- Department of Neurology and Neurosurgery, Montreal Neurological Institute and Hospital, McGill University, Montreal, Quebec, Canada
- Department of Neurosurgery, Kurashiki Central Hospital, University of Kyoto, Okayama, Japan
- Department of Neurosurgery, Nagoya University Hospital, Nagoya University, Nagoya, Japan
- Division of Neurosurgery, St. Michael's Hospital, University of Toronto, Toronto, Ontario, Canada
- Department of Surgery, Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
- Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
- Department of Medicine, Clinical Epidemiology and Biostatistics, Division of Clinical Pharmacology, McMaster University, Hamilton, Canada
Correspondence Address:
Benjamin W. Y. Lo
Department of Medicine, Clinical Epidemiology and Biostatistics, Division of Clinical Pharmacology, McMaster University, Hamilton, Canada
DOI:10.4103/2152-7806.162677
Copyright: © 2015 Lo BWY. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Y. Lo BW, Fukuda H, Nishimura Y, Macdonald RL, Farrokhyar F, Thabane L, H. Levine MA. Pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms: A systematic review. Surg Neurol Int 11-Aug-2015;6:136
How to cite this URL: Y. Lo BW, Fukuda H, Nishimura Y, Macdonald RL, Farrokhyar F, Thabane L, H. Levine MA. Pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms: A systematic review. Surg Neurol Int 11-Aug-2015;6:136. Available from: http://surgicalneurologyint.com/surgicalint_articles/pathophysiologic-mechanisms-of-brain%e2%80%91body-associations-in/
Abstract
Background:Patients with ruptured brain aneurysms and aneurysmal subarachnoid hemorrhage suffer neurological damage from primary injury of the aneurysm rupture itself, as well as a number of secondary injurious processes that can further worsen the affected individual's neurological state. In addition, other body systems can be affected in a number of brain-body associations.
Methods:This systematic review synthesizes prospective and retrospective cohort studies that investigate brain-body associations in patients with ruptured brain aneurysms. The methodologic quality of these studies will be appraised.
Results:Six cohort studies were included in this systemic review. The methodologic quality of each study was assessed. They had representative patient populations, clear selection criteria and clear descriptions of study designs. Reproducible study protocols with ethics board approval were present. Clinical results were described in sufficient detail and were applicable to aneurysmal subarachnoid hemorrhage patients in clinical practice. There were few withdrawals from the study. Limitations included small sample sizes and between-study differences in diagnostic tests and clinical outcome endpoints. Several pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms were clarified through this systematic review. Sympathetic activation of the cardiovascular system in aneurysmal subarachnoid hemorrhage not only triggers the release of atrial and brain natriuretic peptides it can also lead to increased pulmonary venous pressures and permeability causing hydrostatic pulmonary edema. Natriuretic states can herald the onset or worsening of clinical vasospasm as the renin-angiotensin-aldosterone system is activated in a delayed manner.
Conclusions:This systematic review synthesizes the most current evidence of underlying mechanisms of brain related associations with body systems in aneurysmal subarachnoid hemorrhage. Results gained from these studies are clinically useful and shed light on how ruptured brain aneurysms affect the cardiopulmonary system. Subsequent neuro-cardio-endocrine responses then interact with other body systems as part of the secondary responses to primary injury.
