Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1644
Revised: February 1, 2010
Accepted: February 8, 2010
Published online: April 7, 2010
AIM: To explore the expression of leukemia related protein 16 (LRP16) in colorectal carcinoma, and analyze its correlation with clinicopathologic features and prognosis.
METHODS: Immunohistochemistry for LRP16 was performed in 201 cases of colorectal carcinoma and 60 cases of distal normal mucosa. Medical records were reviewed and clinicopathological analysis was performed.
RESULTS: LRP16 expression was detected in 117 of 201 cases of the colorectal carcinoma and in 21 cases of 60 distal normal mucosa. The expression of LRP16 in carcinoma was significantly higher than that in normal mucosa (χ2 = 9.999, P = 0.002). LRP16 protein expression was found in 43.3% (52/120) of carcinoma at stage I and II, and 80.2% (65/81) of carcinoma at stage III and IV (χ2 =27.088, P = 0.001). Correlation between LRP16 expression and clinicopathological factors was significant in differentiation (P = 0.010), tumor size (P = 0.001), infiltrative depth (P = 0.000) and distant metastasis (P = 0.027). The difference of median survival time between cancer patients with LRP16 expression (38.0 mo) and those without was statistically significant (105.0 mo, Log rank = 41.455, P = 0.001). The multivariate survival analysis revealed that LRP16 expression was correlated significantly (Cox’s regression: P = 0.001, relative risk = 2.082) with shortened survival in the patients with colorectal cancer.
CONCLUSION: The expression of LRP16 is related to the degree of differentiation, invasiveness, metastasis and prognosis of colorectal carcinoma.
- Citation: Xi HQ, Zhao P, Han WD. Clinicopathological significance and prognostic value of LRP16 expression in colorectal carcinoma. World J Gastroenterol 2010; 16(13): 1644-1648
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1644
Leukemia related protein 16 (LRP16), which was originally recognized and isolated from human lymphocytes in 1999, was identified as an estrogen-responsive gene. It localizes on chromosome 11q12.1 and encodes nuclear factor[1-5]. It expresses in testicle, ovaries, mucosa of colon, prostate, small intestine, spleen, thymus[2,3] and gastric carcinoma[6]. LRP16 is also an estrogen receptor α (ERα) coactivator. Its expression level is strongly dependent on the estrogen activities. It is involved in estrogen signaling pathway and can strengthen the ERα responsive gene activation.
Colorectal carcinoma is a major cause of cancer-related morbidity and mortality. In the Western countries, it is the second leading cause of death from cancer[7]. The incidence of colorectal cancer has increased in China recently, therefore, some new molecular markers are necessary to raise the efficiency of tumor diagnosis and to predict prognosis of the patients or even for therapeutic application. It has been reported that ER is identified in colorectal cancer[8-10]. However, no immunohistochemical and clinicopathological studies of LRP16 have been performed in colorectal carcinoma. Here, we investigated the expression of LRP16 in colorectal cancer specimens and normal mucosa by an immunohistochemistry (IHC)-based technique and analyzed the relationship between LRP16 expression and the clinicopathological features of Chinese patients with colorectal cancer.
A series of 201 consecutive colorectal carcinoma patients who were treated surgically in Chinese People’s Liberation Army General Hospital (Beijing, China) between August 1999 and September 2003 and 60 cases with distal normal mucosa were recruited in this study. There were 127 men and 74 women with ages ranging from 20 to 81 years (median, 62 years; mean, 57.8 years). The carcinomas were located in the cecum (n = 9), ascending colon (n = 29), transverse colon (n = 14), descending colon (n = 9), sigmoid colon (n = 48) and rectum (n = 92). Of these patients, 24 were grade I, 110 grade II and 67 grade III, according to histological grading; and 48 were stage I, 72 were stage II, 70 were stage III and 11 were stage IV, according to clinical TNM stage revised by International Union Against Cancer in 2003. All the patients were followed up for survival. During the follow-up period from the date of surgery until April 30, 2009, 126 patients died and 75 were alive (median survival time, 51 mo, range, 0.10-115 mo).
The resected specimens were fixed in 10% formalin. The gross appearance and the size of tumor were recorded. Patients’ medical records and histopathology of each specimen were reviewed. The tissues (cancer and normal) were cut into 5-mm slices, embedded in paraffin for histological and immunohistochemical examinations. Each paraffin block was cut at 4 μmol/L in thickness, and sections were mounted onto adhesive-coated slides. Slides were deparaffinized in xylene twice for 10 min and rehydrated through descending concentration of ethanol. Antigen retrieval was performed in 0.01 mol/L citrate buffer (pH 6.0) by microwave oven for 2 min and 30 s at 100°C. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxidase for 15 min. After washing with phosphate-buffered saline (PBS), the sections with primary polyclonal rabbit antibody to human LRP16 (recognized and isolated in 1999 by Department of Molecular Biology of our hospital) that was diluted 1:1000 in blocking solution were incubated at 4°C overnight, in a humidified chamber. After washing three times with PBS, the sections incubated for 30 min with biotinylated secondary antibody (polyperoxidase-anti-mouse/rabbit IgG, Zymed) then washed again with PBS. 3,3’-Diaminobenzidine was used as the chromogen. Sections were then counterstained with hematoxylin for 1 min, raised in water, dehydrated in ascending concentrations of ethanol followed by clearance with xylene, and cover slipped permanently for light microscopy. Normal ovary tissue was used as a positive control, whereas the primary antibody was replaced by PBS as a negative control.
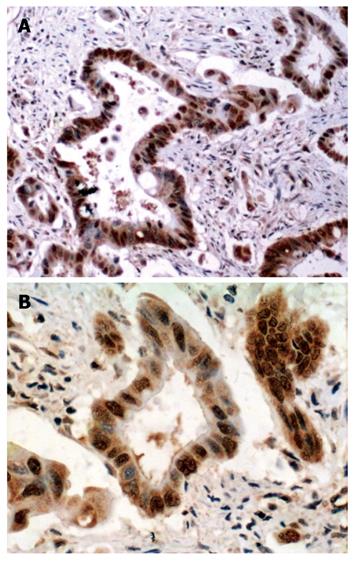
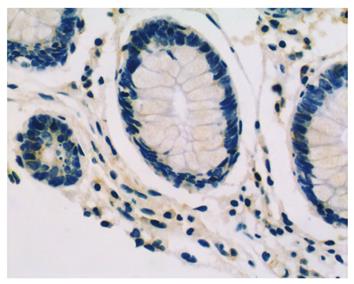
Immunohistochemically stained slides were reviewed by two investigators independently who were blinded to all clinical data. Slides were analyzed under light microscopy using manual methods. Staining was graded for intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong) and percentage of cells stained (0, < 5%; 1, 6%-25%; 2, 26%-50%; 3, > 50%). The final score was determined by the combined staining score (extent + intensity). Score 0 was defined as negative expression (-), scores 1-3 as weak staining pattern (+), and 4-6 as strong expression (++).
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, Illinois). The χ2 test was used to examine the various clinicopathological characteristics and LRP16 expression. Cumulative survival curves were drawn by the Kaplan-Meier method. The difference between the curves was analyzed by the Log-rank test. Multivariate survival analysis was based on Cox proportional hazard model. P < 0.05 was considered statistically significant.
LRP16 staining was performed in 201 colorectal cancer patients and 60 cases of normal tissues by IHC.The result showed that in cancer tissues, LRP16 staining was negative (-) in 41.8% (84/201) cases, weak positive (+) in 21.4% (43/201) cases and strong positive (++) in 36.8% (74/201) cases; whereas in normal mucosas, LRP16 was negative in 65% (39/60) cases, weak positive in 13.3% (8/60) and strong positive in 21.7% (13/60) cases. As a whole, LRP16 expression was detected in 58.2% (117/201) colorectal carcinoma, and in 35% (21/60) distal normal mucosa (Figures 1 and 2). The expression of LRP16 protein was found in cell nucleus or both cell nucleus and cytoplasm. The difference of LRP16 expression between colorectal cancer and normal mucosa was statistically significant (χ2 = 9.998, P = 0.002).
Upon clinicopathological analysis, more LRP16 expression was present in bigger tumors (P = 0.001). When comparing the LRP16 status with clinicopathological variables, we found significant positive correlations between LRP16 expression and degree of differentiation (P = 0.010), depth of invasion (P = 0.001), lymph node metastasis (P = 0.001) and distant metastasis (P = 0.027). The level of LRP16 in cases of low TNM stage (I + II) was lower than that of high stage (III + IV) (χ2 = 27.088, P = 0.001) (Table 1).
| Variables | LRP16 | Statistical value | |
| Positive group (n = 197) | Negative group (n = 139) | ||
| Gender | |||
| Male | 78 (61.4) | 49 (38.6) | χ2 = 1.460a |
| Female | 39 (52.7) | 35 (47.3) | P = 0.227 |
| Age (yr) | |||
| < 45 | 20 (64.5) | 11 (35.5) | χ2 = 0.640a |
| 45 ≤n < 60 | 36 (58.0) | 26 (42.0) | P = 0.726 |
| ≥ 60 | 61 (56.5) | 47 (43.5) | |
| Tumor size (cm) | |||
| d < 5 | 68 (50.0) | 68 (50.0) | χ2 = 13.604a |
| 5 ≤ d < 10 | 42 (72.4) | 16 (27.6) | P = 0.001 |
| d ≥ 10 | 7 (100.0) | 0 (0.0) | |
| Tumor location | |||
| Cecum | 5 (55.6) | 4 (44.4) | χ2 = 6.027a |
| Ascending colon | 22 (75.9) | 7 (24.1) | P = 0.304 |
| Transverse colon | 8 (57.1) | 6 (42.9) | |
| Descending colon | 6 (66.7) | 3 (33.3) | |
| Sigmoid colon | 29 (60.4) | 19 (39.6) | |
| Rectum | 47 (51.1) | 45 (48.9) | |
| Histologic differentiation | |||
| Well differentiated | 12 (50.0) | 12 (50.0) | χ2 = 9.210a |
| Moderately differentiated | 56 (50.9) | 54 (49.1) | P = 0.010 |
| Poorly differentiated | 49 (73.1) | 18 (226.9) | |
| Depth of invasion, T stage | |||
| T1 | 2 (28.6) | 5 (71.4) | χ2 = 25.470a |
| T2 | 25 (36.8) | 43 (63.2) | P = 0.001 |
| T3 | 79 (69.9) | 34 (30.1) | |
| T4 | 11 (84.6) | 2 (15.4) | |
| Lymph node metastasis | |||
| LN = 0 | 58 (45.3) | 70 (54.7) | χ2 = 24.735a |
| LN = 1-3 | 43 (78.2) | 12 (21.8) | P = 0.001 |
| LN > 3 | 16 (88.9) | 2 (11.1) | |
| Distant metastasis | |||
| Negative | 107 (56.3) | 83 (43.7) | χ2 = 5.115a |
| Positive | 10 (90.9) | 1 (9.1) | P = 0.027 |
| TNM stage | |||
| I-II | 52 (43.3) | 68 (57.7) | χ2 = 27.088a |
| III-IV | 65 (80.2) | 16 (19.8) | P = 0.001 |
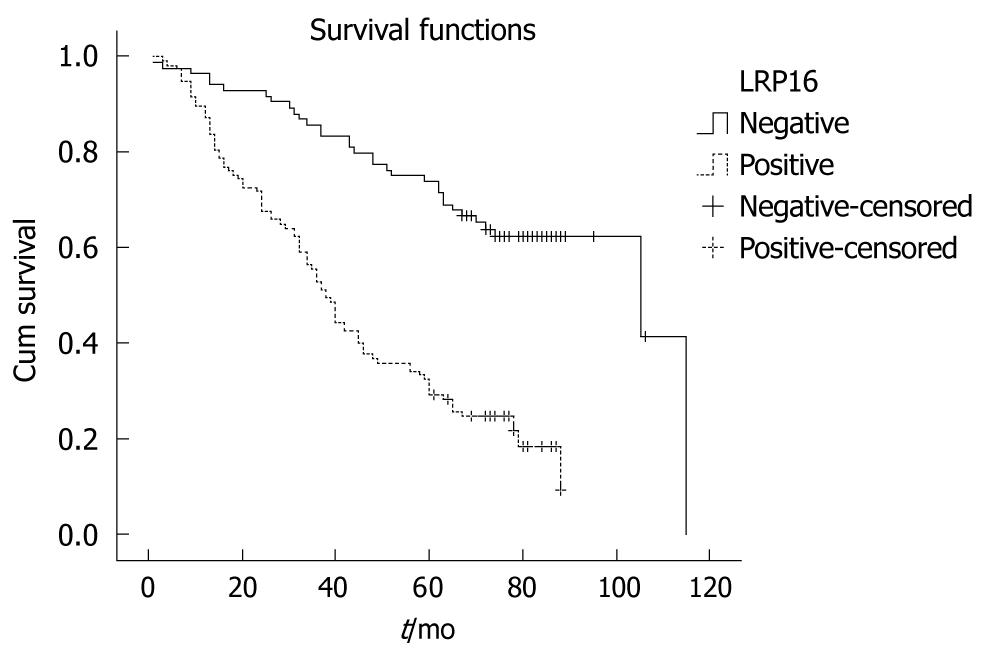
All 201 patients were followed up for survival to assess LRP16 expression as a prognostic factor. Kaplan-Meier survival curves and Log-rank test demonstrated that LRP16 positive cases showed a significantly shortened median survival time (38.0 mo) in comparison with other patients (105.0 mo) (Log rank = 41.455, P = 0.001) (Figure 3). The overall 5-year survival rate of the LRP16 negative patients (71.4%) was higher than that of the LRP16 positive group (29.1%). LRP16 expression appeared as a significant independent prognostic factor (P = 0.001) with a relative risk of 2.082 (confidence interval, 1.3-3.2) in the multivariate survival analysis of the patients. Other independent prognostic factors in multivariate survival analysis included tumor shape, degree of differentiation, depth of invasion and distant metastasis (Table 2).
| Prognostic variables | B | SE | Wald value | P value | RR |
| Gender | 0.261 | 0.197 | 1.756 | 0.185 | 1.298 |
| Age (yr) | 0.191 | 0.124 | 2.366 | 0.124 | 1.211 |
| Tumor location | 0.071 | 0.060 | 1.338 | 0.239 | 1.074 |
| Histologic differentiation | 0.747 | 0.162 | 21.128 | 0.001 | 2.110 |
| Tumor size | 0.123 | 0.188 | 0.433 | 0.510 | 1.131 |
| Depth of invasion | 0.508 | 0.199 | 6.523 | 0.011 | 1.661 |
| Lymph node metastasis | 0.060 | 0.206 | 0.085 | 0.771 | 1.062 |
| Distant metastasis | 1.450 | 0.526 | 7.589 | 0.006 | 4.264 |
| TNM stage | 0.717 | 0.236 | 9.236 | 0.002 | 2.049 |
| LRP16 expression | 0.741 | 0.225 | 10.868 | 0.001 | 2.099 |
LRP16 was originally recognized and isolated from human lymphocytes in 1999[1]. It was identified as an estrogen responsive gene[1-5]. The expression of LRP16 was found in different tissues in varying degrees, including ovary, testicle, prostate, small intestine, spleen, thymus and stomach[2-3,6]. Furthermore, LRP16 is overexpressed in tumors, compared with their matched normal tissues[2]. Some studies have indicated that LRP16 may play an important role in the carcinogenesis and progression of hormone-dependent breast cancer[5,11]. Overexpression of LRP16 significantly stimulated MCF-7 cell proliferation by promoting G1/S transition[4]. The suppression of the endogenous LRP16 in ERα-positive MCF-7 cells not only inhibits cell growth but also significantly attenuates the cellular estrogen-responsive proliferation ability and sensitizes tumor cells to radiation[11-13]. However, some authors thought that expression of LRP16 in ERα-negative cells had no effect on proliferation [11]. The expression of LRP16 gene was dependent on the estrogen activities[14,15], LRP16 was also involved in estrogen signaling and could strengthen the ERα-responsive gene activation, therefore, it is also considered as an ERα coactivator[11]. Previous studies have demonstrated that ERα/PR status, tumor size and auxiliary lymph node metathesis were closely correlated with LRP16 overexpression[16]. ER includes two subtypes, ERα and ERβ. They have been identified in colorectal cancer tissue and normal mucosa[8-10]. It was also reported that the ER expression level of carcinoma was higher than that of non-cancerous colon tissues[10]. Activation of ER signaling pathway plays an important role in multi-tissue development[17-20]. Therefore, we propose that LRP16, a coactivator of ERα, may display an important function in the carcinogenesis and progression of colorectal cancer as in breast cancer.
Although the detailed molecular mechanism involved in this process is unclear, our study have potentially clinical benefits. LRP16 expression that could be detected by IHC may be a useful molecular marker to predict the prognosis in colorectal carcinoma patients. Moreover, LRP16 and ERα could inter-regulate each other, as LRP16 is an ERα coactivator. This study raises the possibility that anti-estrogen therapy could be used in the patients with high LRP16 expression. This information may help us individualize patient care (e.g. progression and prognosis of patients after operation). In this study, LRP16 expression was commonly up-regulated in colorectal carcinoma and was associated with shortened survival time of the patients in univariate and multivariate analyses. However, further investigations and clinicopathological correlation are necessary.
Colorectal cancer is a major cause of cancer–related morbidity and mortality. In the Western world it is the second leading cause of death from cancer. The evidence of colorectal cancer has increased recently in China. Although this disease is curable by surgical interventions together with chemotherapy and radiation in its early stages, the patients are often asymptomatic before the metastasis occurred. Therefore, effective screening and preventive strategies for colorectal cancer are necessary to enhance our capability to predict the clinical outcome of the disease.
Leukemia related protein 16 (LRP16) gene plays an important role in the carcinogenesis and progression of hormone-dependent breast cancer. LRP16 expression was also reported to be associated with invasion, metastasis and prognosis of gastric carcinoma. But no comprehensive description of LRP16 protein expression in colorectal cancer has been reported. In this study, the authors investigated the expression patterns of LRP16 protein in human colorectal cancers and compared the clinical and pathological variables including survival time of the patients.
To date, only limited data on LRP16 protein expression in solid tumors are available. This study is believed to be the first trial for verifying the relationship between LRP16 expression and clinicopathological factors of colorectal carcinoma.
The authors presented some evidences to show a significant association between high LRP16 expression in colorectal cancer and early disease progression or the disease related death, and they believe that the LRP16 expression status detected by immunohistochemistry (IHC) may be a molecular marker to predict the prognosis of colorectal carcinoma patients.
In this paper the authors have analyzed by IHC the expression of LRP16 protein in 201 cases of colorectal carcinoma and 60 cases of distal normal mucosae and they have correlated the expression of this marker with the degree of differentiation, invasiveness, metastasis and prognosis of colorectal carcinoma. The study is well performed and the results are quite interesting, and are potentially helpful for clinical application, the quality of the research and presentation, including photomicrographs and figures, is high, the discussion is clear and well written.
Peer reviewers: Dr. John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom; Dr. Lucia Ricci Vitiani, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena, 299, Rome 00161, Italy
S- Editor Wang JL L- Editor Ma JY E- Editor Zheng XM
| 1. | Yu L, Han WD, Lou FD, Wang QS, Zhao Y, Caligiuri MA. Cloning of leukemia associated gene LRP16 in acute myeloid leukemia. Junyi Jinxiu Xueyuan Xuebao. 2000;21:81-84. [Cited in This Article: ] |
| 2. | Han WD, Yu L, Lou FD, Wang QS, Zhao Y, Shi ZJ, Jiao HY, Zhou JJ. Cloning and Expression Characterization of the Full Length cDNA for a Novel Leukemia-associated Gene LRP16. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao. 2001;17:209-214. [Cited in This Article: ] |
| 3. | Han WD, Lou FD, Yu L, Wang QS, Han XP, Li JX. SAGE pattern of LRP16 gene and its expression in normal blood and leukemic cells. Junyi Jinxiu Xueyuan Xuebao. 2002;23:161-163. [Cited in This Article: ] |
| 4. | Han WD, Mu YM, Lu XC, Xu ZM, Li JX, Yu L, Song HJ, Li M, Lu JM, Pan CY. Estrogen stimulates human breast cancer MCF-7 cell proliferation by up-regulation of LRP16 mRNA via activation of estrogen receptor-alpha. Zhonghua Neifenmi Daixie Zazhi. 2004;20:165-168. [Cited in This Article: ] |
| 5. | Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L, Song HJ, Li M, Lu JM, Zhao YL. Up-regulation of LRP16 mRNA by 17beta-estradiol through activation of estrogen receptor alpha (ERalpha), but not ERbeta, and promotion of human breast cancer MCF-7 cell proliferation: a preliminary report. Endocr Relat Cancer. 2003;10:217-224. [Cited in This Article: ] |
| 6. | Li YZ, Zhao P, Han WD. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J Gastroenterol. 2009;15:4833-4837. [Cited in This Article: ] |
| 7. | Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376-388. [Cited in This Article: ] |
| 8. | Jiang H, Teng R, Wang Q, Zhang X, Wang H, Wang Z, Cao J, Teng L. Transcriptional analysis of estrogen receptor alpha variant mRNAs in colorectal cancers and their matched normal colorectal tissues. J Steroid Biochem Mol Biol. 2008;112:20-24. [Cited in This Article: ] |
| 9. | Di Leo A, Messa C, Cavallini A, Linsalata M. Estrogens and colorectal cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:1-12. [Cited in This Article: ] |
| 10. | Zhou ZW, Wan DS, Wang GQ, Pan ZZ, Lu HP, Gao JH, Ding PR. [Expression of estrogen receptor and progesterone receptor in colorectal cancer: a quantitative study]. Ai Zheng. 2004;23:851-854. [Cited in This Article: ] |
| 11. | Han WD, Zhao YL, Meng YG, Zang L, Wu ZQ, Li Q, Si YL, Huang K, Ba JM, Morinaga H. Estrogenically regulated LRP16 interacts with estrogen receptor alpha and enhances the receptor's transcriptional activity. Endocr Relat Cancer. 2007;14:741-753. [Cited in This Article: ] |
| 12. | Han WD, Zhao YL, Li Q, Mu YM, Li X, Song HJ, Lu ZQ. Inhibition of proliferation of human breast cancer MCF-7 cells by small interference RNA against LRP16 gene. Chin J Cancer Res. 2004;16:239-245. [Cited in This Article: ] |
| 13. | Han WD, Yang D, Li Q, Zhao XL, Ma L, Mu YM. Improvement of radiation sensitivity by inhibiting expression of the human LRP16 gene in tumor cells. Junyi Jinxiu Xueyuan Xuebao. 2005;26:183-185. [Cited in This Article: ] |
| 14. | Zhao YL, Han WD, Li Q, Mu YM, Lu XC, Yu L, Song HJ, Li X, Lu JM, Pan CY. Mechanism of transcriptional regulation of LRP16 gene expression by 17-beta estradiol in MCF-7 human breast cancer cells. J Mol Endocrinol. 2005;34:77-89. [Cited in This Article: ] |
| 15. | Lu XC, Lou FD, Han WD, Zhu XD, Mu YM, Xu ZM, Yu L. [Analysis of LRP16 gene promoter activity]. Zhongguo Shiyan Xueyexue Zazhi. 2006;14:146-149. [Cited in This Article: ] |
| 16. | Liao DX, Han WD, Zhao YL, Pu YD, Mu YM, Luo CH, Li XH. [Expression and clinical significance of LRP16 gene in human breast cancer]. Ai Zheng. 2006;25:866-870. [Cited in This Article: ] |
| 17. | Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res. 2007;16:281-314. [Cited in This Article: ] |
| 18. | Morissette M, Jourdain S, Al Sweidi S, Menniti FS, Ramirez AD, Di Paolo T. Role of estrogen receptors in neuroprotection by estradiol against MPTP toxicity. Neuropharmacology. 2007;52:1509-1520. [Cited in This Article: ] |
| 19. | Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977-1985. [Cited in This Article: ] |
| 20. | Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823-6833. [Cited in This Article: ] |