Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7906
Peer-review started: July 12, 2017
First decision: August 30, 2017
Revised: September 9, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: November 28, 2017
To review evidence on the short-term clinical outcomes of laparoscopic (LRR) vs open rectal resection (ORR) for rectal cancer.
A systematic literature search was performed using Cochrane Central Register, MEDLINE, EMBASE, Scopus, OpenGrey and ClinicalTrials.gov register for randomized clinical trials (RCTs) comparing LRR vs ORR for rectal cancer and reporting short-term clinical outcomes. Articles published in English from January 1, 1995 to June, 30 2016 that met the selection criteria were retrieved and reviewed. The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statements checklist for reporting a systematic review was followed. Random-effect models were used to estimate mean differences and risk ratios. The robustness and heterogeneity of the results were explored by performing sensitivity analyses. The pooled effect was considered significant when P < 0.05.
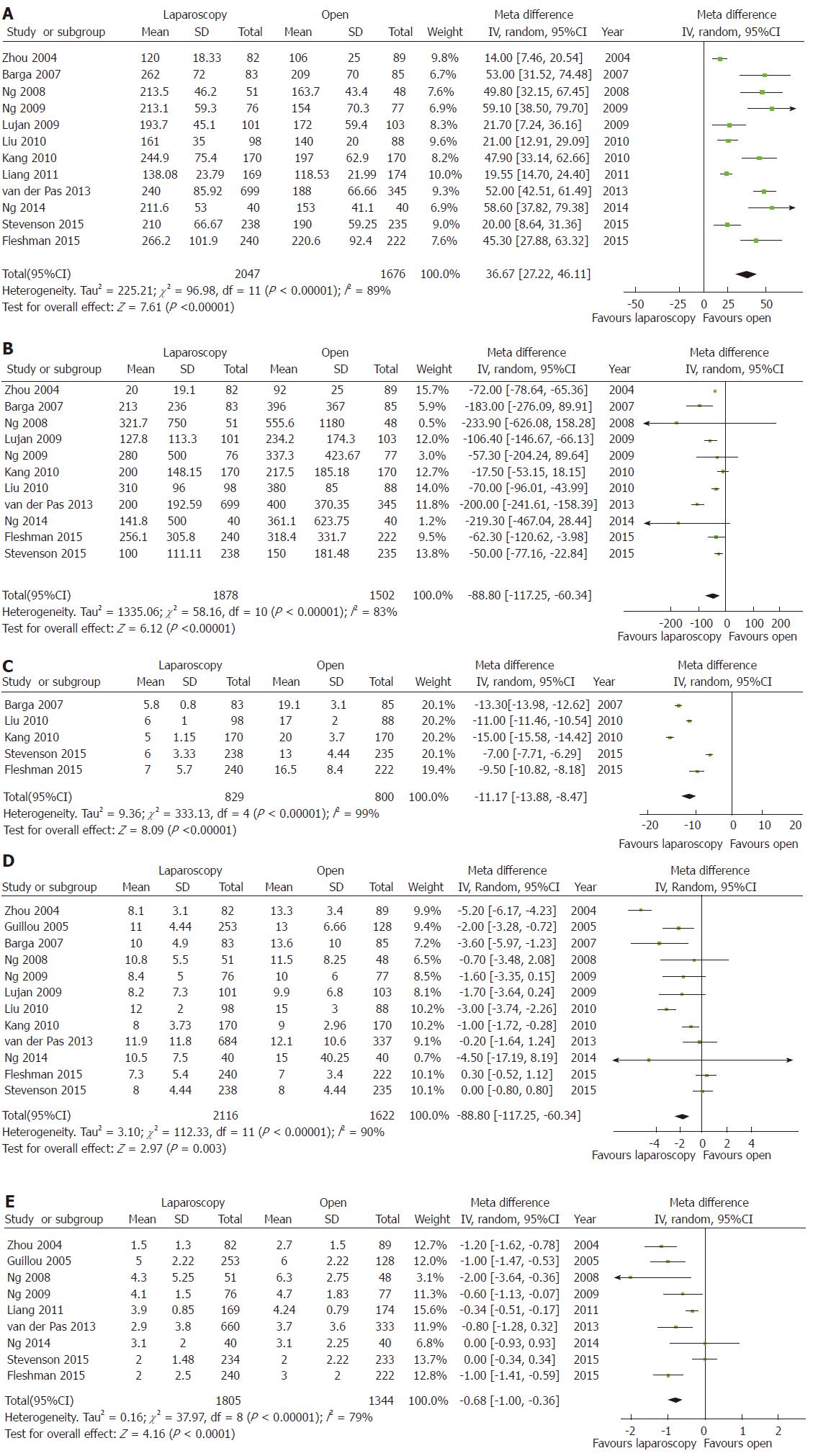
Overall, 14 RCTs were included. No differences were found in postoperative mortality (P = 0.19) and morbidity (P = 0.75) rates. The mean operative time was 36.67 min longer (95%CI: 27.22-46.11, P < 0.00001), the mean estimated blood loss was 88.80 ml lower (95%CI: -117.25 to -60.34, P < 0.00001), and the mean incision length was 11.17 cm smaller (95%CI: -13.88 to -8.47, P < 0.00001) for LRR than ORR. These results were confirmed by sensitivity analyses that focused on the four major RCTs. The mean length of hospital stay was 1.71 d shorter (95%CI: -2.84 to -0.58, P < 0.003) for LRR than ORR. Similarly, bowel recovery (i.e., day of the first bowel movement) was 0.68 d shorter (95%CI: -1.00 to -0.36, P < 0.00001) for LRR. The sensitivity analysis did not confirm a significant difference between LRR and ORR for these latter two parameters. The overall quality of the evidence was rated as high.
