Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11118
Peer-review started: January 24, 2015
First decision: April 14, 2015
Revised: April 23, 2015
Accepted: August 25, 2015
Article in press: August 25, 2015
Published online: October 21, 2015
AIM: To show the efficient generation of hepatocyte-like cells (HLCs) differentiated from the induced pluripotent stem cells (iPSCs) of rats.
METHODS: Hepatic differentiation was achieved using a three-step protocol with several growth factors. First, rat iPSCs were differentiated into definitive endoderm cells using Activin A and Wnt3a treatment. Then fibroblast growth factor 4 and bone morphogenetic protein 2 were added to the culture medium and used to induce hepatic differentiation. Finally, hepatocyte growth factor, Oncostatin M and dexamethasone were used for hepatic maturation. The liver-related markers and functions of HLCs were assessed at the gene and protein levels.
RESULTS: After endodermal induction, the differentiated cells expressed endodermal markers forkhead box protein A2 and SRY-box containing gene 17 at the mRNA and protein levels. After 20 d of culture, the iPSCs were differentiated into HLCs. These differentiated cells expressed hepatic markers including α-fetoprotein, albumin CK8, CK18, CK19, and transcription factor HNF-4α. In addition, the cells expressed functional proteins such as α1-antitrypsin, cytochrome P450 1A2 and CYP 3A4. They acted like healthy hepatic cells, storing glycogen and taking up indocyanine green and low-density lipoproteins. Also, the rates of urea synthesis (20 d 1.202 ± 0.080 mg/dL vs 0 d 0.317 ± 0.021 mg/dL, P < 0.01) and albumin secretion (20 d 1.601 ± 0.102 mg/dL vs 0 d 0.313 ± 0.015 mg/dL, P < 0.01) increased significantly as differentiation progressed.
CONCLUSION: Rat iPSCs can differentiate into HLCs rapidly and efficiently. These differentiated cells may be an attractive resource for treatment of end-stage liver disease.
Core tip: Induced pluripotent stem cells (iPSCs) can differentiate into many kinds of cells, including hepatocytes. However, the most important animal model for studying human diseases, especially liver diseases, is the rat model. This study is the first to show the efficient generation of hepatocyte-like cells (HLCs) differentiated from the iPSCs of rats. Hepatic differentiation was achieved using a three-step protocol. Rat iPSCs were differentiated into HLCs after 20 d of culture. These differentiated cells expressed hepatic markers and exhibited functional hepatic characteristics. These differentiated cells may be an attractive resource for treatment of liver disease.
-
Citation: Sun C, Hu JJ, Pan Q, Cao Y, Fan JG, Li GM. Hepatic differentiation of rat induced pluripotent stem cells
in vitro . World J Gastroenterol 2015; 21(39): 11118-11126 - URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11118
So far, there has been no ideal treatment for end-stage liver disease attributable to chronic hepatitis or cirrhosis. Recent studies have suggested that differentiated hepatocytes derived from transplanted stem cells may be a suitable form of therapy[1,2]. Embryonic stem cells (ESCs) and fetal liver epithelial cells can form mature hepatocytes in vivo. This might reduce the severity of hepatic fibrosis[3,4]. However, the collection of these cells from human embryos involves certain ethical and political issues, which limit their use in clinical settings. According to a previous study, bone marrow-derived liver stem cells may suppress hepatic fibrosis and ameliorate liver function[5]. However, their ability to proliferate is much poorer than that of ESCs, leaving them unable to produce a large number of cells[6]. Bone marrow contains several different components. This low purity produces a non-homogeneous crop of differentiated cells. This affects the treatment after transplantation[7].
