Published online Jan 28, 2009. doi: 10.3748/wjg.15.489
Revised: December 22, 2008
Published online: January 28, 2009
AIM: To investigate the macroscopic and clinico-pathologic features of gastric cancer in patients with biopsy-suggested high grade intraepithelial neoplasia.
METHODS: Patients with biopsy-confirmed gastric high grade intraepithelial neoplasia were reviewed from January 2001 to March 2008. Pathologic sections were re-evaluated by two senior pathologists. Patients with an en-bloc resection of the lesion within two months after the diagnosis of high grade intraepithelial neoplasia were enrolled in the study. Clinical manifestations, endoscopic features, biopsy and surgical pathology of all patients were collected and analyzed. The data acquired were subjected to univariate and multivariate analysis.
RESULTS: Seventy-two superficial gastric lesions with a pathologic diagnosis of high grade intraepithelial neoplasia based on biopsy specimens were enrolled. True high grade intraepithelial neoplasia was finally proved in 16 lesions and gastric cancer in the rest 56 lesions, most of which (96.4%) were differentiated carcinomas. The result of univariate analysis indicated that the size and the presence of marked ulcer plaque or scar in a superficial lesion were independently associated with gastric cancer (P < 0.05), when high grade intraepithelial neoplasia was diagnosed by biopsy pathology. The results of multivariate analysis revealed the size greater than 1.5 cm [odds ratio (OR) 18.400, P < 0.001] and the presence of 5-odd mm ulcer plaque or scar (OR 10.000, P = 0.044) were associated with gastric cancer. Accordingly, the sensitivity, specificity and negative predictive value of multivariate analysis for predicting “true high grade intraepithelial neoplasia” was 87.5%, 89.3% and 96.2%, respectively.
CONCLUSION: Macroscopic findings are of value in differentiation between high grade intraepithelial neoplasia and superficial gastric cancer. This may simplify patient work-up and save costs for patients and healthcare system.
- Citation: Wu W, Wu YL, Zhu YB, Wei Q, Guo Y, Zhu ZG, Yuan YZ. Endoscopic features predictive of gastric cancer in superficial lesions with biopsy-proven high grade intraepithelial neoplasia. World J Gastroenterol 2009; 15(4): 489-495
- URL: https://www.wjgnet.com/1007-9327/full/v15/i4/489.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.489
The reported incidence of severe gastric dysplasia developing into gastric carcinoma varies from 10% to 85% in different studies[12345678910]. These studies were mainly performed in the 1980s[23] and 1990s[4567], except for a recent study drawing a significantly different conclusion compared with the other studies[8]. Although these studies contributed to the current understanding of gastric cancer, their results were obtained mainly through long-term follow-up and re-biopsy. In clinical practice and in our experience, a pathologic diagnosis based on biopsy samples may be inadequate in evaluating the severity of lesions. Besides site and quantity, biopsy bias is inevitably associated with the activity of tissue inflammation and regeneration. For instance, it is well recognized that a biopsy sample containing glandular distortion and atypia on an apparent inflammatory background always leads to a diagnosis of regeneration rather than a diagnosis of neoplasia.
A two-stage evaluation system of intraepithelial neoplasia (IEN) was introduced to gastrointestinal tumors by International Authority for Research on Cancer (IARC) in 2000 to replace the term “dysplasia”[9]. Few relevant studies have been published since then. Our preliminary study on the association between gastric HGIEN and invasive cancer has drawn promising results[10]. Further researches will enhance the clinical understanding of precancerous lesions and help distinguish HGIEN from invasive cancer under routine conventional endoscope, without the aid of complex methodologies like magnifying endoscopy or narrow-band imaging. This would improve the work-up of such patients, potentially saving costs for healthcare system and patients. In the present study, we analyzed the macroscopic and clinicopathologic features of superficial neoplastic lesions in an attempt to identify the macroscopic features independently associated with the presence of cancer.