LRR is associated with lesser blood loss, smaller incision length, and longer operative times compared to ORR. No differences are observed for postoperative morbidity and mortality.
Core tip: There is no consensus on which technique, between laparoscopic rectal resection (LRR) and open rectal resection (ORR), is more beneficial for the patient. A systematic review and meta-analysis exclusively based on randomized clinical trials comparing LRR vs ORR has been performed. The pooled analyses focused on the evaluation and comparison of short-term clinical outcomes and showed that postoperative morbidity and mortality are similar between the two surgical approaches. However, LRR is associated with lesser blood loss and smaller incision length, which may represent clinical advantages for the patient.
- Citation: Martínez-Pérez A, Carra MC, Brunetti F, de’Angelis N. Short-term clinical outcomes of laparoscopic vs open rectal excision for rectal cancer: A systematic review and meta-analysis. World J Gastroenterol 2017; 23(44): 7906-7916
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7906
The oncologic principles for the curative treatment of rectal cancer imply the complete removal of the tumor and the mesorectum[1]. In locally advanced rectal cancers, oncologic outcomes can be improved by tailored multi-disciplinary approaches that combine surgery with neoadjuvant chemoradiation therapy[2].
Laparoscopy is currently useful for the resection of rectal cancer. The results of multi-centric randomized clinical trials (RCTs) have shown that laparoscopic rectal resection (LRR) was associated with more favorable short-term outcomes compared to open rectal resection (ORR)[3,4]. Specifically, the COLOR II trial showed statistically significant differences in favor of LRR in terms of blood loss, bowel recovery, and the length of hospital stay, with no differences between the two approaches in postoperative morbidity and mortality[3]. The COREAN study achieved similar results, and showed less postoperative pain and better physical and intestinal recovery after LRR[4]. In the more recent ACOSOG Z6051 and ALaCaRT trials, LRR was associated with longer operative times, less blood loss, and faster post-surgery bowel movements [ACOSOG] or time to flatus compared to ORR [ALACART], despite no observed group differences in the length of hospital stay[5,6]. Two recent meta-analyses had compared the short-term clinical results of LRR vs ORR based on pooled actualized data from the relevant literature on rectal cancer. They shown, among others advantages, a significant lesser postoperative morbidity[7,8] and mortality[7] for patients undergoing LRR over those who received ORR[7,8]. However, they considered both RCTs and non-RCTs, a critical factor that dampens the strength of the results due to the quality of the selected studies and the risk of bias. Furthermore, the results of a recent RCTs-based meta-analysis focusing exclusively on the pathologic outcomes of LRR vs ORR reignited the debate regarding the oncological safety of laparoscopy for rectal cancer in terms of quality of mesorectal resection[9]. Thus, while waiting for the long-term data of the ongoing RCTs, we conducted a systematic review and meta-analysis on RCTs only to evaluate the best level of evidence available so far on the short-term clinical outcomes of laparoscopic vs open rectal resections in patients with rectal cancer.
A literature search was performed on the following online databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (through PubMed), EMBASE, and Scopus. To increase the probability of identifying all relevant articles, a specific research equation was formulated for each database, using the following keywords and/or MESH terms: rectal/colorectal cancer/carcinoma, treatment, therapy, management, surgery, laparoscopy/laparoscopic surgery, open surgery/laparotomy, and randomized trial/trial. Moreover, the reference lists of the eligible studies and relevant review articles were crosschecked to identify additional pertinent studies. Grey literature was explored on the OpenGrey database and the ClinicalTrials.gov registry was also searched to look for any ongoing RCT whose results might be published in the near future. Articles published in English from January 1, 1995 to June, 30 2016 that met the selection criteria were retrieved and reviewed.
The methodological approach for this systematic review included the development of selection criteria, the definition of search strategies, the assessment of study quality, and an abstraction of relevant data. The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statements checklist for reporting a systematic review was followed[10].
The eligibility and selection criteria were defined before the data search was initiated to ensure the proper identification of all studies that were eligible to be included in the systematic review and meta-analysis. Only RCTs that compared LRR and ORR and reported at least one of the outcomes of interest were retrieved and analyzed. No trial duration limitation was applied. Non-randomized studies, retrospective studies, case reports, review articles, commentaries, and conference abstracts were not considered in the systematic review. Studies that reported the results of surgical teams during their learning curve in laparoscopic rectal resection were excluded.
By applying the PICO framework, the study selection criteria were as follows:
Participants: Adult patients with histologically confirmed rectal cancer that required a surgical resection.
Interventions: Laparoscopic (including laparoscopic-assisted) or open rectal resection. Studies were included independently of the surgical technique (e.g., abdominoperineal resection or anterior resection) and the performance of a primary anastomosis.
Comparisons: LRR vs ORR.
Outcome measures: include short-term surgical and clinical outcomes that were divided into: (1) Intraoperative outcomes: mean operative time (min), intraoperative morbidity rate (%), mean estimated blood loss (ml), mean incision length (cm), ureter injury rate (%), gastrointestinal injury rate (%); and (2) postoperative outcomes: postoperative morbidity rate (%), postoperative mortality rate (%), mean length of hospital stay (days), anastomotic leak rate (%), reoperation rate (%), ileus rate (%), time to bowel recovery (day of first bowel movement in days), wound infection rate (%), chest infection rate (%), urinary infection rate (%).