Induced pluripotent stem cells (iPSCs) sidestep most of the ethical issues surrounding ESCs. They can produce functional hepatocytes and provide new seed cells for the treatment of hepatic diseases[8,9]. Studies have shown that iPSCs can differentiate into neural cells[10,11], osteogenic cells[12,13], cardiac cells[14,15], vascular cells[16,17] and pancreatic cells[18,19]. The differentiation of iPSCs into hepatic lineages has been observed in humans and mice using similar protocols[20-22]. However, the laboratory rat was the first mammalian species domesticated for scientific research, and the rat model is commonly used in research involving hepatic diseases. So far, no reports have been published on the differentiation of rat iPSCs into hepatocyte-like cells (HLCs). In order to develop such technologies in rat models, a method suitable for the differentiation of rat iPSCs into HLCs is here presented and the necessary conditions are given.
In the present study, rat iPSCs were differentiated into HLCs using several growth factors. The characteristics and functions of the HLCs were evaluated. These cells might be suitable for use as a cell resource for transplantation into model animals with liver diseases.
Rat iPSC line M13 was provided by the research group led by Professor Lei Xiao of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences[23]. Rat iPSCs were cultured with a feeder layer of irradiated mouse embryonic fibroblasts (MEF) in iPSC medium (DMEM/F12 containing 20% knockout serum replacement, 2 mmol/L L-glutamine, 1% nonessential amino acids, 0.1 mmol/L β-mercaptoethanol (all from Invitrogen/Gibco, Grand Island, NY, United States) in a humid atmosphere containing 5% CO2. Before differentiation, the cells were cultured on gelatin-coated tissue culture dishes with MEF-conditioned medium.
During the first 5 d, rat iPSCs were cultured in the Roswell Park Memorial Institute 1640 medium (Invitrogen/Gibco) with 25 ng/mL Wnt3a and 100 ng/mL Activin A (both from R and D Systems, Minneapolis, MN, United States) for endodermal induction. Next, the inductive factors were replaced with 30 ng/mL fibroblast growth factor 4 (FGF4, R and D Systems) and 20 ng/mL bone morphogenetic protein 2 (BMP2, R and D Systems) for 5 d. Finally, during the maturation step, the differentiated cells were incubated in the same basal medium containing 10 ng/mL Oncostatin M (OSM, R and D Systems), 20 ng/mL hepatocyte growth factor (HGF, R and D Systems), and 0.1 μmol/L dexamethasone (Dex, Sigma-Aldrich, St. Louis, MO, United States) for another 10 d.
On day 5 after endodermal induction, differentiated cells were tested for SRY-box containing gene 17 (SOX17) and forkhead box protein A2 (FOXA2). On day 20, they were tested for the other markers. Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% TrionX-100, and blocked with 10% goat serum (Sigma-Aldrich) for 1 h at room temperature. Then the cells were incubated overnight with primary antibodies at 4 °C. The primary antibodies against rat FOXA2 (1:200, Chemicon/Millipore, Billerica, MA, United States), SOX17 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States), albumin (ALB, 1:500), α-fetoprotein (AFP, 1:200), α1-antitrypsin (AAT, 1:200), cytokeratin 18 (CK18, 1:200, all from Santa Cruz Biotechnology), cytochrome P450 1A2 (CYP1A2, 1:200, Abcam, La Jolla, CA, United States) and cytochrome P450 3A4 (CYP3A4, 1:200, Abcam) were obtained. Then the cells were incubated with TRITC-conjugated secondary antibody (Invitrogen, Carlsbad, CA, United States) for 1 h at room temperature. The nuclei were then counterstained with 4,6-diamidino-2-phenylindole (DAPI, Roche, Mannheim, Germany). The cells were analyzed using a fluorescence microscope (IX71, Olympus, Japan).
To assess endodermal and hepatic markers, differentiated cells were tested for FOXA2 and SOX17 on day 5 and for other markers on day 20. Trizol reagent was used to extract total RNA from differentiated iPSCs (Invitrogen). Reverse transcription-PCR was used to produce cDNA. GenBank accession numbers, primer sequences, and amplicon size are listed in Table 1 for each gene. The PCR procedure was as follows: cDNA denaturation at 94 °C for 5 min followed by 28 cycles (ALB, 40 cycles) of denaturation at 94 °C for 15 s, annealing at 66 °C for 10 s, and extension at 72 °C for 20 s. The final extension took place at 72 °C and lasted 7 min. PCR products were separated by electrophoresis on agarose gels. The GAPDH housekeeping gene was used as an internal control.
| Gene | Primer | Accession Number | Amplicon size (bp) |
| FOXA2 | TGTCAGGAGCACAAGCGAGG (F) | NM-012743.1 | 194 bp |
| GGGTGGTTGAAGGCGTAATGGTG (R) | |||
| SOX17 | GGCACGGAACCCAACCAGC (F) | NM-001107902.1 | 119 bp |
| CAGTCGTGTCCCTGGTAGGGAAGAC (R) | |||
| AFP | TCTGAAACGCCATCGAAATGCC (F) | NM-012493.2 | 285 bp |
| AATGTAAATGTCGGCCAGTCCCT (R) | |||
| ALB | GGCACAGTGCTTGCAGAATTTCAG (F) | NM-134326.2 | 272 bp |
| CACAGACGGTTCAGGATGGCAG (R) | |||
| CK8 | TGAAGGATGCCAATGCCAAGCTG (F) | NM-199390.1 | 237 bp |
| AACTCAGTCCTCCTGCGTAGCC (R) | |||
| CK18 | ACGATTTCAGTCTCAACGACGCC (F) | NM-053976.1 | 147 bp |
| TCCCTTCTTCTGAGCCTTAGTGCC (R) | |||
| CK19 | GCTCAGCATGAAAGCTGCCCT (F) | NM-199498.1 | 299 bp |
| CGCACTGGTAGCAAGGTAGGAG (R) | |||
| HNF4α | GATGTGCTGCTCCTAGGCAATGAC (F) | NM-001270931.1 | 261 bp |
| CTGCCGGTCGTTGATGTAATCCTC (R) | |||
| GADPH | CCTTCATTGACCTCAACTAC (F) | NM-017008.4 | 594 bp |
| GGAAGGCCATGCCAGTGAGC (R) |
The urea production test was performed using a colorimetric QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA, United States). Undifferentiated cells and differentiated cells on days 5, 10, 15, and 20 were trypsinized and counted with a hemocytometer. The assay was performed in accordance with the manufacturer’s instructions. The sample supernatants were stored at -20 °C. Absorbance was measured using a microplate reader. Urea production was normalized to the total number of cells.
The concentration of rat albumin in the supernatant was determined using a Rat Albumin ELISA Quantitation Kit (ICL, Portland, OR, United States) in accordance with the manufacturer’s instructions. Undifferentiated iPSCs and differentiated cells were trypsinized and counted on days 5, 10, 15, and 20 using a hemocytometer. Albumin secretion was normalized to the total number of cells.
On day 20, differentiated iPSCs were stained using a Periodic acid-Schiff (PAS) staining system (Sigma-Aldrich). The cells were first fixed in 4% paraformaldehyde for 20 min and oxidized with 1% periodic acid solution for 5 min. Then they were washed. The cells were treated with Schiff’s reagent for 15 min at room temperature. The cells were washed with PBS and then stained with hematoxylin for 90 s. They were then examined under a light microscope (IX50, Olympus, Japan).
After differentiation, cells were incubated for 1 h with indocyanine green (ICG, Sigma-Aldrich) in basal medium at 37 °C. Uptake of ICG was detected under a light microscope. The ability of these cells to take up low-density lipoprotein was determined using an LDL Uptake Cell-Based Assay Kit (Cayman Chemical, Ann Arbor, MI, United States) according to the manufacturer’s instructions. The cells were visualized under a fluorescent microscope.
Results are shown as mean ± SD. One-way ANOVA was used for statistical analysis. P < 0.05 was considered significant.
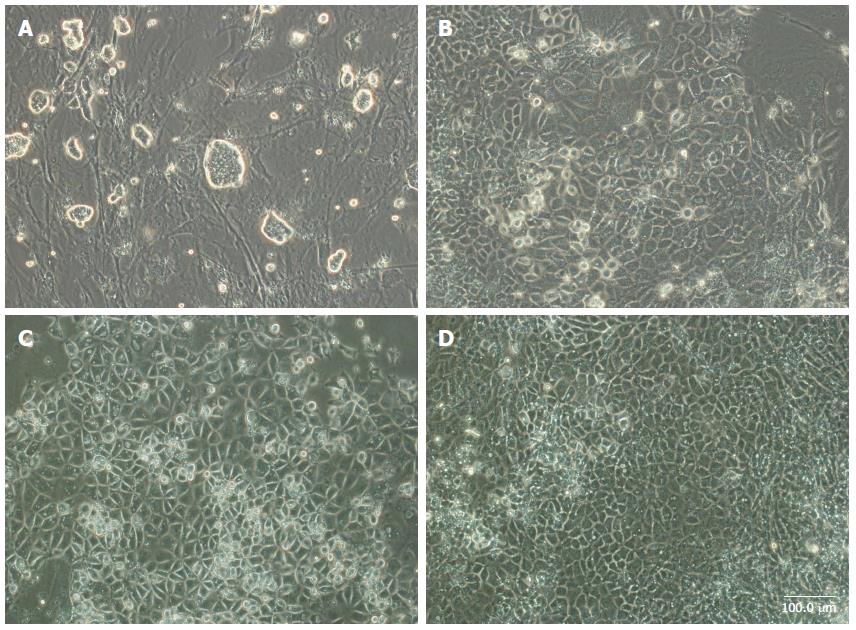
A simple three-stage differentiation method for iPSCs was developed. During the first five days, iPSCs were treated in a medium with Activin A and Wnt3a for endodermal induction. The cells gradually took on flatter and spikier shapes (Figure 1B). Then, the cells were cultured under feeder-free conditions with high concentrations of FGF4 and BMP2 for the next five days. Cells in these colonies had compact, polygonal shapes like those of differentiated hepatocytes (Figure 1C). The medium was replaced with mature medium supplemented with HGF, OSM, and Dex for an additional 10 d. The cells exhibited the morphology of mature hepatocytes with large amounts of cytoplasm and distinct, round nuclei (Figure 1D).
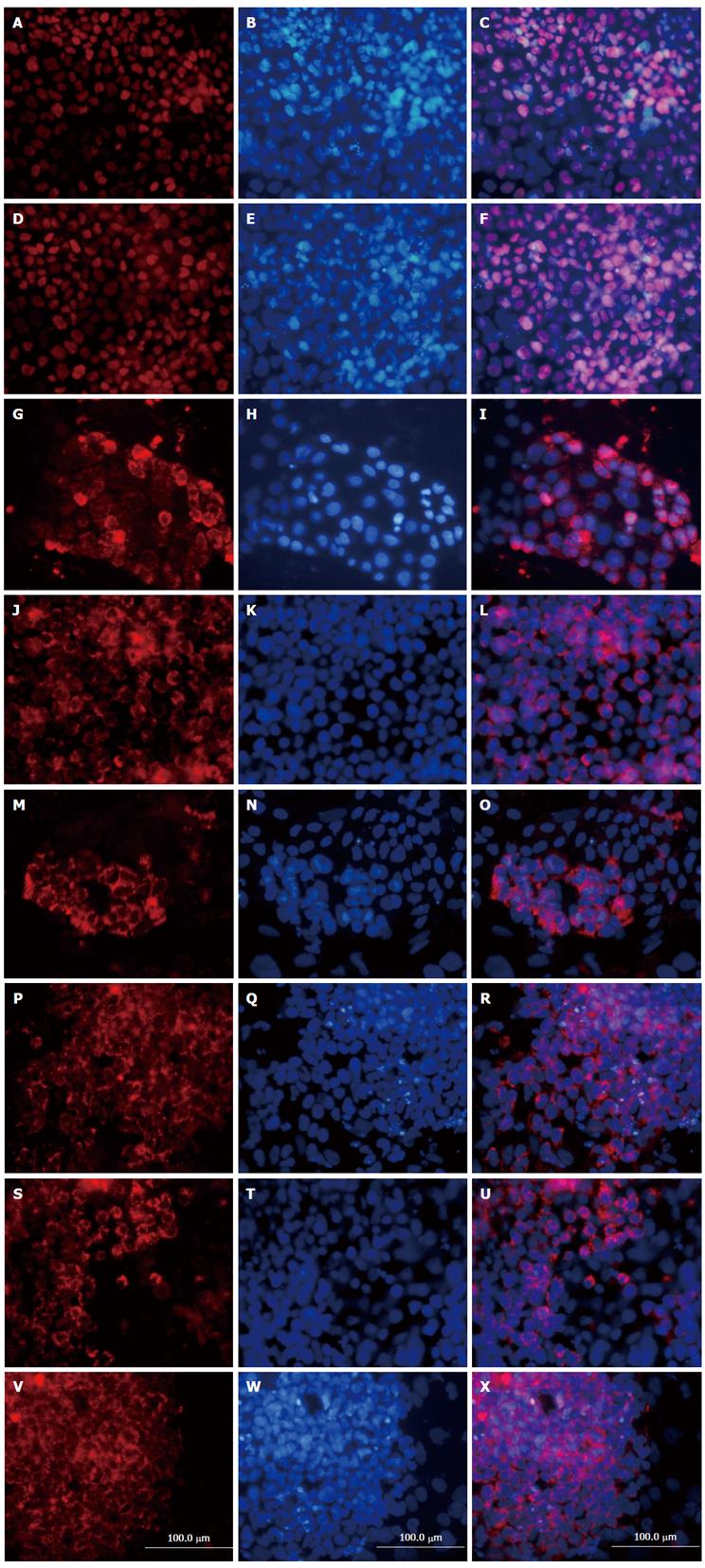
Cell characteristics were determined at different stages of differentiation using immunofluorescence staining. After endodermal induction, approximately 70%-80% of the differentiated cells expressed endodermal markers FOXA2 and SOX17 at the protein level (Figure 2). These results indicate that rat iPSCs could form endoderm efficiently if exposed to Activin A and Wnt3a. After hepatic maturation at stage 3, immunostaining analysis showed that 50%-60% of the cells expressed hepatic markers, including AFP, ALB, CK18, and functional proteins such as AAT, CYP1A2 and CYP 3A4 (Figure 2).
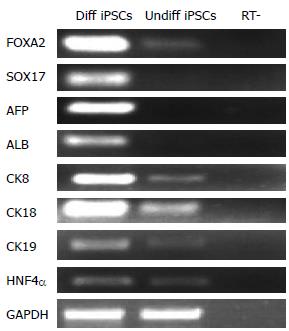
To further analyze the properties of iPSCs that developed into endodermal cells and HLCs, RT-PCR analysis was performed in the cells at various stages of differentiation. After induction of endodermal differentiation, endoderm markers FOXA2 and SOX17 were detected in the cells at stage 1 (Figure 3). This was consistent with the results of the immunostaining assay. After the cells differentiated into HLCs at stage 3, RT-PCR analysis indicated the expression of liver associated genes such as AFP, ALB, CK8, CK18, CK19, and transcription factor HNF-4α (Figure 3). These results collectively indicate that the cells had changed from endodermal to hepatic.
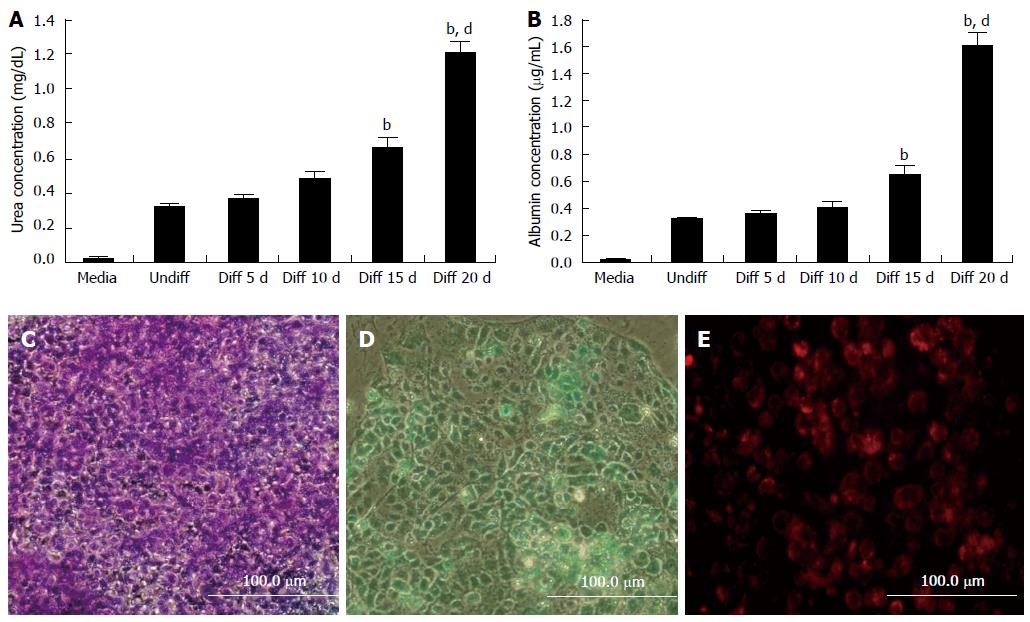
In order to evaluate the liver-specific function of iPSC-derived differentiated cells, the functional properties of HLCs of urea synthesis and albumin secretion were analyzed. The rates of urea synthesis (20 d 1.202 ± 0.080 mg/dL vs 0 d 0.317 ± 0.021 mg/dL, P < 0.01; Figure 4A) and albumin secretion (20 d 1.601 ± 0.102 mg/dL vs 0 d 0.313 ± 0.015 mg/dL, P < 0.01; Figure 4B) increased significantly as differentiation progressed, indicating hepatic maturation.
The ability of the cells to store glycogen was tested. PAS staining was used to confirm the presence of stored glycogen. Differentiated cells showed glycogen staining, indicating glycogen accumulation (Figure 4C).
The iPSCs-HLCs were also found to take up ICG and LDL (Figure 4D and E). These results showed that the iPSC-HLCs had the same function as hepatocytes in vitro.
iPSCs have been used to investigate hepatocyte differentiation and possible stem cell therapies. Studies have shown that human and mouse iPSCs can be differentiated into functional hepatocytes[20,21]. Currently, some of the studies of liver disease and possible therapeutic strategies involve animal models. Most models of liver diseases are based on rodents, especially rats. The present work is the first to demonstrate the efficient generation of HLCs differentiated from iPSCs of rat species. These differentiated cells were found to express the hepatic markers AFP, ALB, CK8, CK18, and CK19 and transcription factor HNF-4α. They were also able to store glycogen, secrete urea and albumin, and take up LDL and ICG. Results showed that these differentiated cells had a gene profile similar to that of HLCs derived from human and mouse iPSCs, as reported previously[20,24].
In the present study, a three-step protocol designed to generate HLCs from rat iPSCs was developed. The process is very simple and highly efficient. It takes two to three weeks and requires only a few inductive factors. The microenvironment in which the hepatocytes develop involves several biological events. Cell growth and differentiation are tightly regulated by a series of intra- and intercellular signals. All the inductive factors used in the process are extracellular signals[24]. As in previous studies, nodal signaling through the transforming growth factor-beta pathway was found to play a crucial role in the development of the endoderm. Activin A initiated this nodal pathway, causing the phosphorylation of Smad2 and Smad3. It also interacted with Smad4 and then activated gene-specific transcription factors in the nucleus[25]. Several studies have demonstrated that Activin A can be used during the formation of definitive endoderm. Hay and colleagues were able to enhance the endodermal differentiation of ESCs using Wnt3a in combination with Activin A. They postulated that Activin A plus Wnt3a would induce the rapid formation of a relatively consistent hepatic endoderm and cells that are more capable of acting like hepatocytes than other combinations of inductive factors[26]. In the present differentiation system, the iPSCs quickly became spiky in shape, and the formation of endoderm was induced using Activin A combined with Wnt3a, as previously reported[22,27].
FGFs and BMPs were found to play significant roles in liver specification. FGFs from the cardiac mesoderm and BMPs from mesenchymal cells of the septum transversum have been shown to be essential for the induction of foregut endoderm cells into hepatocytes[28]. Cai et al[29] found that both FGF4 alone and BMP2 alone in the culture medium had little effect on definitive endoderm cells into liver cells, but both together led to a pronounced increase in the number of cells expressing ALB in vitro. They also demonstrated that the induction of definitive endoderm is essential for the effectiveness of FGF4 and BMP2 in hepatic induction[29]. Another study showed that FGF4 plus BMP2 can generate hepatic cells from human iPSCs[20]. These factors were used in the present protocol at stage 2 of differentiation. Cells were driven toward commitment to hepatic cells.
To generate mature liver cells, the iPSCs were subjected to a process meant to foster in vitro maturation. At this stage, cells are generally grown in media that had previously been optimized for the culture of hepatocytes with the addition of factors thought to promote the maturation or proliferation of hepatocytes during liver development. OSM has been reported to facilitate the differentiation of liver progenitor cells into hepatocytes[30]. HGF enhances hepatic maturation and proliferation[27]. Dex is a synthetic glucocorticoid hormone. It is active in induction of enzymes involved in gluconeogenesis[24]. In the present work, during the part of maturation that involves these factors, differentiated cells presented a cuboidal morphology and showed the properties of hepatic cells.
The enzymes CYP1A2 and CYP3A4 are critical to drug metabolism. The present study showed that rat iPSC-derived HLCs expressed the hepatocyte function-specific proteins CYP1A2 and CYP3A4. This suggested that these cells gain liver-specific properties and may be used in drug metabolism tests and toxicity screens.
In summary, the data collected here suggest that rat iPSCs can differentiate into HLCs in a rapid and efficient manner through a three-stage process involving the application of inductive factors. Differentiated cells may be an attractive resource for treatment of end-stage liver disease.
Recent studies have suggested that differentiated hepatocytes derived from transplanted stem cells may be a suitable form of therapy. Embryonic stem cells (ESCs) and bone marrow-derived liver stem cells can form mature hepatocytes and reduce the severity of hepatic fibrosis. However, the ethical issues of ESCs and the low purity of bone marrow stem cells limit their use in clinical settings. Induced pluripotent stem cells (iPSCs) sidestep most of the ethical issues surrounding ESCs. The differentiation of iPSCs into hepatic lineages has been observed in humans and mice. So far, no reports have been published on the differentiation of rat iPSCs into hepatocyte-like cells (HLCs).
The laboratory rat was the first mammalian species domesticated for scientific research, and the rat model is commonly used in research involving hepatic diseases. This study is the first to show the efficient generation of HLCs differentiated from the iPSCs of rats.
Hepatic differentiation was achieved using a three-step protocol. Rat iPSCs were differentiated into HLCs after 20 d of culture. These differentiated cells expressed hepatic markers and exhibited functional hepatic characteristics.
This study suggests that rat iPSCs can differentiate into HLCs in a rapid and efficient manner through a three-stage process involving the application of inductive factors. Differentiated cells may be an attractive resource for treatment of end-stage liver disease.
Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from adult cells. Pluripotent stem cells hold great promise in the field of regenerative medicine. Because they can propagate indefinitely, as well as give rise to every other cell type in the body, they represent a single source of cells that could be used to replace those lost to damage or disease.
The manuscript is the first to demonstrate the efficient generation of HLCs differentiated from iPSCs of rat species.
P- Reviewer: Liu L S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Iwamoto T, Terai S, Hisanaga T, Takami T, Yamamoto N, Watanabe S, Sakaida I. Bone-marrow-derived cells cultured in serum-free medium reduce liver fibrosis and improve liver function in carbon-tetrachloride-treated cirrhotic mice. Cell Tissue Res. 2013;351:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Huang CK, Lee SO, Lai KP, Ma WL, Lin TH, Tsai MY, Luo J, Chang C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology. 2013;57:1550-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Moriya K, Yoshikawa M, Ouji Y, Saito K, Nishiofuku M, Matsuda R, Ishizaka S, Fukui H. Embryonic stem cells reduce liver fibrosis in CCl4-treated mice. Int J Exp Pathol. 2008;89:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Zheng JF, Liang LJ, Wu CX, Chen JS, Zhang ZS. Transplantation of fetal liver epithelial progenitor cells ameliorates experimental liver fibrosis in mice. World J Gastroenterol. 2006;12:7292-7298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Sun C, Li DG, Chen YW, Chen YW, Wang BC, Sun QL, Lu HM. Transplantation of urokinase-type plasminogen activator gene-modified bone marrow-derived liver stem cells reduces liver fibrosis in rats. J Gene Med. 2008;10:855-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2009;6:724-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14327] [Cited by in F6Publishing: 13579] [Article Influence: 848.7] [Reference Citation Analysis (0)] |
| 9. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7589] [Cited by in F6Publishing: 6999] [Article Influence: 411.7] [Reference Citation Analysis (0)] |
| 10. | Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 598] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 11. | Ishizuka T, Goshima H, Ozawa A, Watanabe Y. β1-adrenoceptor stimulation enhances the differentiation of mouse induced pluripotent stem cells into neural progenitor cells. Neurosci Lett. 2012;525:60-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109:643-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Kao CL, Tai LK, Chiou SH, Chen YJ, Lee KH, Chou SJ, Chang YL, Chang CM, Chen SJ, Ku HH. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010;19:247-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Blazeski A, Zhu R, Hunter DW, Weinberg SH, Zambidis ET, Tung L. Cardiomyocytes derived from human induced pluripotent stem cells as models for normal and diseased cardiac electrophysiology and contractility. Prog Biophys Mol Biol. 2012;110:166-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Rana P, Anson B, Engle S, Will Y. Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicol Sci. 2012;130:117-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Joo HJ, Kim H, Park SW, Cho HJ, Kim HS, Lim DS, Chung HM, Kim I, Han YM, Koh GY. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118:2094-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Homma K, Sone M, Taura D, Yamahara K, Suzuki Y, Takahashi K, Sonoyama T, Inuzuka M, Fukunaga Y, Tamura N. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells. Atherosclerosis. 2010;212:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 19. | Higuchi Y, Shiraki N, Kume S. In vitro models of pancreatic differentiation using embryonic stem or induced pluripotent stem cells. Congenit Anom (Kyoto). 2011;51:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 944] [Cited by in F6Publishing: 904] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 22. | Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Yang Y, Zhang J, Wang GY, Liu W, Qiu DB, Hei ZQ, Ying QL, Chen GH. Efficient derivation of functional hepatocytes from mouse induced pluripotent stem cells by a combination of cytokines and sodium butyrate. Chin Med J (Engl). 2011;124:3786-3793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 25. | Sulzbacher S, Schroeder IS, Truong TT, Wobus AM. Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors-the influence of differentiation factors and culture conditions. Stem Cell Rev. 2009;5:159-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105:12301-12306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 307] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 27. | Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Behbahan IS, Duan Y, Lam A, Khoobyari S, Ma X, Ahuja TP, Zern MA. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev. 2011;7:748-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Snykers S, De Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |