian J Gastroenterol 32: 330-334.
- Sainsbury A, Sanders DS, Ford AC (2013) Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin Gastroenterol Hepatol 11: 359-365.
- Rana SV, Sinha SK, Lal S, Sikander A, Singh K (2007) Small intestinal bacterial overgrowth in North Indian patients with celiac disease. Trop Gastroenterol 28: 159-161.
- Zugna D, Richiardi L, Stephansson O, Pasternak B, Ekbom A (2014) Risk of Congenital Malformations among Offspring of Mothers and Fathers With Celiac Disease: A Nationwide Cohort Study.Clinical Gastroenterology and Hepatology 12: 1108-1116.
- Brusca I (2015) Overview of biomarkers for diagnosis and monitoring of celiac disease. Adv Clin Chem 68: 1-55.
- Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, et al. (2012) Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol 46: 680-685.
- A Sapone, KM Lammers, G Mazzarella, I Mikhailenko, M Carteni, et al. (2010) Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 152: 75-80.
- Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, et al. (2014) Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol 109: 741-746.
- Fasano A, Catassi C (2001) Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 120: 636-651.
- Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, et al. (2003) Risk of malignancy in patients with celiac disease. Am J Med 115: 191-195.
- Smedby KE, Akerman M, Hildebrand H, Glimelius B, Ekbom A, et al. (2005) Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut 54: 54-59.
- Halfdanarson TR, Rubio-Tapia A, Ristow KM, Habermann TM, Murray JA et al. (2010) Patients With Celiac Disease and B-Cell Lymphoma Have a Better Prognosis Than Those With T-Cell Lymphoma. Clinical gastroenterology and Hepatology 8: 1042-1127.
- Zintzaras E, Germenis AE (2006) Performance of antibodies against tissue transglutaminase for the diagnosis of celiac disease: meta-analysis. Clin Vaccine Immunol 13: 187-192.
- Husby S, Koletzko S, Korponay-Szabó IR, Mearin, ML, Phillips A, et al. (2012) European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 54: 136-60.
- Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, et al. (2012) Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr 54: 229-241.
- Tonutti E, Visentini D, Picierno A, Bizzaro N, Villalta D, et al. (2009) Diagnostic efficacy of the ELISA test for the detection of deamidated anti-gliadin peptide antibodies in the diagnosis and monitoring of celiac disease. J Clin Lab Anal 23: 165-171.
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology (2013) ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 108: 656-676.
- Evans KE, Aziz I, Cross SS, Sahota GR, Hopper AD, et al. (2011) A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol 106: 1837-1742.
- Gonzalez S, Gupta A, Cheng J, Tennyson C, Lewis SK, et al. (2010) Prospective study of the role of duodenal bulb biopsies in the diagnosis of celiac disease. Gastrointest Endosc 72: 758-765.
- Bonamico M, Thanasi E, Mariani P, Nenna R, Luparia RP, et al. (2008) Duodenal bulb biopsies in celiac disease: a multicenter study. J Pediatr Gastroenterol Nutr 47: 618-622.
- Green PH, Cellier C (2007) Celiac disease. N Engl J Med 357: 1731-1743.
- Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, et al. (2010) Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology 139: 112-119.
- Marsh MN (1992) Gluten, major histocompatibility complex, and the small intestine.A molecular and immunobiologic approach to the spectrum of gluten sensitivity (celiac sprue).Gastroenterology 102: 330-354.
- Oberhuber G (2000) Histopathology of celiac disease. Biomed Pharmacother 54: 368-372.
- Corazza GR, Villanacci V, Zambelli C, Milione M, Luinetti O, et al. (2007) Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol 5: 838-843.
- Salmi TT, Collin P, Korponay-Szabó IR, Laurila K, Partanen J, et al. (2006) Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 55: 1746-1753.
- Kaukinen K, Partanen J, Mäki M, Collin P (2002) HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol 97: 695-699.
- Barret M, Malamut G, Rahmi G, Samaha E, Edery J, et al. (2012) Diagnostic yield of capsule endoscopy in refractory celiac disease. Am J Gastroenterol 107: 1546-1553.
- Atlas DS, Rubio-Tapia A, Van Dyke CT, Lahr BD, Murray JA (2011) Capsule endoscopy in nonresponsive celiac disease. Gastrointest Endosc 74: 1315-1322.
- Haapalahti M, Kulmala P, Karttunen TJ, Paajanen L, Laurila K, et al. (2005) Nutritional status in adolescents and young adults with screen-detected celiac disease. J Pediatr Gastroenterol Nutr 40: 566-570.
- Tikkakoski S, Savilahti E, Kolho KL (2007) Undiagnosed coeliac disease and nutritional deficiencies in adults screened in primary health care. Scand J Gastroenterol 42: 60-65.
- Dickey W (2002) Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol 14: 425-427.
- Halfdanarson TR, Kumar N, Hogan WJ, Murray JA (2009) Copper deficiency in celiac disease. J Clin Gastroenterol 43: 162-164.
- Lerner A, Gruener N, Iancu TC (1993) Serum carnitine concentrations in coeliac disease. Gut 34: 933-935.
- Mukherjee R, Kelly CP, Schuppan D (2012) Nondietary therapies for celiac disease. Gastrointest Endosc Clin N Am 22: 811-831.
- Lähdeaho ML, Kaukinen K, Laurila K, Vuotikka P, Koivurova OP, et al. (2014) Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 146: 1649-1658.
- Kelly CP, Green PH, Murray JA, Dimarino A, Colatrella A, et al. (2013) Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther 37: 252-262.
- Leffler DA, Kelly CP, Green PH, Fedorak RN, DiMarino A, et al. (2015) Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 148: 1311-1319.
- Kelly CP, Bai JC, Liu E, Leffler DA (2015) Advances in diagnosis and management of celiac disease. Gastroenterology 148: 1175-1186.
- Pekki H, Kurppa K, Mäki M, Huhtala H, Sievänen H, et al. (2015) Predictors and Significance of Incomplete Mucosal Recovery in Celiac Disease After 1 Year on a Gluten-Free Diet. Am J Gastroenterol 110: 1078-1085.
- Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, et al. (2007) Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 5: 445-450.
- Abdulkarim AS, Burgart LJ, See J, Murray JA (2002) Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 97: 2016-2021.
- O'Mahony S, Howdle PD, Losowsky MS (1996) Review article: management of patients with non-responsive coeliac disease. Aliment Pharmacol Ther 10: 671-680.
- Tursi A, Brandimarte G, Giorgetti G (2003) High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol 98: 839-843.
- Fine KD, Meyer RL, Lee EL (1997) The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology 112: 1830-1838.
- Trier JS, Falchuk ZM, Carey MC, Schreiber DS (1978) Celiac sprue and refractory sprue. Gastroenterology 75: 307-316.
- Daum S, Cellier C, Mulder CJ (2005) Refractory coeliac disease. Best Pract Res Clin Gastroenterol 19: 413-424.
- Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, et al. (2000) Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 356: 203-208.
- Goerres MS, Meijer JW, Wahab PJ, Kerckhaert JA, Groenen PJ, et al. (2003) Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment Pharmacol Ther 18: 487-494.
- Gillett HR, Arnott ID, McIntyre M, Campbell S, Dahele A, et al. (2002) Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology 122: 800-805.
- Vivas S, Ruiz de Morales JM, Ramos F, Suárez-Vilela D (2006) Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N Engl J Med 354: 2514-2515.
- Al-Toma A, Goerres MS, Meijer JW, von Blomberg BM, Wahab PJ, et al. (2006) Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol 4: 1322-1327.
- Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, et al. (2007) Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 109: 2243-2249.
- Di Sabatino A, Biagi F, Gobbi PG, Corazza GR (2012) How I treat enteropathy-associated T-cell lymphoma. Blood 119: 2458-2468.
- Bossart R, Henry K, Booth CC, Doe WF (1975) Subepithelial collagen in intestinal malabsorption. Gut 16: 18-22.
- Freeman HJ, Webber DL (2009) Free perforation of the small intestine in collagenous sprue. World J Gastroenterol 15: 4446-4448.
- Freeman HJ (2003) Free perforation due to intestinal lymphoma in biopsy-defined or suspected celiac disease. J Clin Gastroenterol 37: 299-302.
- Robert ME, Ament ME, Weinstein WM (2000) The histologic spectrum and clinical outcome of refractory and unclassified sprue. Am J Surg Pathol 24: 676-687.
- Freeman HJ (2003) Collagenous sprue associated with an extensive T-cell lymphoma. J Clin Gastroenterol 36: 144-146.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14807
- [From(publication date):
December-2015 - Jun 04, 2024] - Breakdown by view type
- HTML page views : 13624
- PDF downloads : 1183