Initially, titles and abstracts of the retrieved studies were independently and blindly screened for relevance by two reviewers (AM-P and NdeA) according to the 2010 CONSORT Statement for RCTs (Http://http://www.consort-statement.org). To enhance sensitivity, records were removed only if both reviewers excluded the record at the initial screening level. Subsequently, both reviewers performed a full-text analysis of the selected articles.
Both reviewers independently assessed the risk of bias using the Cochrane “Risk of Bias” tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions[11]. Additionally, the Grading of Recommendations Assessment Development and Evaluation (GRADE) system was used to grade the “body of evidence” that emerged from the review[12]. All disagreements between the two reviewers in the selection and evaluation processes were resolved by discussion with a third reviewer (FB).
Data from the included studies were processed using qualitative and quantitative analyses. For binary outcome data, the relative risk (RR) and 95% CI were estimated using the Mantel-Haenszel method. For continuous data, the mean differences (MD) and 95%CIs were estimated using inverse variance weighting. Outcome measures (mean and median values, standard deviations, interquartile ranges) were extracted for each surgical treatment. If necessary and possible, outcome variables were calculated based on the data available in the individual studies. If the SE was provided instead of a SD, the SD was calculated based on the sample size (SE = SD/variables were calculated based on the data available in the individual studies. Whether neither mean or SD values were reported, they were estimated from the median, ranges, interquartile ranges (IQR) or P values[13,14]. Heterogeneity was assessed by the I2 statistic[11,15,16]. I2 values of 25%, 50%, and 75% were considered as low, moderate, and high, respectively[11,16].
The pooled estimates of the mean differences were calculated using random effects models to consider potential inter-study heterogeneity and to adopt a more conservative approach. Then, the robustness of the results and the potential sources of heterogeneity were explored by performing sensitivity analyses (e.g., subgroup analyses; comparison using a fixed-effects model). The pooled effect was considered significant if P < 0.05. The meta-analysis was performed using Review Manager (RevMan, version 5.3, Cochrane Collaboration, Copenhagen, Denmark).
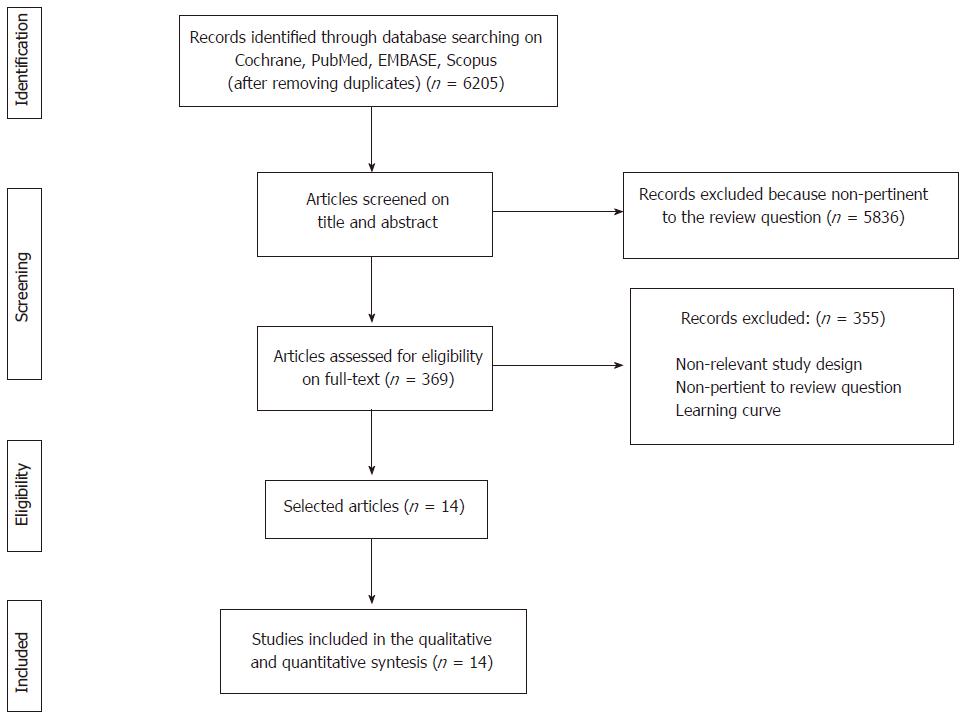
Overall, the combined search identified 6205 articles, of which 5836 were rejected based upon the title and abstract evaluation. The remaining 369 articles underwent full-text evaluation; 355 were excluded because they were not RCTs, presented duplicate data of other RCTs included in the systematic review, did not report the outcomes of interest, or presented the results of laparoscopic rectal resections during the surgeon’s learning curve. No additional study was identified through the manual search, reference lists crosschecks, grey literature or on ClinicalTrials.gov. Finally, 14 eligible articles were found and were included in the qualitative and quantitative analyses. The flowchart of the literature search and the study selection process is shown in Figure 1.
The 14 selected studies were published between 2003 and 2015. They included patients who had surgery between September 1993 and November 2014. Nine studies were performed in single centers[17-25], whereas 5 were multi-centric studies[3-6,26]. Overall, these studies analyzed a total of 4132 patients who underwent either open (n = 1819) or laparoscopic (n = 2313) rectal resections. In this latter group, 13.8% of patients (range: 0%-33.9%) required a conversion from laparoscopy to open surgery. Table 1 displays the baseline characteristics of patients who underwent LRR or ORR.
| Ref. | Number of centers involved (country) in the RCT and in the study period | Inclusion criteria | Exclusion criteria | n | Surgical approach | Types of procedure | Preoperative treatment | ||||
| Lap (n) | Conversion rate (%) | Open (n) | Lap (%) | Open (%) | Lap (%) | Open (%) | |||||
| Fleshman et al[5], 2015 | 35 (United States-Canada) Oct 2008-Sep 2013 | S. II-III rectal cancer ≤ 12 cm from AV | 1-11 | 462 | 240 | 11.25 | 222 | LAR (74.6) APR (24.2) H (0.4) TPC (0.8) | AR (76.1) APR (21.2) TPC 6 (2.7) | CRT (95) RT (3.3) CT (1.7) | CRT (91.2) RT (5.5) CT (3.4) |
| Stevenson et al[6], 2015 | 24 (Australia-N. Zeal) Mar 2010-Nov 2014 | T1-3 rectal cancer ≤ 15 cm from AV | 1, 2, 4, 7, 10, 12, 13 | 473 | 238 | 8.82 | 235 | LAR (89) APR (11) | AR (90) APR (10) | RT (50) | RT (49) |
| Ng et al[20], 2014 | 1 (Hong Kong) Aug 2001-Aug2007 | Rectal cancer low margin 5-12 cm AV | 13, 16, 17, 23, 24, 25 | 80 | 40 | 7.5 | 40 | LAR (100) | AR (100) | 0 | 0 |
| van der Pas et al[3], 2013 | 30 (Europe- Canada-South Korea) Jan 2004-May 2010 | T1-3 rectal cancer ≤ 15 cm from AV | 1, 2, 9, 10, 13-22 | 1044 | 699 | 16.4 | 345 | LAR (70) APR (29) U (1) | AR (77) APR (23) | RT (59) CT (32) | RT (58) CT (34) |
| Liang et al[24], 2011 | 1 (China) May 2004-April 2008 | Rectal cancer | 16, 25, 26, 31, 34, 35 | 343 | 169 | 0.59 | 174 | LAR (50.9) APR (49.1) | AR (59.8) APR (40.2) | 0 | 0 |
| Kang et al[4], 2010 | 3 (South Korea) Apr 2006-Aug 2009 | T1-3 rectal cancer ≤ 9 cm from AV | 1, 5, 10, 13, 16, 21, 23, 26 | 340 | 170 | 1.18 | 170 | LAR (85.9) APR (14.1) | AR (88.8) APR (11.2) | CRT (100) | CRT (100) |
| Liu et al[18], 2010 | 1 (China) Feb 2005-Oct 2008 | Rectal cancer | 16, 17, 23 | 186 | 98 | 0 | 88 | 1LAR (69.4) H 14 (14.3) APR (12.2) O 4 (4.1) | AR (67) H (12.5) APR (15.9) O 4 (4.5) | 0 | 0 |
| Ng et al[21], 2009 | 1 (Hong Kong) Sep 1993-Oct 2002 | Rectal cancer low margin 12-15 cm AV | 1, 16, 23, 24, 27, 28 | 153 | 76 | 30.26 | 77 | LAR (100) | AR (100) | 0 | 0 |
| Luján et al[19], 2009 | 1 (Spain) Jan 2002-Feb 2007 | Mid or low rectal cancer | 1, 13, 18, 29 | 204 | 101 | 7.92 | 103 | LAR 77 (76.2) APR 24 (23.8) | AR (78.6) APR (21.4) | CRT (72.3) | CRT (74.8) |
| Ng et al[22], 2008 | 1 (Hong Kong) Sep1994-Feb 2005 | Low rectal cancer | 13, 16, 23, 24, 30 | 99 | 51 | 9.8 | 48 | APR (100) | APR (100) | 0 | 0 |
| Braga et al[17], 2007 | 1 (Italy) Period n.a. | Rectal cancer | 1, 2, 10, 13, 31 | 168 | 83 | 7.23 | 85 | LAR (91.6) APR (8.4) | AR (87) APR (13) | CRT (16.9) | CRT (14.1) |
| Guillou et al[26], 2005 | 27 (United Kingdom) Jul 1996 - Jul 2002 | Colorectal cancer (excl. transverse) | 11, 16, 17, 21, 32, 33 | 381 | 253 | 33.88 | 128 | AR 167 (66) APR (25) S (3) LH (2) O (3) U (1) | AR (62) RC 1 (1) S 7 (5) APR (27) O 4 (3) U (2) | NA | NA |
| Zhou et al[23], 2004 | 1 (China) Jun 2001 - Sep 2002 | 1.5 cm above AV to peritoneal reflection | 1, 13, 29 | 171[24] | 82 | na | 89 | LAR (100) | AR (100) | 0 | 0 |
| Araujo et al[25], 2003 | 1 (Brazil) Sep 1997-Sep 2000 | Low rectal cancer not responding RCT | 1 | 28 | 13 | 0 | 15 | APR (100) | APR (100) | CRT (100) | CRT (100) |
Mean operative time was significantly longer, the estimated blood loss and the mean incision length significantly lower for LRR than ORR (Figure 2A-C). Conversely, no significant differences were observed for ureteric or gastrointestinal injury rates, or for overall intraoperative morbidity (Table 2).