One hundred and four lesions in 103 consecutive patients were pathologically diagnosed as HGIEN of gastric mucosa based on biopsy samples obtained by EGD procedures in Shanghai Ruijin Hospital from January 2001 to March 2008. The charts, EGD reports and histologic sections were reviewed. Histopathologically, suspected stromal invasion was identified in 4 lesions. Endoscopically, lesions with an appearance strongly suggestive of advanced gastric cancer (i.e. Borrmann-type gastric cancer) were found in 21 patients, including fungating type in 1 patient, ulcerated in 7 and infiltrative ulcerated in 13 patients. These patients, excluded from the study, were later proved to have advanced gastric cancer. Seventy-nine superficial lesions were confirmed in 78 patients. Of these 78 patients, 6 refused interventional treatment while the remaining one was not a candidate for resection because of severe co-morbidities and poor overall condition. Seventy-one patients (59 males, 12 females, range 35-82 years, and a mean age of 60.5 ± 9.9 years) with 72 superficial neoplastic lesions were finally enrolled in the present study.
All patients gave their written consent to undergo endoscopic or surgical resection with the knowledge that the lesion may not be carcinomatous or infiltrative and the widely recognized guideline in treating GI neoplastic lesions with a biopsy diagnosis of HGIEN was not well established. Patients for endoscopic treatment were well informed about possibilities of incomplete resection, tumor recurrence, hemorrhage and other complications beforehand.
EGD procedures were carried out on each patient prior to resection using either EG 410HR or EG 590WR video endoscope (Fujinon, Saitama, Japan). Characteristics of the lesion, including its location, size, contour, and ulceration if present, as well as changes in mucosal rugae and endoscopic type, were documented. If an ulcer or ulcer scar was noted, descriptive terms and an estimation of the size of ulcer plague or scar was obtained. Lesions were classified according to the Paris endoscopic classification of superficial neoplastic lesions[11]. “Superficial” lesions are defined when endoscopic appearance suggests either a small cancer or a noninvasive neoplastic lesion[11]. Recognition of superficial gastric lesions was also based on our experience in the endoscopic diagnosis of early gastric cancer. Biopsy was obtained and all patients were diagnosed as HGIEN based on biopsy samples.
Abdominal US and enhanced CT scan were performed and distant metastasis was ruled out in all patients. All patients received en-bloc resection within two months after the initial diagnosis of HGIEN. Surgery was preferentially carried out given its advantage in curative resection irrespective of tumor size and invasion depth. Endoscopic resection was strictly limited to type 0-Ior 0-IIa lesions with fine deformability and no ulceration. Lesions less than 15 mm in size were subjected to EMR using a strip biopsy method[12] and otherwise to endoscopic submucosal dissection (ESD) using the insulated-tip knife[13].
The diagnosis of HGIEN was confirmed by two senior pathologists (Y-B. Z & Q. W). The IARC diagnostic criterion for HGIEN was applied[1], in which HGIEN is characterized by increasing architectural distortion with glandular crowding and prominent cellular atypia. An increased proliferative activity was present throughout the epithelium where no stromal invasion occurred. A lesion suspected of stromal invasion was ruled out of this series. Standardized sectioning of the resected specimen was carried out according to the guideline by JGCA[14]. Photograph of the specimen was taken before sectioning and a schematic map of sectioning was drawn. “True HGIEN” was diagnosed when each section of the en-bloc resected specimen met the criteria of HGIEN. Gastric carcinoma is diagnosed when the tumor invades to the lamina propria[1].
Endoscopic and clinicopathologic variables that might be predictive of gastric cancer were analyzed, including age, sex, history of melena or weight loss, time frame between the diagnosis of HGIEN and resection, endoscopic type (0-I/0-IIa, 0-IIc, 0-IIa + IIc/0-IIc + IIa, 0-IIc + III/0-III; mixed type), exact lesion size, location, mucosal rugae changes and presence of marked ulcer plaque or ulcer scar on the lesion. Continuous variables (age, time frame, exact lesion size) were compared using group t test when the variables were normally distributed and Wilcoxon test when a normal distribution was not observed. Categorical variables [sex, history of melena or weight loss, endoscopic type (0-I/0-IIa, 0-IIc, 0-IIa + IIc/0-IIc + IIa, 0-IIc + III/0-III; mixed type), lesion size with a cut-off value of 1.5 cm, location, mucosal ruga changes and presence of marked ulcer plaque or ulcer scar] were compared using χ2 test or continuity-adjusted χ2 test. Multiple logistic regression analysis was used to identify the factors independently associated with gastric cancer. P < 0.05 was considered statistically significant. All analyses were performed using the SAS software (SAS Institute, Cary, NC, USA).
Of the 71 patients, 69 were presented with symptoms while the other 2 were asymptomatic. The time of symptoms ranged 1 wk-11 years. The indications for EGD are presented in Table 1.
| Indications for endoscopy | Patients (n) |
| Abdominal pain | 391 |
| Abdominal discomfort | 102 |
| Abdominal distension | 7 |
| Melena | 6 |
| Retroxyphoid pain | 6 |
| Asymptomatic physical check-up | 2 |
| Anemia | 1 |
Of the 71 patients, 69 were proved to have isolated HGIEN lesions in the stomach, one was found to have two biopsy-proven HGIEN lesions both in antrum and in fundus, and the other one was found to have gastric polyposis involving antrum and corpus. Of the 72 lesions, 28 were located in antrum, 20 in angularis, 10 in corpus, 1 in fundus and 11 in cardia. Antrum, angularis and corpus were involved in another 2 lesions.
All patients received either surgical or endoscopic resection within two months of initial diagnosis. The time frame between initial diagnosis of HGIEN and resection was 19.2 ± 11.8 d. Open gastrectomy and laparoscopic-assisted gastrectomy were performed on 53 and 14 patients, respectively. Three patients received EMR and one received ESD. All surgical and endoscopic resections were proved curative with horizontal and vertical R0 margins.
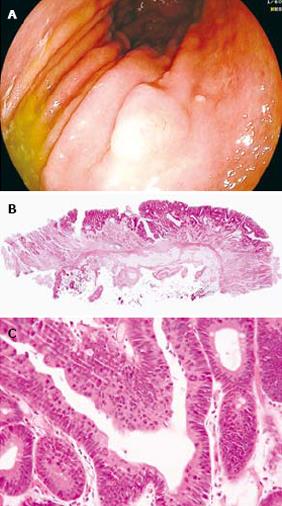
Of the 72 lesions, 16 (22.2%) were pathologically confirmed as true HGIEN of gastric mucosa, 51 (70.8%) were early gastric cancer and 5 (6.9%) were advanced gastric cancer. Among the early gastric cancer, 34 were intramucosal and 17 were submucosal cancer.. Advanced gastric cancer in the 5 cases invaded the superficial layer of muscularis propria. Differentiated adenocarcinoma was found in 54/56 lesions (96.4%) while undifferentiated adenocarcinoma was found in 2/56 lesions (3.6%). A case of true HGIEN of gastric mucosa is presented in Figure 1.
All lesions were divided into “true HGIEN” group (n = 16) and gastric cancer group (n = 56), according to their final diagnosis. A comparison of endoscopic and clinicopathological parameters of the two groups is shown in Table 2.
| Parameter | True HGIEN group (n = 16) | Gastric cancer group (n = 56) | P value |
| Age (yr) | 60.6 ± 7.5 | 60.5 ± 10.4 | 0.987 |
| Male sex | 11 (91.7) | 49 (87.5) | 0.163 |
| History of melena or weight loss | 2 (12.5) | 10 (17.9) | 0.899 |
| History of previous target biopsy | 6 (37.5) | 17 (30.4) | 0.589 |
| Time frame (d) | 19.7 ± 11.8 | 19.0 ± 11.9 | 0.955 |
| Macroscopic type | |||
| 0-I/0-IIa | 4 (25.0) | 9 (16.1) | 0.652 |
| 0-IIc | 9 (56.2) | 26 (46.4) | 0.488 |
| 0-IIa + IIc/IIc + IIa | 3 (18.8) | 13 (23.2) | 0.970 |
| 0-IIc + III/0-III | 0 (0) | 8 (14.3) | 0.249 |
| Mixed type1 | 3 (18.8) | 19 (33.9) | 0.393 |
| Details of endoscopic findings | |||
| Lesion size (cm) | 1.2 ± 0.4 | 2.6 ± 2.1 | < 0.0012 |
| > 1.5 cm | 2 (12.5) | 46 (82.1) | < 0.0012 |
| Location (distal stomach) | 10 (62.5) | 38 (67.8) | 0.688 |
| Mucosal ruga changes | 0 (0) | 5 (8.9) | 0.496 |
| Marked ulcer plaque3 or ulcer scar | 1 (6.2) | 34 (60.7) | < 0.0012 |
Twenty-three patients previously underwent EGD procedure within past 2 years. Of the 23 patients, one had normal EGD and 22 were identified to have 23 lesions. Targeted biopsy revealed high grade intraepithelial neoplasia in 17 cases, low-grade intraepithelial neoplasia in 2 and no neoplasia in 4 cases, respectively. Six out of the 23 lesions were finally proved to be true HGIEN of gastric mucosa.
Since endoscopy is an essential method in the diagnosis of gastrointestinal neoplasm and endoscopic appearance of a lesion plays an important role in predicting its characteristics and its invasion depth or metastasis[15], macroscopic findings under conventional endoscopy were analyzed in this study, including macroscopic type, lesion size, location, mucosal ruga changes (e.g. convergence, tapering, abruption, etc) and presence of ulcer plaque or ulcer scar, all of which are easily accessible in daily practices. Location was categorized as proximal (body, cardia and fundus of stomach) or distal (antrum and angularis). Presence of marked ulcer plaque/ulcer scar was defined as one or several ulcer plaques/ulcer scars at least 5 mm in size noted on the lesion.
The results of univariate analysis indicate that gastric cancer was associated with the size of lesion and the presence of marked ulcer plaque or ulcer scar (P < 0.05). Abruption of mucosal rugae was noted around 5 lesions, all of which were proved to be gastric cancer, so did the 8 lesions presenting with an ulcerated type (0-IIc + III or 0-III). However, no statistical significance was identified in these parameters.
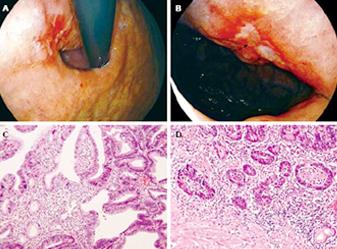
Multivariate analysis revealed that a lesion larger than 1.5 in size (OR 18.400, 95% CI 3.364-100.653, P < 0.001) and the presence of marked ulcer plaque or ulcer scar (OR 10.000, 95% CI 1.062-94.111, P = 0.044) were associated with gastric cancer (Table 3). The value of ulcer plaque in the diagnosis of invasive cancer is demonstrated in Figure 2.
| Variable | Odds ratio (95% CI) | P value |
| Age (yr) | - | 0.773 |
| Gender (male) | - | 0.328 |
| Endoscopic findings | ||
| 0-IIc + III/0-III | - | 0.548 |
| Mixed type2 | - | 0.947 |
| Lesion size > 1.5 cm | 18.400 (3.364-100.653) | < 0.0011 |
| Mucosal ruga changes | - | 0.685 |
| Marked ulcer plaque3 or ulcer scar | 10.000 (1.062-94.111) | 0.0441 |
A macroscopic criterion for true HGIEN of gastric mucosa was accordingly proposed as a lesion ≤ 1.5 cm in size, and absence of 5-odd mm ulcer plaque or ulcer scar on the premise that HGIEN was suggested by biopsy pathology. A total 20 lesions in this series met the above criteria, of which, 14 were finally proved to be “true HGIEN” of gastric mucosa, and 6 were proved to be intramucosal gastric cancer. The sensitivity, specificity and negative predictive value of multivariate analysis for predicting “true HGIEN” was 87.5%, 89.3% and 96.2%, respectively.
Dysplasia is now replaced by IEN to indicate intraepithelial neoplastic changes with the absence of stromal invasion. It was reported that 0%-23% of mild dysplasia might progress to invasive cancer while the incidence of severe dysplasia is estimated to be 70%-85% in Western studies[123456716] and 10% in Japanese reports[8]. Most of these studies focusing on long-term follow-up results had a limited number of patients enrolled. Furthermore, the initial patient status is usually decided by endoscopy, which may fall into the pit of underestimation caused by inevitable biopsy bias.
China is an East Asian country with a high risk of gastric cancer[9]. The incidence of age-adjusted gastric cancer in males and females was 46.5 and 21.0 per 100 000 in Shanghai, China in 1996[17]. The number decreased to 27.4 and 14.0 per 100 000 in 2002 and 2004, still significantly higher than that in most Western countries and Japan[18]. The number of gastric cancer patients per year in the authors” institution, however, has increased significantly in recent years with a total of 2355 cases of surgically proved gastric cancer from January 2001 to March 2008[19]. In addition, intestinal-type gastric carcinoma, a consequence of HGIEN if left untreated[20], is found more common in high risk areas than in low risk areas of gastric cancer. In this study, all patients received curative resection at a mean interval of 19 d between the diagnosis of HGIEN and resection, and invasive carcinoma was found in up to 77.8% lesions. It was reported, however, that the time frame between initial diagnosis of severe dysplasia and identification of gastric cancer, was between four and twenty-three months on average[245678921]. It implys that most gastric lesions diagnosed as HGIEN (severe dysplasia) are underestimated and a lot biopsy-proven “HGIEN” lesions actually are gastric cancer in the meantime. The process of HGIEN advancing to gastric cancer may not be what meets the eye through repeated
biopsy.
Although the diagnosis of HGIEN was carefully considered in the study to rule out possible invasion in biopsy samples, it was almost inevitable to get rid of biopsy bias. Multiple causes lead to biased biopsy sampling, including missed target and inadequate tissue amount. Active inflammation of the tissue sample may conceal neoplastic architectural distortion and lead to false negative results. Short-course (2 wk) proton pump inhibitor treatment is suggested for such condition and re-biopsy should be performed. A combination of well-differentiated cancer tissue and absent muscularis mucosa is a typical case in a biopsy sample leading to a diagnosis of HGIEN. The reason why there were not so many well-differentiated adenocarcinomas in this series is probably due to the fact that gastric cancer tends to be less differentiated as it penetrates into deeper layer[22]. It was reported that as high as 11% of biopsy-proven differentiated early gastric cancer turns out to be undifferentiated at surgery[23]. Cryptal dysplasia is another case in which atypia originates from deeper portion of the mucosa and penetrates downward; the epithelium is sometimes spared with little trace of IEN[21]. In this series, only 3.6% of the lesions were proved to be undifferentiated gastric cancer, indicating that this histological type is unlikely to present in biopsy-proven HGIEN.
Macroscopically, superficial changes or markedly ulcerated appearance can be found in a lesion diagnosed as HGIEN by biopsy. However, all “true HGIEN” lesions in this series were less than 20 mm in size while 87.5% of which were less than 15 mm in size. To draw a definite line between HGIEN and gastric cancer is almost unworkable under endoscope, yet a marked lesion suggestive of Borrmann-type gastric cancer always turns out to be invasive cancer[3]. We hereby propose a macroscopic criterion for true HGIEN which can better differentiate true HGIEN from superficial gastric cancer. The criterion involves endoscopic and biopsy parameters easy to be measured in daily practices with conventional endoscope and without the aid of complicated techniques. The relatively low predictive value (70%) for true HGIEN is primarily attributed to the overlapping macroscopic feature of HGIEN and early gastric cancer, both of which can be small and inconspicuous, yet the former seems much unlikely to be a large one. Since HGIEN is a precancerous change without stromal invasion or lymph node metastasis, endoscopic en-bloc resection, both preserving gastric function and improving quality of life of patients, is considered sufficient and curative.
The authors hold that an ulcerated lesion with biopsy-proved HGIEN should always turn out to be invasive cancer, which is supported by the theory of ulcerated early gastric cancer progression proposed by Kyoichi[24], in which the Hauser’s cancer is considered a sporadic case. In this series, although the comparison of ulcerated type between the groups did not reach a level of statistical significance, all the patients (8/8) were proved to have invasive cancer. The lack of significance could be attributed to inadequate population size. Same explanation also goes to the issue of mucosal ruga changes, which has been recognized as signs indicating submucosal tumor invasion[2425]. Experienced pathologists are warranted in making the proper diagnosis of HGIEN without confusion of regenerative changes associated with erosion or healing ulcer or cases suggestive of gastric cancer with inadequate tissue.
In conclusion, a superficial gastric lesion diagnosed as HGIEN by biopsy is most likely to be an early gastric cancer at the time of diagnosis. Lesions greater than 1.5 cm in size and presence of 5-odd mm ulcer plaque or ulcer scar are independently associated with invasive cancer, especially differentiated gastric carcinoma in such a setting. Combining endoscopic characteristics with biopsy results could greatly enhance the diagnostic accuracy of superficial gastric neoplastic lesions, thus reducing the risk of unnecessary gastrectomy, simplifying patient work-up and improving the quality of life of patients. Further investigations are, however, necessary to validate these contentions and to clarify the impact of repeated biopsy on the diagnosis of true HGIEN lesions.
Intraepithelial neoplasia has been proposed by the WHO to replace dysplasia in gastrointestinal epithelial neoplastic changes. However, differential diagnosis between high grade intraepithelial neoplasia and invasive cancer is still confusing when the diagnosis is based on biopsy pathology.
Both intraepithelial neoplasia and early gastric cancer can be presented as superficial lesions. Conventional endoscopy provides direct observation of the lesion, yet the appearance of intraepithelial neoplasia and early gastric cancer resembles each other. It is unhelpful to macroscopically differentiate intraepithelial neoplasia from early cancer. Biopsy pathology may also fail to differentiate intraepithelial neoplasia from early cancer. This study focused on the macroscopic features of gastric lesions with a biopsy diagnosis of high grade intraepithelial neoplasia, suggesting that certain macroscopic features can help for better differentiation.
Conventional endoscopy observation has not been considered to play a role in differential diagnosis between intraepithelial neoplasia and early cancer. Likewise, gastric pit pattern observation either by magnifying endoscopy or by NBI lacks the accuracy to better characterize the lesion. Our study suggested that under typical conditions conventional endoscopy would be capable of differentiating between high grade intraepithelial neoplasia and gastric cancer.
The results of this study enhance the value of routine conventional endoscopy in the diagnosis of clinically confusing conditions. It could simplify the work-up of patients with a biopsy diagnosis of high grade intraepithelial neoplasia and save costs for patients and healthcare system.
Intraepithelial neoplasia means to replace dysplasia for precancerous status with cellular and structural atypia. High grade intraepithelial neoplasia denotes increasing architectural distortion with prominent cell atypia without invasion to lamina propria.
The authors investigated the macroscopic features of gastric superficial lesions with a biopsy diagnosis of high grade intraepithelial neoplasia and compared endoscopic findings with surgical results. It revealed that a lesion > 1.5 cm in size and the presence of ulcer/scar are associated with invasive cancer. The results are of interest and value for better patient work-up.
| 1. | Saraga EP, Gardiol D, Costa J. Gastric dysplasia. A histological follow-up study. Am J Surg Pathol. 1987;11:788-796. [Cited in This Article: ] |
| 2. | Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31:977-983. [Cited in This Article: ] |
| 3. | Kokkola A, Haapiainen R, Laxen F, Puolakkainen P, Kivilaakso E, Virtamo J, Sipponen P. Risk of gastric carcinoma in patients with mucosal dysplasia associated with atrophic gastritis: a follow up study. J Clin Pathol. 1996;49:979-984. [Cited in This Article: ] |
| 4. | Rugge M, Farinati F, Baffa R, Sonego F, Di Mario F, Leandro G, Valiante F. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107:1288-1296. [Cited in This Article: ] |
| 5. | Di Gregorio C, Morandi P, Fante R, De Gaetani C. Gastric dysplasia. A follow-up study. Am J Gastroenterol. 1993;88:1714-1719. [Cited in This Article: ] |
| 6. | Fertitta AM, Comin U, Terruzzi V, Minoli G, Zambelli A, Cannatelli G, Bodini P, Bertoli G, Negri R, Brunati S. Clinical significance of gastric dysplasia: a multicenter follow-up study. Gastrointestinal Endoscopic Pathology Study Group. Endoscopy. 1993;25:265-268. [Cited in This Article: ] |
| 7. | Rugge M, Farinati F, Di Mario F, Baffa R, Valiante F, Cardin F. Gastric epithelial dysplasia: a prospective multicenter follow-up study from the Interdisciplinary Group on Gastric Epithelial Dysplasia. Hum Pathol. 1991;22:1002-1008. [Cited in This Article: ] |
| 8. | Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390-396. [Cited in This Article: ] |
| 9. | Hamilton SR, Aaltonen LA. World health organization classification of tumours: Pathology and genetics of tumours of the digestive system. IARC Press: Lyon 2000; 38-52. [Cited in This Article: ] |
| 10. | Wu W, Guo Y, Wei Q, Wu Y, Wang X, Zhu Z, Yuan P, Zhu Z, Hu W, Yuan Y. Comparison between biopsy confirmed high grade intraepithelial neoplasia of the stomach and relevant surgical results. [abstract]. Ann Oncol. 2007;18:vii100. [Cited in This Article: ] |
| 11. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-S43. [Cited in This Article: ] |
| 12. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [Cited in This Article: ] |
| 13. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [Cited in This Article: ] |
| 14. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. [Japanese] 13th ed. Kanehara: Tokyo 1999; 22-25. [Cited in This Article: ] |
| 15. | Namieno T, Koito K, Higashi T, Takahashi M, Yamashita K, Kondo Y. Assessing the suitability of gastric carcinoma for limited resection: endoscopic prediction of lymph node metastases. World J Surg. 1998;22:859-864. [Cited in This Article: ] |
| 16. | Testino G. Gastric precancerous changes: carcinogenesis, clinical behaviour immunophenotype study and surveillance. Panminerva Med. 2006;48:109-118. [Cited in This Article: ] |
| 17. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [Cited in This Article: ] |
| 18. | Wang X, Wu CX, Zheng Y, Wang JJ. [Time trends and characteristics of gastric cancer incidence in urban Shanghai]. Zhonghua Liuxingbingxue Zazhi. 2007;28:875-880. [Cited in This Article: ] |
| 19. | Wu W, Wu YL, Sun PH, Huang W, Jiang FX, Zhu YB, Guo Y, Wei Q, Yan M, Zhu ZZ. The trend of surgical proportion of early gastric cancer: our experience (in Chinese with English abstract). Chin J Gastroenterol Hepatol. 2008;17:205-208. [Cited in This Article: ] |
| 20. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [Cited in This Article: ] |
| 21. | Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut. 1999;45:784-790. [Cited in This Article: ] |
| 22. | Ikeda Y, Mori M, Kamakura T, Haraguchi Y, Saku M, Sugimachi K. Increased incidence of undifferentiated type of gastric cancer with tumor progression in 912 patients with early gastric cancer and 1245 with advanced gastric cancer. Cancer. 1994;73:2459-2463. [Cited in This Article: ] |
| 23. | Namieno T, Koito K, Higashi T, Shimamura T, Yamashita K, Sato N, Kondo Y. Assessing the suitability of gastric carcinoma for limited resection: histologic differentiation of endoscopic biopsy. World J Surg. 1998;22:865-868. [Cited in This Article: ] |
| 24. | Kyoichi N. The structure of gastric cancer [Japanese] 3rd ed. Igaku-Shoin: Tokyo 2005; 1. [Cited in This Article: ] |
| 25. | The editorial committee of stomach and intestine. The atlas of stomach and intestine [Japanese]. Igaku-Shoin: Tokyo 2001; 180-187. [Cited in This Article: ] |