Keywords: Aneurysmal subarachnoid hemorrhage, brain-body interactions, cardiopulmonary interactions, neuro-cardio-endocrine interactions, systematic review
INTRODUCTION
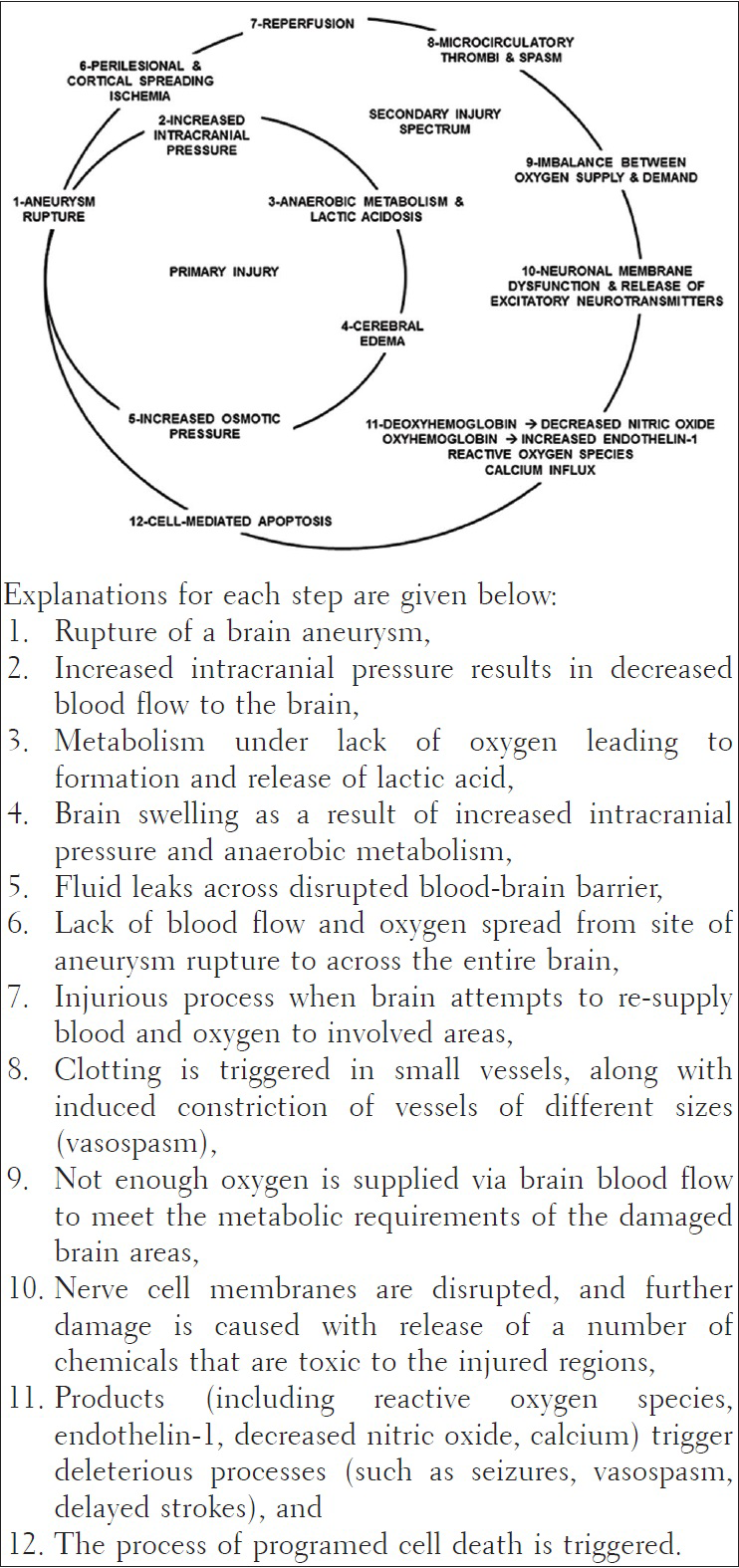
Brain injury spectrum after aneurysmal subarachnoid hemorrhage
Patients with ruptured brain aneurysms and aneurysmal subarachnoid hemorrhage suffer neurological damage from primary injury of the aneurysm rupture itself, as well as a number of secondary injurious processes that can further worsen the affected individual's neurological state. Secondary injurious processes can be related to the nervous system such as re-bleeding from the ruptured aneurysm, brain swelling, occurrence of a delayed stroke, brain blood vessel vasospasm leading to seizures, strokes and an increase in brain-spinal fluid causing hydrocephalus.
Brain-body associations in aneurysmal subarachnoid hemorrhage
When brain aneurysms rupture, other body systems can be affected in a number of brain-body associations. Previous literature attempted to characterize cardiac and pulmonary manifestations in aneurysmal subarachnoid hemorrhage.[
Stunned myocardium and contraction band necrosis, and Altered pulmonary capillary permeability and pulmonary edema.
Yet, patients with ruptured brain aneurysms manifest clinical observations to show the involvement of other body organs including endocrine and renal abnormalities.
Objectives
The purpose of this systematic review was to synthesize studies that investigate the pathophysiologic mechanisms of brain-body associations in patients with ruptured brain aneurysms. This systematic review also critically appraises the methodologic quality of studies that attempt to derive brain-body associations in aneurysmal subarachnoid hemorrhage.
METHODS
This systematic review was designed based on a predefined protocol.
Study eligibility criteria
Studies that were eligible for this systematic review included:
Studies of adult patients with ruptured brain aneurysms, These patients received diagnostic tests to investigate pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage, These patients also received reference standard investigations to document multi-organ deteriorations after brain aneurysm rupture, and Prospective and retrospective cohort studies and randomized controlled trials investigating pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage.
We excluded the following studies:
Those that do not characterize pathophysiologic mechanisms of brain-body associations, Those that do not distinguish between aneurysmal and nonaneurysmal subarachnoid hemorrhage, and Studies based on expert opinions.
Eligible studies were limited to those published from January 1, 2000 to March 31, 2014. We truncated eligible studies to this time period because of advancements in:
Investigations to diagnose aneurysmal subarachnoid hemorrhage, such as computed tomography (CT) angiogram, Diagnostic tests to investigate pathophysiologic mechanisms of brain-body associations, such as advanced pulse contour analysis for cardiopulmonary parameters and blood assays for neuroendocrine markers, and Surgical and neurocritical care treatment differences such as availability of minimally invasive endovascular techniques.
Literature search
Two reviewers (Benjamin Lo [BL], Hitoshi Fukuda [HF]) independently searched a number of electronic databases. Relevant studies were identified from Ovid MEDLINE, Ovid EMBASE, Web of Science, the Cumulative Index to Nursing and Allied Health Literature, without language restrictions. To include gray literature, we also searched Proceedings First and Papers First. We used the following combination of search terms:
“Aneurysmal subarachnoid hemorrhage” and “cardiopulmonary,” “Aneurysmal subarachnoid hemorrhage” and “renal,” “Aneurysmal subarachnoid hemorrhage” and “gastrointestinal,” “Aneurysmal subarachnoid hemorrhage” and “immune” and “hematologic,” “Aneurysmal subarachnoid hemorrhage” and “brain-body associations.”
Study selection and data collection process
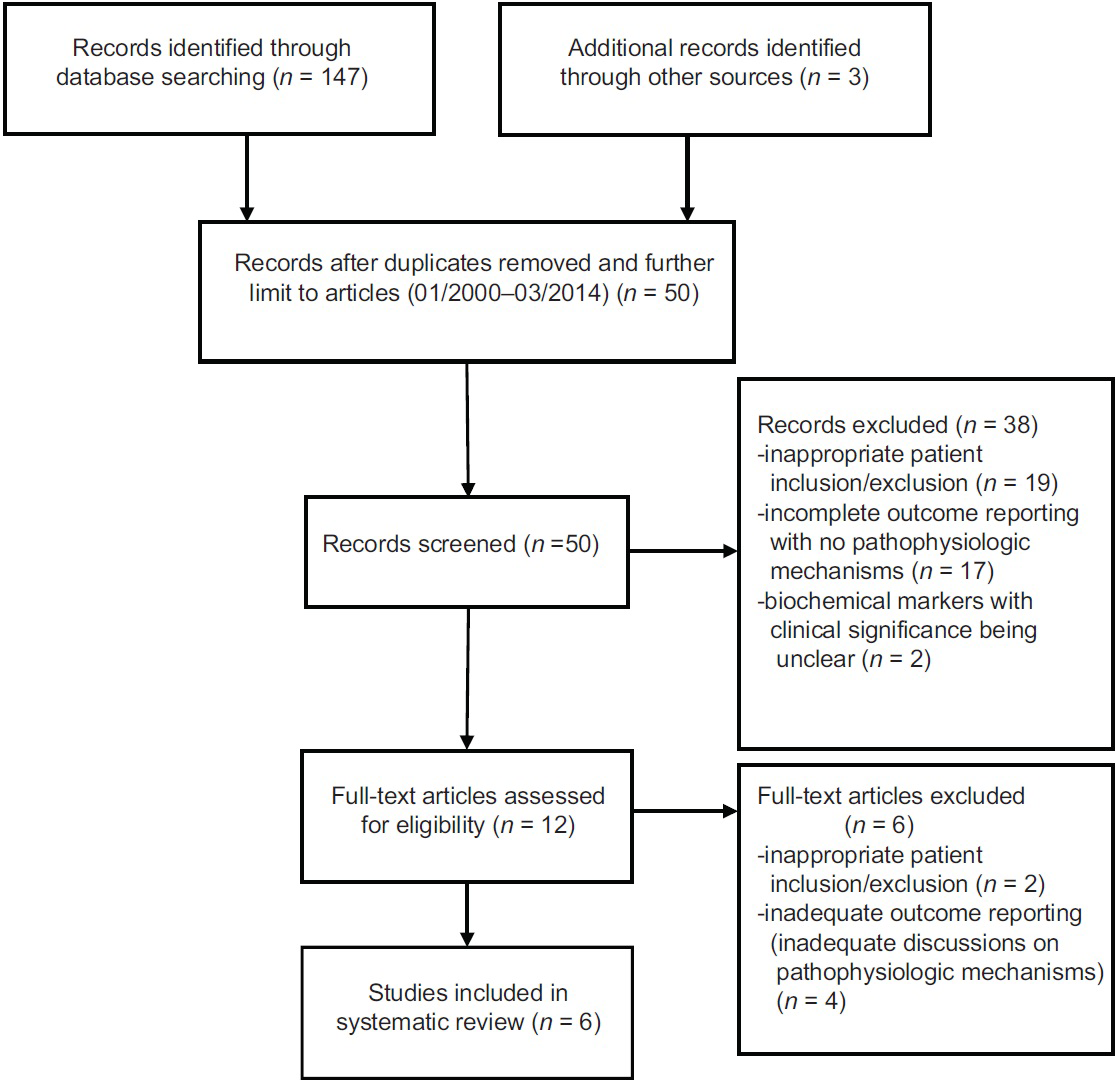
Both investigators (BL and HF) reviewed all titles and abstracts, and full reports of all potentially relevant trials. The initial literature search (January 1, 2000–March 31, 2014) yielded 150 citations [
Inappropriate patient inclusion and exclusion criteria, Inadequate outcome reporting, and Discussions are lacking pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage.
Investigators BL and HF then independently applied the inclusion criteria to the full reports. Each trial report was examined carefully for its methodologic quality. As outlined in the “methodologic quality assessment” section, each article was appraised in nine areas. Of the 54 items assessed in six articles, BL and HF reached agreement on 43 items, disagreed on 8 items and were unsure on 3 items (kappa statistic = 0.82, 95% CI 0.71–0.93). Disagreements were resolved through consensus discussions and YN, the third reviewer.
For data collection, the reviewers (BL, HF) extracted relevant data using a data extraction form, piloted on a sample of included studies. Disagreements were resolved by consensus discussions and YN, the third reviewer.
Assessment of pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage
For this systematic review, the following items were reviewed in order to clarify pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage:
Study design – including description of study protocol, study setting, and recruitment, Patient population – including representative of cohort, inclusion and exclusion criteria, and sample size, Investigations – including reference tests to document organ dysfunction and diagnostic tests to document how ruptured brain aneurysms affect other organs, Outcome – including discussions on pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage, and Ethical conduct of study – including institutional ethics board approval and funding declarations.
Methodologic quality assessment
For this systematic review, we sampled the QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies Tool– Revised Version).[
Whether subjects included are representative of those treated in clinical practice, Clear study designs and descriptions of study protocols, inclusion and exclusion criteria, study settings, and recruitment, Inclusion of study findings with adequate discussions of pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage, Clear descriptions of reference standards to document multi-organ dysfunction, Whether diagnostic tests to investigate, brain-body associations were described in sufficient detail to permit test replication, Whether clinical data for study subjects are reproducible for those treated in clinical practice, Reporting of noninterpretable test results, Explanation of study withdrawals, and Whether the measure is clinically useful.
RESULTS
Study search and selection
The initial literature search (January 1, 2000 – March 31, 2014) yielded 150 citations [
A meta-analysis was not feasible in this review to statistically pool outcomes obtained from the included studies clarifying pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms. Marked between-study differences was noted in the included studies with: (1) Differing diagnostic tests and reference value ranges, (2) differing clinical outcome endpoint measures, and (3) scarcity of studies investigating each type of diagnostic investigation. Diagnostic tests of serum and cerebrospinal fluid markers were studied in four out of six studies, radiographic investigations in two out of six studies, and invasive pulmonary artery thermodilution parameters in two out of six studies. In addition, there is no standardization of diagnostic tests between investigating centers.
Study results and synthesis of results
This systemic review identified five prospective cohort studies and one retrospective study for inclusion in the analysis. Of the six methodologically rigorous studies identified for this systematic review, pathophysiologic mechanisms were clarified between the brain and the following organ systems:
Cardiac system – six out of six studies, Pulmonary system – three out of six studies, Endocrine system – three out of six studies, Renal system (including electrolyte and fluid balance) – three out of six studies, Immune and hematologic systems – zero studies, and Gastrointestinal system – zero studies.
Assessment of pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage for included studies
Article 1: Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation
In this prospective cohort study, Audibert et al. investigated endocrinologic responses, as well as sodium and water regulation after severe aneurysmal subarachnoid hemorrhage.[
With maintenance of euvolemia and salt balance, only 1 out of 19 patients experienced severe clinical vasospasm, Levels of brain natriuretic peptide increased between days 1 and 3 after aneurysmal rupture, Levels of atrial natriuretic peptide increased between days 4 and 6 after aneurysmal rupture, with associated increases in levels of renin, aldosterone and angiotensin II, and Levels of vasopressin increased between days 10 and 12 after aneurysmal rupture.
This study clearly describes the relationship between brain aneurysmal rupture and delayed activation of the renin-angiotensin-aldosterone system. It had institutional ethics board approval, and clear declaration of funding sources. However, its main weaknesses included small study sample size, patient selection, and center referral biases.
Article 2: Neuro-cardio-endocrine response to acute subarachnoid hemorrhage
In this prospective cohort study, Espiner et al. investigated relationships between neurologic cardiac and endocrine responses after severe aneurysmal subarachnoid hemorrhage.[
Brain natriuretic peptide and atrial natriuretic peptide levels in plasma increased in the first 3 days postaneurysmal rupture. They returned to normal levels by day 7. Cerebrospinal fluid levels of brain natriuretic peptide and atrial natriuretic peptide did not show any changes. Abnormal electrocardiograms were noted in 6 out of 7 patients Urinary sodium levels increased within the first 3 days then remained stable. Plasma sodium levels gradually decreased Plasma vasopressin levels were increased on presentation then fell to normal levels after 2 days. Plasma aldosterone and renin levels increased at day 10–12. Cortisol levels fell abruptly on day 3 and maintained low levels to day 10 Initial elevations of epinephrine, norepinephrine, and endothelin fell by day 3 and remained at subnormal levels.
This study thoroughly describes the various relationships between the brain, neuroendocrine and renal systems. It had institutional ethics board approval and clear declaration of funding sources. However, its main weakness is the treatment of patients with admission dose of dexamethasone which further delayed activation of the renin-angiotensin-aldosterone system. Patients also demonstrated depressed adrenal-hypothalamic cortisol axis. This study also had small study sample size. Other weaknesses included patient selection and center referral biases.
Article 3: Subarachnoid hemorrhage complicated with neurogenic pulmonary edema and takatsubo-like cardiomyopathy
In this retrospective cohort study, Inamasu et al. characterized cardiopulmonary dysfunctions in aneurysmal subarachnoid hemorrhage.[
Of 16 patients with neurogenic pulmonary edema, 14 had takatsubo-like cardiomyopathy. All exhibited electrocardiographic changes (including ST segment abnormalities, QT prolongation, and T-wave inversions). They were all intubated and were on vasopressors Neurogenic pulmonary edema was significantly associated with posterior circulation aneurysms (P = 0.004). 9 out of 16 patients died (7 from the primary aneurysmal rupture, 1 from rebleeding and 1 from vasospasm-induced strokes).
This study clearly demonstrated relationships between neurogenic pulmonary edema and takotsubo-like cardiomyopathy. It had institutional ethics board approval, and clear declaration of funding sources. However, its main weaknesses included small study sample size, patient selection, and center referral biases.
Article 4: Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage
In this retrospective cohort study, Naidech et al. investigated relationships between cardiac troponin rise after aneurysmal subarachnoid hemorrhage and its association with cardiopulmonary complications, delayed cerebral ischemia and death.[
Cardiac enzyme elevation was noted in patients who were older (average age 55 years), worse admission neurologic status, more blood on CT scan on admission, more physiologic dysfunction, and hypertensive history Cardiac enzyme elevation was associated with risk of hypotension requiring vasopressors, left ventricular systolic dysfunction on echocardiogram, delayed cerebral ischemia from vasospasm and death.
This study demonstrated associations between cardiac troponin elevation and cardiovascular-related morbidity and mortality after aneurysmal subarachnoid hemorrhage. It had institutional ethics board approval, and clear declaration of funding sources. However, this study did not distinguish between aneurysmal and nonaneurysmal subarachnoid hemorrhage. Furthermore, it did not discuss pathophysiologic mechanisms of brain-body associations in detail.
Article 5: Clinical significance of elevated natriuretic peptide levels and cardiopulmonary parameters after subarachnoid hemorrhage
In this prospective cohort study, Nakamura et al. investigated the relationships between natriuretic peptides and changes in cardiopulmonary parameters.[
Plasma levels of brain natriuretic peptides peaked on postaneurysm rupture day 1. Plasma levels of atrial natriuretic peptide peaked on postaneurysm rupture day 2, and remained increased up to day 6 Natriuretic peptides caused sodium and water loss with plasma sodium levels falling between day 3 and 10 There were no changes in cardiac indices and slight increases in extravascular lung water indices. There were no changes in intrathoracic blood volume indices.
This study thoroughly discussed relationships between elevated natriuretic peptides and cardiopulmonary parameters after aneurysmal subarachnoid hemorrhage. It had institutional ethics board approval, and clear declaration of funding sources. However, its main weaknesses included small study sample size, patient selection, and center referral biases.
Article 6: Circulatory characteristics of normovolemia and normotension therapy after subarachnoid hemorrhage, focusing on pulmonary edema
In this prospective cohort study, Sato et al. characterized circulatory parameters after severe aneurysmal subarachnoid hemorrhage to reveal mechanisms of pulmonary edema.[
7 out of 49 patients experienced neurogenic pulmonary edema Patients with neurogenic pulmonary edema had a lower cardiac function index and lower global ejection fraction. They had higher global end diastolic volume index Even though neurogenic pulmonary edema patients had net negative water balance and low central venous pressures, they had higher extravascular lung water index.
This study thoroughly discussed circulatory changes in aneurysmal subarachnoid hemorrhage patients with neurogenic pulmonary edema. It had institutional ethics board approval, and clear declaration of funding sources. However, its main weaknesses included small study sample size, patient selection, and center selection biases.
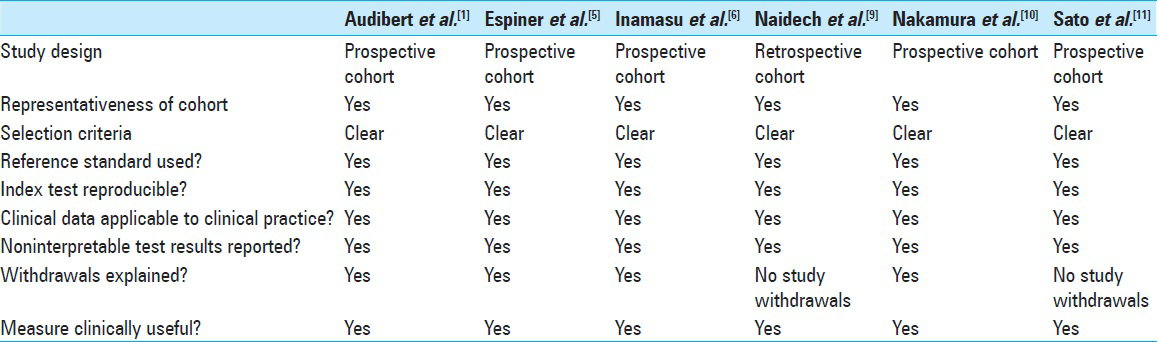
Methodological quality of included studies
In summary, all six studies have strong methodologic quality [
Results gained from these studies are clinically useful and shed light on how ruptured brain aneurysms affect the cardiopulmonary system. Subsequent neuro-cardio-endocrine responses then interact with other body systems, including the renal system, as part of the secondary responses to primary injury.
DISCUSSION
This systematic review gathers the most current methodologically rigorous evidence on known pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage. The following pathophysiologic discussion summaries this current state of knowledge.[
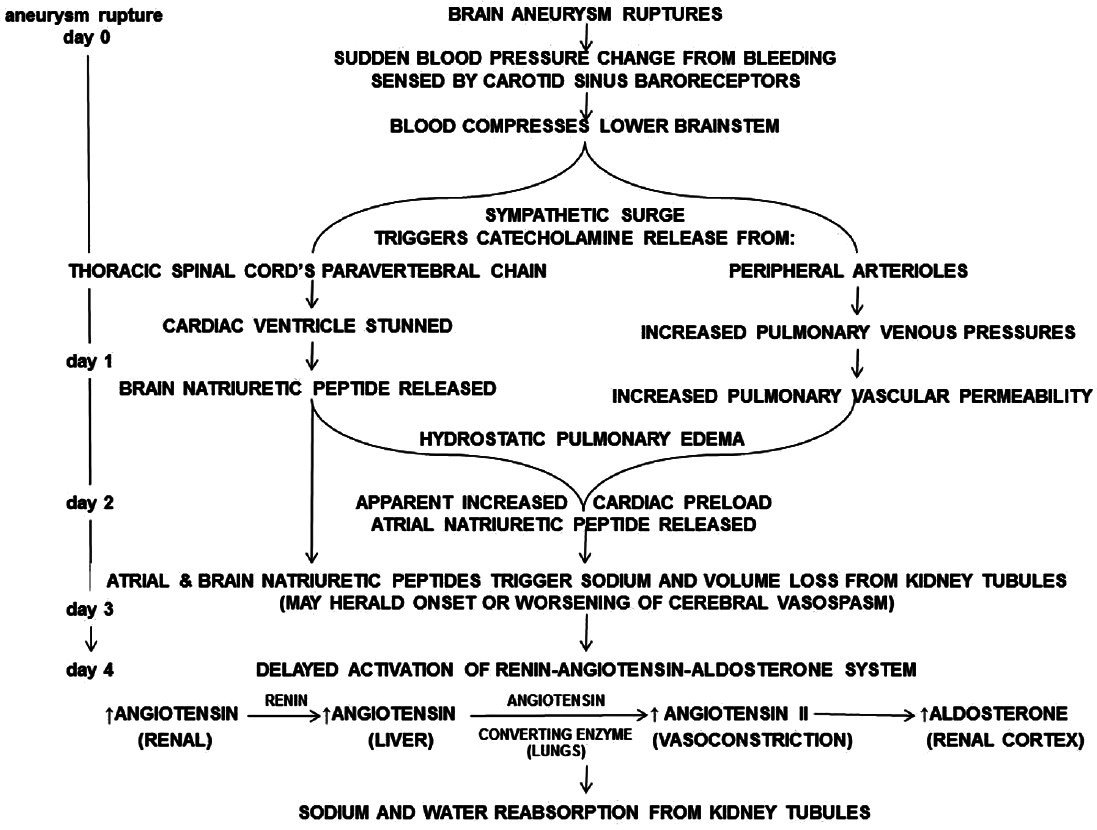
Rupture of brain aneurysms can lead to overactivity of the sympathetic nervous system. This triggers a sympathetic surge of catecholamine release from the thoracic spinal cord's paravertebral chain. Pathologically, the cardiac ventricle is stunned.[
Together, atrial and brain natriuretic peptides then act on renal tubules triggering sodium and volume loss. Without appropriate resuscitation, plasma sodium levels can fall drastically by postrupture day 4–6.[
Clinical implications
This systematic review elucidates mechanisms of how ruptured brain aneurysms affect neuroendocrine, heart, lung, as well as fluid, and electrolyte balance in the affected patients. Recognition of pathophysiologic mechanisms of brain-body associations is essential in preventing complications after treatment of ruptured brain aneurysms. The clinician should be vigilant about:
Maintenance of sodium and water balance, Potential negative impact of agents which interfere with the renin-angiotensin-aldosterone system (including corticosteroids and angiotensin-converting enzyme inhibitors), Ventilatory support to overcome pulmonary edema, and Inotropic and vasopressor support for the stunned myocardium.
Limitations
This systematic review included studies with small sample sizes. Highly selected patient populations were included, with short-term follow-up. Differing diagnostic tests were used to clarify pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms, with no standardization of diagnostic tests between investigating centers. Because of these limitations, individual patient variability in physiologic responses may not be adequately captured.
Patients with underlying comorbidities such as gastrointestinal diseases were not included to clarify the effects of other comorbidities on pathophysiologic mechanisms of brain-body associations in aneurysmal subarachnoid hemorrhage patients. In addition, there is scarce epidemiologic evidence to demonstrate the clinical associations between ruptured brain aneurysms and the gastrointestinal, immune and hematologic systems.
CONCLUSION
This systematic review synthesizes the most current evidence of underlying mechanisms of brain related associations with body systems in aneurysmal subarachnoid hemorrhage. However, literature is still lacking on some key mechanisms including the reasons for early-onset cerebral edema in the aneurysmal subarachnoid hemorrhage patient, which is an important cause of early hospital associated mortality. In addition, the mechanisms of late hospital associated mortality are not well described in large epidemiologic aneurysmal subarachnoid hemorrhage populations. There is also insufficient epidemiologic evidence of associations between the brain-gastrointestinal and brain-immune systems.
References
1. Audibert G, Steinmann G, de Talancé N, Laurens MH, Dao P, Baumann A. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009. 108: 1922-8
2. Bederson JB.editors. AANS Publications Committee. Subarachnoid Hemorrhage: pathophysiology and Management. Park Ridge, Ill: American Association of Neurological Surgeons; 1997. p.
3. Beseoglu K, Holtkamp K, Steiger HJ, Hänggi D. Fatal aneurysmal subarachnoid haemorrhage: Causes of 30-day in-hospital case fatalities in a large single-centre historical patient cohort. Clin Neurol Neurosurg. 2013. 115: 77-81
4. Ebihara T, Kinoshita K, Utagawa A, Sakurai A, Furukawa M, Kitahata Y. Changes in coagulative and fibrinolytic activities in patients with intracranial hemorrhage. Acta Neurochir Suppl. 2006. 96: S69-73
5. Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2002. 56: 629-35
6. Inamasu J, Nakatsukasa M, Mayanagi K, Miyatake S, Sugimoto K, Hayashi T. Subarachnoid hemorrhage complicated with neurogenic pulmonary edema and takotsubo-like cardiomyopathy. Neurol Med Chir (Tokyo). 2012. 52: 49-55
7. Junttila EK, Koskenkari J, Romppainen N, Ohtonen PP, Karttunen A, Ala-Kokko TI. Risk factors for 1-year mortality in patients with nontraumatic intracranial hemorrhage requiring intensive care. Acta Anaesthesiol Scand. 2011. 55: 1052-60
8. Kudo K, Konta T, Degawa N, Saito S, Kondo R, Kayama T. Relationship between kidney damage and stroke types in Japanese patients. Clin Exp Nephrol. 2012. 16: 564-9
9. Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005. 112: 2851-6
10. Nakamura T, Okuchi K, Matsuyama T, Fukushima H, Seki T, Konobu T. Clinical significance of elevated natriuretic peptide levels and cardiopulmonary parameters after subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2009. 49: 185-91
11. Sato Y, Isotani E, Kubota Y, Otomo Y, Ohno K. Circulatory characteristics of normovolemia and normotension therapy after subarachnoid hemorrhage, focusing on pulmonary edema. Acta Neurochir (Wien). 2012. 154: 2195-202
12. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011. 155: 529-36
13. Zacharia BE, Ducruet AF, Hickman ZL, Grobelny BT, Fernandez L, Schmidt JM. Renal dysfunction as an independent predictor of outcome after aneurysmal subarachnoid hemorrhage: A single-center cohort study. Stroke. 2009. 40: 2375-81
14. Zygun DA, Doig CJ, Gupta AK, Whiting G, Nicholas C, Shepherd E. Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003. 18: 238-44