| Outcome variables | Number of studies (Number of patients) | RR or MD | 95%CI(Low; High) | P value | Heterogeneity, I2(P value) | Sensitivity analysis by including the largest multi-centric RCTs | ||||
| Number of studies (Number of patients) | RR or MD | 95%CI (Low; High) | P value | Heterogeneity, I2 (P value) | ||||||
| Intraoperative outcomes | ||||||||||
| Operative time | 12 (3723) | 36.67 | 27.22; 46.11 | <0.00001 | 89% (< 0.00001) | 4 (2319) | 41.18 | 24.88; 57.48 | < 0.00001 | 85% (0.0002) |
| Intraoperative morbidity | 4 (1909) | 0.97 | 0.74; 1.27 | 0.82 | 8% (0.35) | 2 (1500) | 1.02 | 0.60; 1.72 | 0.94 | 61% (0.11) |
| Estimated blood loss | 11 (3380) | -88.8 | -117.25; -60.34 | < 0.00001 | 83% (< 0.00001) | 4 (2319) | -82.1 | -158.87; -5.34 | 0.04 | 94% (< 0.00001) |
| Incision length | 5 (1629) | -11.17 | -13.88; -8.47 | < 0.00001 | 99% (< 0.00001) | 3 (1275) | -10.51 | -16.16; -4.85 | 0.0003 | 99% (< 0.00001) |
| Ureter injury | 5 (2256) | 1.23 | 0.20; 7.72 | 0.82 | 51% (0.11) | 2 (1500) | 2.59 | 0.66; 10.11 | 0.17 | 0% (0.67) |
| Gastrointestinal injury | 4 (2052) | 1.14 | 0.25; 5.17 | 0.86 | 73% (0.02) | 2 (1500) | 1.05 | 0.13; 8.22 | 0.96 | 86% (0.007) |
| Postoperative outcomes | ||||||||||
| Postoperative morbidity | 12 (3313) | 0.98 | 0.88; 1.09 | 0.75 | 16% (0.3) | 3 (1844) | 1.02 | 0.91; 1.15 | 0.69 | 0% (0.65) |
| Postoperative mortality | 13 (3751) | 0.65 | 0.34; 1.23 | 0.19 | 0% (1) | 4 (2319) | 0.60 | 0.27; 1.33 | 0.21 | 0% (0.89) |
| Length hospital stay | 12 (3738) | -1.71 | -2.84; -0.58 | 0.003 | 90% (< 0.00001) | 4 (2296) | -0.25 | -0.90; 0.39 | 0.44 | 52% (0.10) |
| Anastomotic leak | 9 (2253) | 0.97 | 0.69; 1.34 | 0.84 | 0% (0.6) | 3 (1351) | 1.19 | 0.79; 1.80 | 0.41 | 0% (0.53) |
| Reoperation rate | 7 (2468) | 0.93 | 0.64; 1.34 | 0.69 | 3% (0.4) | 3 (1844) | 1.19 | 0.77; 1.86 | 0.43 | 0% (0.42) |
| Ileus | 10 (2930) | 0.77 | 0.55; 1.06 | 0.11 | 0% (0.66) | 3 (1875) | 0.79 | 0.43; 1.44 | 0.44 | 62% (0.07) |
| Bowel recovery | 9 (3149) | -0.68 | -1.00; -0.36 | < 0.0001 | 79% (< 0.00001) | 3 (1922) | -0.59 | -1.24; 0.07 | 0.08 | 87% (0.0005) |
| Wound infection | 10 (2684) | 0.81 | 0.61; 1.09 | 0.16 | 0% (0.5) | 1 (1042) | 0.82 | 0.43;1.47 | 0.5 | NA |
| Chest infection | 7 (1252) | 1.55 | 0.82; 2.93 | 0.17 | 0% (0.61) | 0 (0) | - | - | - | - |
| Urinary infection | 7 (1075) | 0.89 | 0.50; 1.57 | 0.68 | 15% (0.32) | 0 (0) | - | - | - | - |
The mean length of hospital stay was reported in 12 studies[3-6,17-23,26]. The overall MD was -1.71 d (95%CI: -2.84 to -0.58, P < 0.003) in favor of laparoscopy, with a high heterogeneity (I2 = 90%). Bowel recovery, described as the day of the first bowel movement, was reported in 9 studies[3,5,6,17,20-24,26]. The overall MD was -0.68 d (95%CI: -1.00 to -0.36, P < 0.00001) in favor of laparoscopy, with a high heterogeneity (I2 = 79%) (Figure 2D and E). Anastomotic leak[3-5,17,19-21,23,24], postoperative morbidity[3-5,17-23,25,26], and mortality[3-6,17-24] rates showed no significant differences between LRR and ORR (Table 2).
Sensitivity analyses performed to test the impact of using fixed-effect models showed the same results for all variables that for random effect models. Subgroup analysis was also performed by including the four largest multi-centric trials only (namely, the ACOSOG Z6051, AlaCaRT, COLOR II, and COREAN trials[3-6]). These 4 studies comprised 2319 patients (56.2% of the total). Although being a high-populated multi-centric RCT, the UK MRC-CLASICC trial[26] was not included in the sensitivity analysis because it was conducted in the early years of laparoscopic surgery and included both colon and rectal cancers. The estimated blood loss and the length of incision were significantly lower with an operative time that was significantly higher for LRR compared with ORR, but the heterogeneity remained high. For the postoperative variables, the length of hospital stay and bowel recovery did not reach statistical significance in favor of LRR. Heterogeneity decreased to moderate for length of hospital stay and remained high for bowel recovery (Table 2).
The assessment of study quality and the risk of bias, according to the Cochrane Collaboration tool for RCTs, are shown in supplemental table 1. Overall, 10 studies were classified as a low risk of bias[3-6,17,19-22,26], 1 at an unknown risk of bias[24] and 3 studies at a high risk of bias[18,23,25]. By applying the GRADE system, the overall quality of the evidence was rated as high.
This systematic review and meta-analysis focuses on the short-term clinical outcomes of laparoscopic vs open resection for the treatment of rectal cancer and shows that there are no differences in postoperative morbidity and mortality between the two approaches. However, LRR is associated with significantly longer operative time, lesser blood loss, and smaller incision than ORR. The length of hospital stay and the time to bowel recovery are shorter for LRR in the overall analysis but are not significantly different when considering the major RCTs only.
Previous meta-analyses have reported contrasting results about the benefits associated with the use of laparoscopy for rectal cancer instead of conventional open surgery. In 2013, Arezzo et al[27] analyzed 8 RCTs and 15 non-RCTs and showed a significantly lower postoperative mortality and morbidity in LRR than ORR. A more recent meta-analysis by Zhao et al[28] and the latest Cochrane review[29], both based on RCTs only, showed no differences in overall morbidity and mortality. However, they found better outcomes for laparoscopy in terms of blood loss, length of hospital stay, wound infection, and bowel recovery compared to open surgery. Noticeably, the above-mentioned meta-analyses were performed before the two largest and most recent RCTs being published, namely the ACOSOG Z6501 and ALaCaRT trials[5,6], which did not confirm the non-inferiority of laparoscopy and questioned the oncological safety of laparoscopy for rectal cancer. Indeed, the topic remains highly debated. Two recent meta-analyses published by Chen et al[8] and by Zheng et al[7] were performed to assess the outcomes of laparoscopy vs open surgery by including data from RCTs and non-RCTs. The study by Chen et al[8] demonstrated longer operative time, lesser blood loss, lesser overall complications, faster bowel recovery, shorter hospitalization, and major distal resection margin for laparoscopic surgery than open surgery[8]. However, there was found a considerable and arbitrary lack of data from the most populated RCTs[3-6] (which represented more than 50% of the patients included) for all the short-term variables analyzed, such as distance of distal resection margin[3,4,6], CRM involvement[5], lymph node harvest[3], operative time[3,4,6], hospital stay[4,6], and estimated blood loss[3,4,6]. Similarly, the meta-analysis by Zheng et al[7] included 38 studies and 13,408 patients, but only less than a third of patients (3978, 29.7%) were treated in RCTs. Moreover, the 32.9% of the included patients (4405 patients) were coming from a unique multi-centric observational study involving 72 Spanish hospitals[30]. Despite the eager of pooling data to gain power and answer the hot question about the advantages of laparoscopic rectal resection, caution should be paid when interpreting meta-analytic results. Contrasting result may be generated by using different statistical models, or when pooling together RCTs with non-RCTs. In general, the choice of the effect model should be assessed prior to start the data analysis and based on the researcher’s understanding of whether or not all the included studies share a common treatment effect. For surgical literature, a random-effect model seems to be more appropriate than a fixed-effect[31,32] due to the nature of the data retrieved from studies performed by researchers operating independently. Deciding between the models after performing the analysis or based upon the level of heterogeneity found (e.g., whether the I2 is higher than 40%[29] or 50%[7,8,27] or reaches significance[33]) is strongly discouraged[31,32]. Most importantly, the quality of a meta-analysis is strictly dependent on the quality of the original studies included and robustness of the findings should be tested by sensitivity analyses.
The present systematic review and meta-analysis aimed to analyze the best level of evidence available for LRR vs ORR, thus only high-quality RCTs were included. By applying a strict methodology, the present findings confer fewer advantages to LRR over ORR, especially when only the largest multi-centric RCTs were considered, when compared to the results of previous meta-analyses.
The main intra-operative benefit of LRR, confirmed by both the pooled data analysis and the sensitivity analysis, is a lower blood loss. This might justify the use of laparoscopy for rectal cancer resections despite longer operative times. Indeed, the amount of blood loss has been shown to be an independent predictor of adverse surgical outcomes, such as intra- and postoperative complications, cancer recurrence, and poorer survival[34,35]. Although the reasons why intraoperative blood loss would be associated with morbidity and poor survival remain unclear, some evidence supports that blood loss triggers stress and immune reactions, which may lead to an increased susceptibility of infections and cancer recurrence. Thus, minimizing blood loss, and the consequent risk of blood transfusion by meticulous and gentle dissections in the anatomical planes, may contribute to better outcomes of oncological surgery. However, it remains to be assessed whether the difference observed between the two surgical approaches (i.e., 88.80 mL) is clinically relevant, and may potentially impact on the postoperative and long-term outcomes.
Other markers of surgical quality are the postoperative complication rates and the time to bowel recovery. Based on the pooled data analyses from the major RCTs[3-6], laparoscopy was not associated with a significantly different incidence of postoperative complications, time to bowel recovery or hospital stay compared to open surgery. Concerning bowel recovery, it can be measured with multiple clinical variables, such as the time to the first flatus, the time to a liquid or solid diet, or the time to the first bowel movement. Globally, bowel recovery was not significantly different between LRR and ORR but it must be noted that benefits in at least one of the recovery variables considered (e.g., time to flatus or time to regular diet) were found in all RCTs. Thus, caution should be paid before drawing definitive conclusions; differences among studies did not allow pooling data for all variables (e.g., the COREAN study[4] expressed bowel recovery in hours rather than days and could not be included in the meta-analysis), except for the time to first bowel movement. Despite the non-significant results in the sensitivity analysis, bowel recovery is probably faster after LRR than ORR, but further studies are needed to confirm this finding.
The evidence emerging from this systematic review and meta-analysis can be considered of high quality since it is based exclusively upon RCTs[36], most of which with low risk of bias. However, some limitations must be acknowledged. The pooled data analyses showed high degrees of heterogeneity; this may be linked to multiple factors, such as different sample size (e.g., some studies presented less than 100 patients per group[17,18,20-23,25]), different tumor characteristics (e.g., only upper[21] or lower rectal cancer[22,25]), and different study designs (e.g., non-inferiority study) or protocols. For instance, neoadjuvant treatments were not performed in all studies, and therapies were not standardized. It has been hypothesized that major pathologic responses might translate into greater postoperative morbidities because of the effects of neoadjuvant chemoradiation therapy on pelvic tissues[37]. To date, only a few studies have addressed the influence of the pathologic response to neoadjuvant chemoradiation therapy on intraoperative and short-term morbidity, with contrasting results[37-40], but its impacts could neither be confirmed nor ruled out in this meta-analysis. Finally, the results of this meta-analysis cannot be generalized to the application of LRR and ORR for all types of rectal cancer. Indeed, T4 rectal cancers were excluded from most of the studies[3,4,6,17,19,22,23]. Thus, the outcomes of laparoscopy for this specific subset of tumors cannot be assumed, although a recent propensity score-matched study showed that LRR also achieved similar outcomes to ORR in pT4 rectal cancer patients[41].
The short-term benefits of laparoscopy must be counterbalanced with its safety. Indeed, uncertainty persists concerning the oncological appropriateness of laparoscopy for rectal cancer. A recent meta-analysis[9] focused on the pathologic outcomes of LRR vs ORR and showed that LRR was associated with a significantly higher rate of non-complete mesorectal excision compared with ORR, which represents a critical issue on the choice of the surgical approach. Innovative techniques, such as transanal-TME and robotics, are receiving worldwide attention in the latest years and they may represent a valuable alternative to laparoscopy, especially if they are proved to be oncologically safe, clinically advantageous for the patient, and maybe less challenging for the surgeon[42-45]. Nevertheless, data on the long-term outcomes of the ongoing RCTs are pending and they may be crucial in the definitive assessment of the role of laparoscopy in rectal cancer resection.
In conclusion, LRR and ORR show similar rates of intra- and postoperative complications, as well as morbidity and mortality. However, LRR is associated with a significantly higher operative time, lesser blood loss, and smaller incision length than ORR.
Laparoscopy is widely used for the resection of rectal cancer. The associated short-term benefits for the patient (e.g., fewer postoperative morbidity) have been highlighted in several studies, but with contrasting results. We conducted a systematic review and meta-analysis by selecting only randomized clinical trials (RCTs) that evaluated the short-term clinical outcomes of laparoscopic rectal resection (LRR) vs open rectal resection, (ORR) in patients with rectal cancer.
The short-term advantages of laparoscopic rectal resection remain under debate due to controversial results, especially when analyzing the most recent RCTs. Pooled data analyses of the available literature represents the best way to summarize the current evidence and support the development and widespread of the most advantageous surgical approach.
The main objective of the present systematic review and meta-analysis was to analyze the current literature of RCTs on the surgical treatment for rectal cancer to compare the short-term outcomes of laparoscopy vs open surgery. The analysis of the literature has also highlighted the level of evidence and risk of bias inherent in the available studies, which should be used to design future research on the treatment of rectal cancer.
This is a systematic literature review and meta-analysis that was conducted by following the guidelines of the Cochrane Collaboration as well as the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statements checklist. Literature search was performed on different databases for articles published in English from January 1, 1995 to June, 30 2016. Random-effect models were used to estimate mean differences and risk ratios between LRR and ORR. The robustness and heterogeneity of the results were explored by performing sensitivity analyses.
Overall, 14 RCTs were analyzed. The mean operative time was longer for LRR than ORR, whereas the mean estimated blood loss and the mean incision length were lower for LRR than ORR. No differences between the two surgical approaches were found in postoperative mortality, morbidity, length of hospital stay, and time to bowel recovery. Although the overall quality of evidence was judged as high, not all the studies evaluated the same parameters. Thus, future research should use standardized definitions of postoperative outcomes in order to increase comparability and decrease heterogeneity among studies.
LRR is associated with lesser blood loss, smaller incision length, and longer operative times compared to ORR. No differences are observed for postoperative morbidity and mortality. The short-term advantages of laparoscopic rectal resection are mainly represented by a significantly lower intraoperative blood loss and better cosmetic results compared to open surgery.
The overall level of evidence supporting these findings is high.
Further studies should evaluate alternative minimally-invasive surgical techniques (e.g., transanal TME or Robotics) and compare them with laparoscopic and open approaches.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aytac E, De Nardi P, García-Flórez LJ, Seow-Choen F S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1867] [Cited by in F6Publishing: 1814] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 2. | Lee M, Gibbs P, Wong R. Multidisciplinary Management of Locally Advanced Rectal Cancer--An Evolving Landscape? Clin Colorectal Cancer. 2015;14:251-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1030] [Cited by in F6Publishing: 883] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 4. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 682] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 5. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 6. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 688] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 7. | Zheng J, Feng X, Yang Z, Hu W, Luo Y, Li Y. The comprehensive therapeutic effects of rectal surgery are better in laparoscopy: a systematic review and meta-analysis. Oncotarget. 2017;8:12717-12729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Chen K, Cao G, Chen B, Wang M, Xu X, Cai W, Xu Y, Xiong M. Laparoscopic versus open surgery for rectal cancer: A meta-analysis of classic randomized controlled trials and high-quality Nonrandomized Studies in the last 5 years. Int J Surg. 2017;39:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Martínez-Pérez A, Carra MC, Brunetti F, de’Angelis N. Pathologic Outcomes of Laparoscopic vs Open Mesorectal Excision for Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Surg. 2017;152:e165665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7773] [Cited by in F6Publishing: 7377] [Article Influence: 526.9] [Reference Citation Analysis (0)] |
| 11. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21506] [Article Influence: 1654.3] [Reference Citation Analysis (2)] |
| 12. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11058] [Cited by in F6Publishing: 12672] [Article Influence: 792.0] [Reference Citation Analysis (0)] |
| 13. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4895] [Cited by in F6Publishing: 6178] [Article Influence: 325.2] [Reference Citation Analysis (0)] |
| 14. | Higgins JP, Green S. The Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed. 2011;. [Cited in This Article: ] |
| 15. | Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1088] [Cited by in F6Publishing: 1050] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 16. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39087] [Cited by in F6Publishing: 42097] [Article Influence: 2004.6] [Reference Citation Analysis (1)] |
| 17. | Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Liu FL, Lin JJ, Ye F, Teng LS. Hand-assisted laparoscopic surgery versus the open approach in curative resection of rectal cancer. J Int Med Res. 2010;38:916-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Ngo DK, Leung WW, Leung KL. Laparoscopic-assisted versus open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc. 2014;28:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Hon SS. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum. 2009;52:558-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418-2425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, Li G, Wang W, Feng Y, Guo J. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. 2011;21:381-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Araujo SE, da Silva eSousa AH Jr, de Campos FG, Habr-Gama A, Dumarco RB, Caravatto PP, Nahas SC, da Silva J, Kiss DR, Gama-Rodrigues JJ. Conventional approach x laparoscopic abdominoperineal resection for rectal cancer treatment after neoadjuvant chemoradiation: results of a prospective randomized trial. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2360] [Cited by in F6Publishing: 2210] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 27. | Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc. 2013;27:1485-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Zhao JK, Chen NZ, Zheng JB, He S, Sun XJ. Laparoscopic versus open surgery for rectal cancer: Results of a systematic review and meta-analysis on clinical efficacy. Mol Clin Oncol. 2014;2:1097-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie JP, Wiggers T, Breukink S. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2014;CD005200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 2009. John Wiley & Sons Ltd, West Sussex, United Kingdom. . [Cited in This Article: ] |
| 32. | Higgins JP, Green S. Cochrane Handbook for Systematic Review of Intervention. West Sussex PO19 8SQ, England: Wiley-Blackwell 2008; . [Cited in This Article: ] |
| 33. | Zhao D, Li Y, Wang S, Huang Z. Laparoscopic versus open surgery for rectal cancer: a meta-analysis of 3-year follow-up outcomes. Int J Colorectal Dis. 2016;31:805-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Okamura R, Hida K, Hasegawa S, Sakai Y, Hamada M, Yasui M, Hinoi T, Watanabe M; Japan Society of Laparoscopic Colorectal Surgery. Impact of intraoperative blood loss on morbidity and survival after radical surgery for colorectal cancer patients aged 80 years or older. Int J Colorectal Dis. 2016;31:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Egenvall M, Mörner M, Påhlman L, Gunnarsson U. Degree of blood loss during surgery for rectal cancer: a population-based epidemiologic study of surgical complications and survival. Colorectal Dis. 2014;16:696-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Mahid SS, Hornung CA, Minor KS, Turina M, Galandiuk S. Systematic reviews and meta-analysis for the surgeon scientist. Br J Surg. 2006;93:1315-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Horisberger K, Hofheinz RD, Palma P, Volkert AK, Rothenhoefer S, Wenz F, Hochhaus A, Post S, Willeke F. Tumor response to neoadjuvant chemoradiation in rectal cancer: predictor for surgical morbidity? Int J Colorectal Dis. 2008;23:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Duldulao MP, Lee W, Le M, Wiatrek R, Nelson RA, Chen Z, Li W, Kim J, Garcia-Aguilar J. Surgical complications and pathologic complete response after neoadjuvant chemoradiation in locally advanced rectal cancer. Am Surg. 2011;77:1281-1285. [PubMed] [Cited in This Article: ] |
| 39. | Maggiori L, Bretagnol F, Aslam MI, Guedj N, Zappa M, Ferron M, Panis Y. Does pathologic response of rectal cancer influence postoperative morbidity after neoadjuvant radiochemotherapy and total mesorectal excision? Surgery. 2014;155:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Landi F, Espín E, Rodrigues V, Vallribera F, Martinez A, Charpy C, Brunetti F, Azoulay D, de’Angelis N. Pathologic response grade after long-course neoadjuvant chemoradiation does not influence morbidity in locally advanced mid-low rectal cancer resected by laparoscopy. Int J Colorectal Dis. 2017;32:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | de’Angelis N, Landi F, Vitali GC, Memeo R, Martínez-Pérez A, Solis A, Assalino M, Vallribera F, Mercoli HA, Marescaux J. Multicentre propensity score-matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc. 2017;31:3106-3121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | de’Angelis N, Portigliotti L, Brunetti F. Robot-assisted rectal cancer surgery deserves a fair trial. Colorectal Dis. 2015;17:824-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | de’Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400:945-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 45. | Burke JP, Martin-Perez B, Khan A, Nassif G, de Beche-Adams T, Larach SW, Albert MR, Atallah S. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Colorectal Dis. 2016;18:570-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |