Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1576
Revised: December 14, 2002
Accepted: December 22, 2002
Published online: July 15, 2003
AIM: To study whether patients with excess deoxycholic acid (DCA) differ from those with normal percentage of DCA with respect to biliary lipid composition and cholesterol saturation of gallbladder bile.
METHODS: Bile was collected during operation through puncturing into the gallbladder from 122 cholesterol gallstone patients and 46 gallstone-free subjects undergoing cholecystectomy. Clinical data, biliary lipids, bile acid composition, presence of crystals and nucleation time were analyzed.
RESULTS: A subgroup of gallstone patients displayed a higher proportion of DCA in bile than gallstone free subjects. By choosing a cut-off level of the 90th percentile, a group of 13 gallstone patients with high DCA levels (mean 50 percent of total bile acids) and a large group of 109 patients with normal DCA levels (mean 21 percent of total bile acids) were obtained. The mean age of the patients with high DCA levels was higher than that of the group with normal levels (mean age: 62 years vs 45 years) and so was the mean BMI (28.3 vs 24.7). Plasma levels of cholesterol and triglycerides were slightly higher in the DCA excess groups compared with those in the normal DCA group. There was no difference in biliary lipid composition, cholesterol saturation, nucleation time or occurrence of cholesterol crystals in bile between patients with high and normal levels of DCA.
CONCLUSION: Gallstone patients with excess DCA were of older age and had higher BMI than patients with normal DCA. The two groups of patients did not differ with respect to biliary lipid composition, cholesterol saturation, nucleation time or occurrence of cholesterol crystals. It is concluded that DCA in bile does not seem to contribute to gallstone formation in cholesterol gallstone patients.
- Citation: Gustafsson U, Sahlin S, Einarsson C. High level of deoxycholic acid in human bile does not promote cholesterol gallstone formation. World J Gastroenterol 2003; 9(7): 1576-1579
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1576.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1576
The two major primary bile acids formed in human liver are cholic acid (CA) and chenodeoxycholic acid (CDCA). During the enterohepatic circulation, CA and CDCA are partly 7a-dehydroxylated by microbial enzymes, yielding deoxycholic acid (DCA) and lithocholic acid, respectively[1]. Only a small amount of lithocholic acid is absorbed from the intestine, whereas DCA is efficiently reabsorbed mainly via active transport in the distal part of ileum but also by passive diffusion along the small intestine and probably also from the proximal part of colon. DCA constitutes usually 10%-35% of bile acids in the bile. However, individuals may vary widely in the proportion of DCA in bile from 0 to more than 50% of biliary bile acids. These wide inter-individual variations in DCA probably reflect the difference in colonic flora.
According to some previous studies, the proportion of DCA in bile of patients with cholesterol gallstone is higher than that of stone-free subjects and it has been suggested that DCA may play a role in cholesterol gallstone formation[2-8]. Other authors have found a normal percentage of DCA in patients with cholesterol gallstones[9-13]. An increased proportion of DCA in the bile acid pool of gallstone patients has been associated with higher levels of 7a-dehydroxylating faecal bacteria[6,14]. In a recent study, Thomas et al[8] found an increased 7a-hydroxylating activity in the caecal aspirates of patients with cholesterol gallstones, which was associated with slower colonic transit time and increased percentage of DCA in fasting serum. Berr et al[6] have reported about a subgroup of cholesterol gallstone patients with DCA excess and increased 7a-dehydroxylating activity and levels of faecal 7a-dehydroxylating bacteria which may trigger cholesterol gallstone disease.
In a recent study including large series of gallstone patients and gallstone free subjects, we could not find a significantly higher proportion of biliary DCA in cholesterol gall-stone patients compared to pigment stone patients or gallstone-free subjects[13]. No correlation was found between the percentage of DCA and cholesterol saturation in gallbladder bile. However, a subgroup of the patients with cholesterol gallstones had a high percentage of DCA.
The purpose of this study was to study whether patients with excess DCA differed from patients with a normal percentage of DCA with respect to biliary lipid composition, cholesterol saturation, nucleation time and occurrence of cholesterol crystals in gallbladder bile.
The patients in the present study were included in a recent report from our group dealing with lipid composition of gallbladder bile in large series of patients with cholesterol gallstones and pigment stones and gallstone free subjects undergoing elective cholecystectomy[13]. In this study, only cholesterol gallstone patients (n = 122) and gallstone-free subjects (n = 46) in which data on biliary bile acid composition were available were included. Data on the patients are shown in Table 1. None of the patients had clinical or laboratory evidence of diabetes mellitus, alcohol abuse or other diseases that affect the function of the kidney or the liver. The patients were not on any medications known to affect lipid metabolism. None of the patients received or had been treated with antibiotics prior to or during cholecystectomy. The gallstone-free patients were cholecystectomized either because of polyps, cholesterolosis or adenomyomatosis or because of frequent, recurrent right upper biliary colic and other symptoms suggestive of gallbladder dysfunction.
| Group | n | Female/male ratio(% Female) | Median age years (range) | Body mass index kg/m2(n) | Plasma cholesterol mmol/L (n) | Plasma triglycerides mmol/L (n) |
| Total gallstone | 122 | 96/26 (79) | 49 (18-83) | 25.0 ± 0.3 (111) | 5.7 ± 0.1 (81) | 1.5 ± 0.1 (82) |
| High DCA | 13 | 10/3 (77) | 62b (37-74) | 28.3 ± 1.3b (10) | 6.4 ± 0.3a (11) | 2.2 ± 0.4a (10) |
| Low DCA | 109 | 86/23 (79) | 45 (18-83) | 24.7 ± 0.4 (101) | 5.6 ± 0.1 (70) | 1.5 ± 0.1 (72) |
| Gallstone free | 46 | 38/18 (83) | 45 (20-71) | 23.2 ± 0.5c (45) | 5.8 ± 0.2 (36) | 1.4 ± 0.1 (36) |
Informed consent was obtained from each patient before operation. The ethical aspects of the study were approved by the Ethical Committée at Karolinska Institutet, Stockholm.
All patients were hospitalized in the surgical ward. The operations were done after an overnight fasting. After opening the abdomen or in the case of a laparoscopic procedure following application of pneumoperitoneum and establishing four laparoscopic ports, the gall bladder was completely emptied of bile with a sterile needle and syringe to avoid possible stratification of bile[15]. Indicative of a functioning gallbladder was the presence of dark concentrated bile in the gallbladder and no evidence of impacted stones in the neck of the cystic duct at operation. The gall bladder was then removed. The gallstones were classified as cholesterol stones by visual inspection and when necessary by analysis in the laboratory[16,17]. All gall bladders were routinely examined by a pathologist.
Gallbladder bile was extracted with chloroform-methanol (2/1, w/w) for measurement of cholesterol and phospholipids. Cholesterol was measured by an enzymatic method[18] and phospholipids by the method of Rouser et al[19]. The total bile acid concentration in one aliquot of the bile sample was measured using a 3-alpha-hydroxysteroid dehydrogenase assay[20]. Cholesterol saturation was calculated as a percentage of the predicted cholesterol solubility at the respective biliary lipid concentration and composition as described by Carey[21].
Aliquots of bile were hydrolyzed in 1 mol/L potassium hydroxide at 110 °C for 12 h. The conjugated bile acids were extracted with diethyl ether after acidification to pH1 with hydrochloric acid. After preparation of trimethylsilyl ethers, the bile acids were analyzed by gas-liquid chromatography using 1% Hi-Eff BP 8 as the stationary phase. A Pye Unicam gas chromatograph (Pye Unicam Ltd, Cambridge, UK) equipped with a 1.5 m × 4 mm column was used[22].
Bile samples were examined for typical rhomboid monohydrate cholesterol crystals by polarising light microscopy on pre-warmed slides. Nucleation time (NT) was determined by the method of Holan et al[23] with minor modifications[24].
Data were presented as mean ± SEM. The statistical significance of differences was evaluated with Student's t-test. Nucleation time was evaluated by Wilcoxon's sum of rank test.
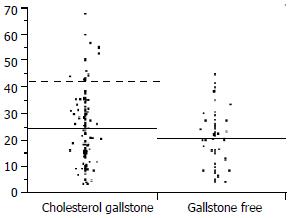
As can be seen in Figure 1, a subgroup of gallstone patients displayed a higher proportion of DCA in bile than gallstone-free subjects. Choosing a cut-off level of the 90th percentile, a group of 13 gallstone patients with high DCA-levels was obtained. The proportion of DCA in the high DCA-group averaged 50% compared with 21% in the normal DCA-group.
The mean age of the patients with high DCA-levels was significantly higher than that of the patients with normal DCA-levels (mean ages: 62 years vs 45 years, Table 1). The mean body mass index (BMI) was also higher in the high DCA-group, 28.3, compared to 24.7 in the normal DCA-group (P < 0.005). Plasma levels of cholesterol and triglycerides were slightly higher in the high DCA-group.
The results are summarized in Table 2. There was no difference in biliary lipid composition, total lipid concentration or cholesterol saturation of bile between patients with high and normal levels of DCA.The molar percentage of cholesterol in cholesterol gallstone patients was significantly higher (7.8 mol %), compared to gallstone-free subjects (5.8 mol %). The total biliary lipid concentration was significantly lower in cholesterol gallstone patients compared to gallstone-free patients. Also the mean cholesterol saturation was significantly higher in gallstone patients (112%) compared to gallstone-free subjects (79%), (P < 0.0001).
| Group (n) | Single stone% (n) | Crystals positive%(n) | Median nucleation time days, (range)(n) | Cholesterol mol% | Bile acid mol% | Phospholipids mol% | Total lipid concentration g/dl | Cholesterol saturation% |
| Total gallst one(122) | 29 (116) | 79 (112) | 6(1-56)(46) | 7.8 ± 0.3 | 68.6 ± 0.6 | 23.6 ± 0.4 | 9.4 ± 0.4 | 112 ± 4 |
| High DCA (13) | 42 (12) | 73 (11) | 14 (2-28) (4) | 8.1 ± 0.6 | 68.1 ± 1.6 | 23.8 ± 1.2 | 8.2 ± 1.0 | 116 ± 10 |
| Low DCA (109) | 28 (104) | 79 (101) | 3 (1-56) (42) | 7.8 ± 0.3 | 68.7 ± 0.6 | 23.6 ± 0.5 | 9.6 ± 0.5 | 112 ± 5 |
| Gallstone free (46) | - | 11a (45) | 17a(1-35)(24) | 5.8 ± 0.3a | 71.3 ± 0.8 | 22.9 ± 0.7 | 13.1 ± 0.6a | 79 ± 3a |
There was a tendency to shorter nucleation time in patients with normal DCA-levels compared to those with high DCA-levels, but the difference did not reach statistical significance. The proportion of patients with crystals in their bile was the same between patients with high and normal levels of DCA. Nucleation time was significantly shorter in cholesterol gallstone patients compared to that in gallstone-free patients, 6 d vs. 17 d (P < 0.0001). The proportion of patients with bile positive for cholesterol crystals was significantly higher among gallstone patients (79%) compared to gallstone-free subjects (11%), (P < 0.0001).
The bile acid composition is summarized in Table 3. The proportion of cholic acid was significantly lower and that of chenodeoxycholic acid was higher in the gallstone patients compared to the gallstone-free patients. There was no significant difference in the proportion of deoxycholic acid, lithocholic acid or ursodeoxycholic acid between gallstone patients and gallstone-free subjects.
| Group | n | Cholic acid% | Deoxycholic acid% | Chenodeoxy-cholic acid% | Lithocholic acid% | Ursodeoxy-cholic acid% |
| Total gallstone | 122 | 38.9 ± 1.0 | 24.2 ± 1.2 | 34.5 ± 0.9 | 1.0 ± 0.1 | 1.3 ± 0.2 |
| High DCA | 13 | 22.7 ± 1.7 | 50.0 ± 2.1 | 24.5 ± 1.7 | 1.4 ± 0.4 | 1.3 ± 0.9 |
| Low DCA | 109 | 40.8 ± 1.0 | 21.1 ± 0.9 | 35.7 ± 0.9 | 0.9 ± 0.1 | 1.3 ± 0.2 |
| Gallstone free | 46 | 46.1 ± 1.6b | 20.7 ± 1.6 | 30.6 ± 1.0a | 0.6 ± 1.6 | 1.7 ± 0.4 |
In the present study, a subgroup of cholesterol gallstone patients with a high proportion of DCA in the gallbladder bile, mean 50%, was compared to gallstone patients with a normal proportion of DCA, mean 21%. The high DCA-level was not associated with either higher cholesterol content or cholesterol saturation of bile. The proportion of patients with cholesterol crystals in the bile did not differ between patients with high and normal levels of DCA. Nor was the nucleation time shortened in patients with high DCA content. If anything, there was a tendency to longer nucleation time in patients with high DCA-levels although the difference was not significant. These results were in agreement with some previous studies, showing that a high proportion of DCA in human gallbladder bile was not associated with a higher risk for formation of cholesterol gallstones[9-13].
The gallstone patients with a high level of DCA were of older age than those with a normal level of DCA. The reason why old age is associated with a high percentage of DCA in bile is not quite apparent. Slow colonic transit has been reported to lead to high biliary proportions of DCA, probably by increasing the intestinal residence time of bile salts[2]. Recently, Thomas et al[8] reported that slow colonic transit in combination with increased number of colonic gram-positive anaerobes and greater 7a-dehydroxylating activity favours enhanced DCA-formation in gallstone patients. Berr et al[6] also found that a group of gallstone patients with DCA-excess had higher 7a-dehydroxylating activity and increased counts of 7a-dehydroxylating bacteria in feces compared with a group of patients with normal DCA, but this difference was not due to a slow intestinal transit time. In agreement with that, van der Werf et al[25] have previously shown that elderly persons (55-75 years old) have a higher fractional turnover of cholic acid and a higher deoxycholic acid input rate from the large bowel than young adults (20-30 years old) and this is not due to any difference in gut transit time.
Another finding of this study was that high level of DCA was also associated with high BMI. This could indicate a potential role of BMI as a confounder when analysing biliary DCA levels in studies on cholesterol gallstone patients.
To conclude, gallstone patients with excess DCA are of older age and have higher BMI than patients with normal DCA. The two groups of patients do not differ with respect to biliary lipid composition, cholesterol saturation, nucleation time or occurrence of cholesterol crystals. These findings further underline that DCA in bile does not significantly contribute to cholesterol gallstone formation.
We greatly appreciate the skilful technical assistance by Ms Ingela Arvidsson and Ms Lisbet Benthin.
Edited by Xu XQ
| 1. | Carey MC, Duane WC. Enterohepatic circulation. The liver: biology and pathobiology. New York: Raven Press 1994; 719-767. [Cited in This Article: ] |
| 2. | Marcus SN, Heaton KW. Intestinal transit, deoxycholic acid and the cholesterol saturation of bile--three inter-related factors. Gut. 1986;27:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 122] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Berr F, Schreiber E, Frick U. Interrelationships of bile acid and phospholipid fatty acid species with cholesterol saturation of duodenal bile in health and gallstone disease. Hepatology. 1992;16:71-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Hussaini SH, Pereira SP, Murphy GM, Dowling RH. Deoxycholic acid influences cholesterol solubilization and microcrystal nucleation time in gallbladder bile. Hepatology. 1995;22:1735-1744. [PubMed] [Cited in This Article: ] |
| 5. | Shoda J, He BF, Tanaka N, Matsuzaki Y, Osuga T, Yamamori S, Miyazaki H, Sjövall J. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995;21:1291-1302. [PubMed] [Cited in This Article: ] |
| 6. | Berr F, Kullak-Ublick GA, Paumgartner G, Münzing W, Hylemon PB. 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology. 1996;111:1611-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Azzaroli F, Mazzella G, Mazzeo C, Simoni P, Festi D, Colecchia A, Montagnani M, Martino C, Villanova N, Roda A. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am J Gastroenterol. 1999;94:2453-2459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Thomas LA, Veysey MJ, Bathgate T, King A, French G, Smeeton NC, Murphy GM, Dowling RH. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | van Erpecum KJ, Portincasa P, Stolk MF, van de Heijning BJ, van der Zaag ES, van den Broek AM, van Berge Henegouwen GP, Renooij W. Effects of bile salt and phospholipid hydrophobicity on lithogenicity of human gallbladder bile. Eur J Clin Invest. 1994;24:744-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Noshiro H, Chijiiwa K, Makino I, Nakano K, Hirota I. Deoxycholic acid in gall bladder bile does not account for the shortened nucleation time in patients with cholesterol gall stones. Gut. 1995;36:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Miquel JF, Núñez L, Amigo L, González S, Raddatz A, Rigotti A, Nervi F. Cholesterol saturation, not proteins or cholecystitis, is critical for crystal formation in human gallbladder bile. Gastroenterology. 1998;114:1016-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Jüngst D, Müller I, Kullak-Ublick GA, Meyer G, Frimberger E, Fischer S. Deoxycholic acid is not related to lithogenic factors in gallbladder bile. J Lab Clin Med. 1999;133:370-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Gustafsson U, Sahlin S, Einarsson C. Biliary lipid composition in patients with cholesterol and pigment gallstones and gallstone-free subjects: deoxycholic acid does not contribute to formation of cholesterol gallstones. Eur J Clin Invest. 2000;30:1099-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Wells JE, Berr F, Thomas LA, Dowling RH, Hylemon PB. Isolation and characterization of cholic acid 7alpha-dehydroxylating fecal bacteria from cholesterol gallstone patients. J Hepatol. 2000;32:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Tera H. Stratification of human gallbladder bile in vivo. Acta Chir Scand Supplement. 1960;256:4-65. [Cited in This Article: ] |
| 16. | Trotman BW, Ostrow JD, Soloway RD. Pigment vs cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig Dis. 1974;19:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 134] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Whiting MJ, Down RH, Watts JMCK. Biliary crystals and granules, the cholesterol saturation index, and the prediction of gallstone type. Surg Gastroenterol. 1982;1:17-21. [Cited in This Article: ] |
| 18. | Roda A, Festi D, Sama C, Mazzella G, Alini R, Roda E, Barbara L. Enzymatic determination of cholesterol in bile. Clin Chim Acta. 1975;64:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 131] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2676] [Cited by in F6Publishing: 2635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 20. | Fausa O, Skålhegg BA. Quantitative determination of bile acids and their conjugates using thin-layer chromatography and a purified 3alpha-hydroxysteroid dehydrogenase. Scand J Gastroenterol. 1974;9:249-254. [PubMed] [Cited in This Article: ] |
| 21. | Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945-955. [PubMed] [Cited in This Article: ] |
| 22. | Angelin B, Einarsson K, Leijd B. Biliary lipid composition during treatment with different hypolipidaemic drugs. Eur J Clin Invest. 1979;9:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Holan KR, Holzbach RT, Hermann RE, Cooperman AM, Claffey WJ. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979;77:611-617. [PubMed] [Cited in This Article: ] |
| 24. | Sahlin S, Ahlberg J, Angelin B, Reihnér E, Einarsson K. Nucleation time of gall bladder bile in gall stone patients: influence of bile acid treatment. Gut. 1991;32:1554-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | van der Werf SD, Huijbregts AW, Lamers HL, van Berge Henegouwen GP, van Tongeren JH. Age dependent differences in human bile acid metabolism and 7 alpha-dehydroxylation. Eur J Clin Invest. 1981;11:425-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |