Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.628
Peer-review started: May 28, 2015
First decision: September 11, 2015
Revised: September 20, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 14, 2016
Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) has been applied to pancreaticobiliary lesions since the 1990s and is in widespread use throughout the world today. We used this method to confirm the pathological evidence of the pancreaticobiliary lesions and to perform suitable therapies. Complications of EUS-FNA are quite rare, but some of them are severe. Operators should master conventional EUS observation and experience a minimum of 20-30 cases of supervised EUS-FNA on non-pancreatic and pancreatic lesions before attempting solo EUS-FNA. Studies conducted on pancreaticobiliary EUS-FNA have focused on selection of suitable instruments (e.g., needle selection) and sampling techniques (e.g., fanning method, suction level, with or without a stylet, optimum number of passes). Today, the diagnostic ability of EUS-FNA is still improving; the detection of pancreatic cancer (PC) currently has a sensitivity of 90%-95% and specificity of 95%-100%. In addition to PC, a variety of rare pancreatic tumors can be discriminated by conducting immunohistochemistry on the FNA materials. A flexible, large caliber needle has been used to obtain a large piece of tissue, which can provide sufficient histological information to be helpful in classifying benign pancreatic lesions. EUS-FNA can supply high diagnostic yields even for biliary lesions or peri-pancreaticobiliary lymph nodes. This review focuses on the clinical aspects of EUS-FNA in the pancreaticobiliary field, with the aim of providing information that can enable more accurate and efficient diagnosis.
Core tip: Since the first attempts in 1990th, the instruments and methodology associated with endoscopic ultrasonography-guided fine needle aspiration have been largely improved for greater safety and efficacy of the procedure and accuracy of diagnosis. Choices of suitable needle and puncture method (fanning, suction, stylet, number of the passes) are critical for the better diagnostic yields of the pancreaticobiliary lesions as well as improving endosonographic skills.
- Citation: Matsubayashi H, Matsui T, Yabuuchi Y, Imai K, Tanaka M, Kakushima N, Sasaki K, Ono H. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol 2016; 22(2): 628-640
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.628
Until the 1990s, the diagnostic accuracy of pancreaticobiliary lesions was limited because of the tissue sampling procedures used, which were mostly endoscopic retrograde cholangiopancreatography (ERCP) or occasionally extra-abdominal approaches [e.g., computed tomography (CT)-guided or ultrasonography (US)-guided procedures]. Forceps biopsy[1] and brush cytology[2] have been applied during ERCP to provide confirmatory histological evidence. However, diagnosis of pancreatic carcinoma (PC), even in recent studies, is limited by a sensitivity of 49%-66% for pancreatic duct brushing cytology, with a complication of 3%-6% of post-ERCP pancreatitis[3,4], and pancreatic duct forceps biopsy has seldom been reported since the 1990s[5]. In contrast, these methods are routinely performed for bile duct carcinoma (BDC) and have achieved excellent diagnostic yields [forceps biopsy (77%-92%)[1,6] and brush cytology (75%-79%)[2,7]].
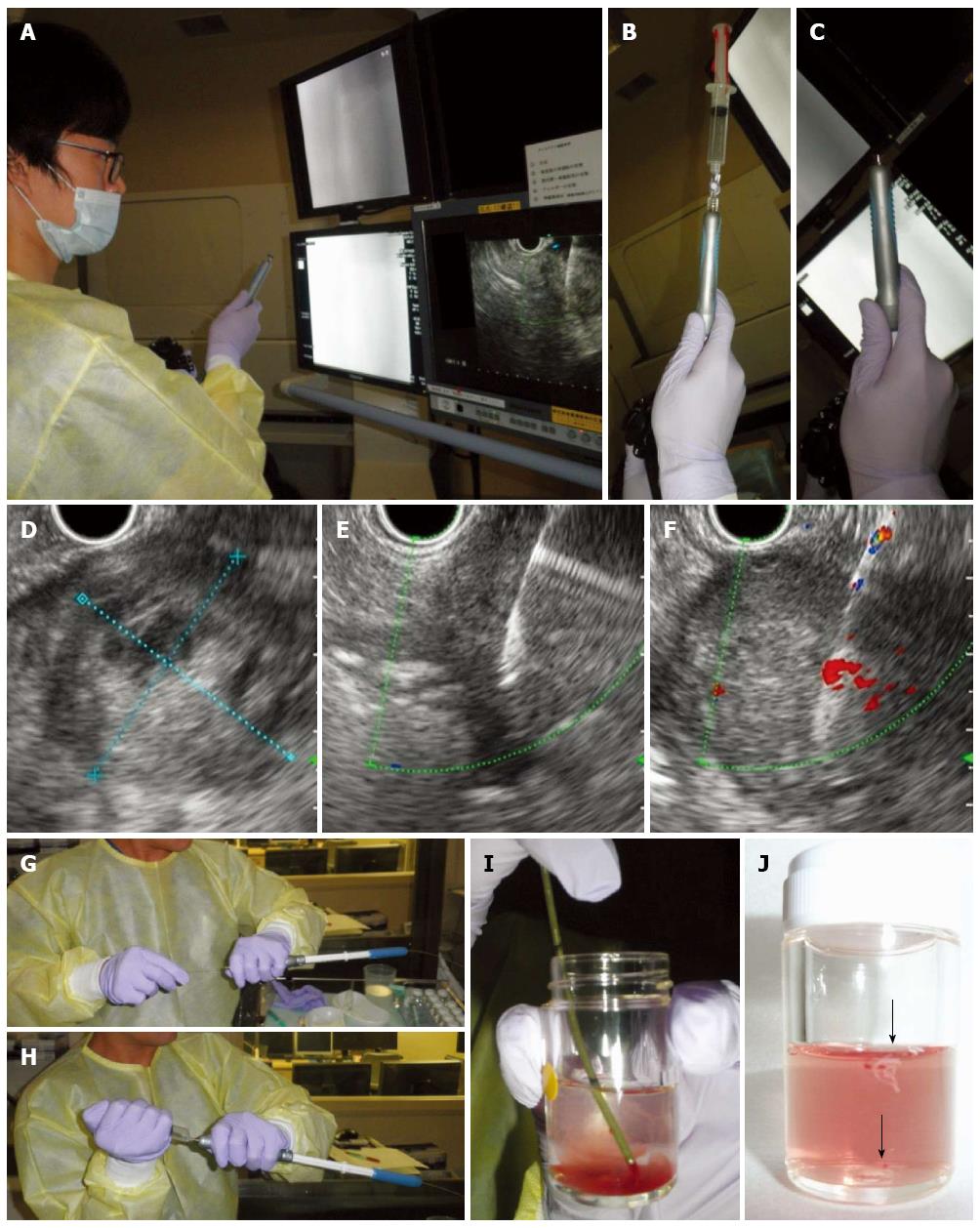
Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) is a safe and efficient diagnostic tool that provides pathological results for the lesions[8-10]. This method was first reported by Harada et al[11] and Caletti et al[12] in 1991, who attempted to obtain tissues from para-esophageal lymph nodes of the dog and human gastric submucosal tumors. Today, most of the lesions in or around the gastrointestinal tracts are targets of EUS-FNA[8]. The standard technique simply consists of the following steps: (1) visualization of the target by EUS; (2) selection of the puncture line; (3) needle puncture; (4) removal of the stylet; (5) suction by vacuum syringe; (6) back-and-forth movement of the needle; (7) removal of the needle; and (8) expulsion of the sample from the needle using the stylet[13] (Figure 1). The diagnostic capability of this method for cytopathological diagnosis of PC is highly sensitive and accurate[9,14-17] (sensitivity: 85%; specificity: 98%, by a meta-analysis performed in 2012)[17]; however, it is affected by various factors, such as scope position[13], needle type[18-21], FNA methodology[22-25], characteristics of the lesions, environments surrounding the lesions, on-site pathologist[8,13,15,19,24]. Recently, the instruments associated with EUS-FNA have been improved, including videoscopes[26] and FNA needles[21,27,28], as have the methodologies[19,22,25,29]. Associated studies have confirmed the improvements of EUS-FNA in the pancreaticobiliary field.
EUS-FNA is an excellent diagnostic tool for obtaining cytopathological evidence from pancreatic mass lesions. The reported sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for detecting PCs were 79%-98%, 71%-100%, 96%-100%, 33%-85%, and 82%-98%, respectively[9,15,16,21,22,24,25,29-36]. False negative and false positive rates were 12%-14%[30,37] and 0%-5%[30,31,37,38], respectively.
EUS-FNA can usually be performed safely. When the pancreatic solid mass is targeted, the most common complication is mild pancreatitis and the complication rate ranges from 0%[9,15] to 3.4%[10,28,29,35,39,40]. Targeting of small masses (≤ 20 mm) and endocrine tumors are reported as incurring risks of complications[39]. Rare but serious complications have been reported, such as severe bleeding (0.2%)[41], rupture of pseudoaneurysm[42], pancreatic pseudocyst[43], abscess[35], and cancer seeding[44,45]. Bleeding is a concern with this procedure; however, a prospective study performed on patients taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) did not show increased level of bleeding events, suggesting that EUS-FNA is safe in patients taking these anticoagulants[46].
Infectious complication, bacteremia or sepsis is scarcely caused by EUS-FNA for the pancreatobiliary solid lesions. Barawi et al[47] and Levy et al[48] prospectively examined blood cultures after EUS-FNA near the gastrointestinal tract and reported 5.8% (3/52)[48]-6.0% (6/100)[47] of culture-positive cases. However, all these patients were asymptomatic and the detected bacteria were coagulase negative Straphylococcus, Streptococcus viridans and so forth, considered to be contaminations. Other studies have noted bacteria-proven sepsis or febrile event only in small proportion of EUS-FNA procedures (0%-1%)[9,10,31,49]. Although the use of antibiotic prophylaxis is still arguable in the cases of cystic lesions, however at least, is not recommended for EUS-FNA of solid pancreatic lesions[48,50].
Tumor seeding is a late complication that is possibly induced by EUS-FNA, and several case reports have demonstrated gastric and/or peritoneal dissemination in cases with cancer at the pancreatic body and tail[44,45,51]. However, to date, no significant effects of EUS-FNA suggestive of increased levels of dissemination or worsening of survival have been found by several retrospective studies. For example, Ngamruengphong et al[52] analyzed 2034 patients with surgically resected PC in the Surveillance Epidemiology and End Results (SEER) medical database of the United States during 1998 and 2009; 498 (24%) of these patients underwent EUS-FNA. The study demonstrated a marginally improved prognosis in the EUS-FNA group than in the non-EUS-FNA group, even when the data were adjusted for the tumor site. However, this finding may be simply reflect the current advances in surgery, as cases with PC now tend to undergo more presurgical EUS-FNA. Japanese studies analyzing 82-107 cases of resected PC[53,54] reported no worsening of the incidence of peritoneal dissemination[54] and overall survival[53] in cases where EUS-FNA was used than when it was not (peritoneal dissemination: 17% vs 17%[54], overall survival: 1042 d vs 557 d; better in the EUS-FNA (+) group, P < 0.05[53]). Similar results were obtained in a study that included unresectable cases [217 cytopathologically confirmed PC cases divided into an ERCP group (161 cases) and an EUS-FNA group (56 cases)], and a similar occurrence was noted for peritoneal carcinomatosis in the ERCP group (15% during 545 d) and in the EUS-FNA group (18% during 599 d) (P = 0.85)[55].
The technical difficulty of EUS-FNA is affected by the location, size[34], hardness, necrosis, and vascularity of the target lesion, by large vessels lining the lesion, by the stability of the scope position[13] and by the needle size[18]. Acute angulation of the scope tip, torsion of the scope shaft, and intensive elevation of the needle sheath hamper smooth needle movement and increase the difficulty of the procedure. Puncture of the pancreas from the stomach is easily performed when the target is large, without disturbing the large vessels on the puncture line. However, a transduodenal puncture often needs angulation of the scope and needle elevation. In these cases, a 22 or 25 gauge (G) needle[18] is suitable and a flexible 19G needle[20,56] may also work.
Before starting the EUS-FNA procedure, the operator should master the convex-type EUS. In 2001, the American Society of Gastrointestinal Endoscopy recommended that an operator trainee conduct 150 supervised EUS procedures (including 75 pancreaticobiliary indications) and 60 cases of FNA (including 25 pancreatic FNAs) before the determination of competency[57]. The sensitivity of the cytopathological diagnosis of PC increases with the operator’s experience and is reported to reach 80% after 20-30 cases of supervised EUS-FNA training[58,59]. Accordingly, a minimum of 20-30 cases of supervised EUS-FNA on non-pancreatic and pancreatic lesions is recommended by the European Society of Gastrointestinal Endoscopy[60].
Selection of FNA needles and puncture methods are important factors associated with the efficacy and accuracy of EUS-FNA diagnosis of solid pancreatic lesions.
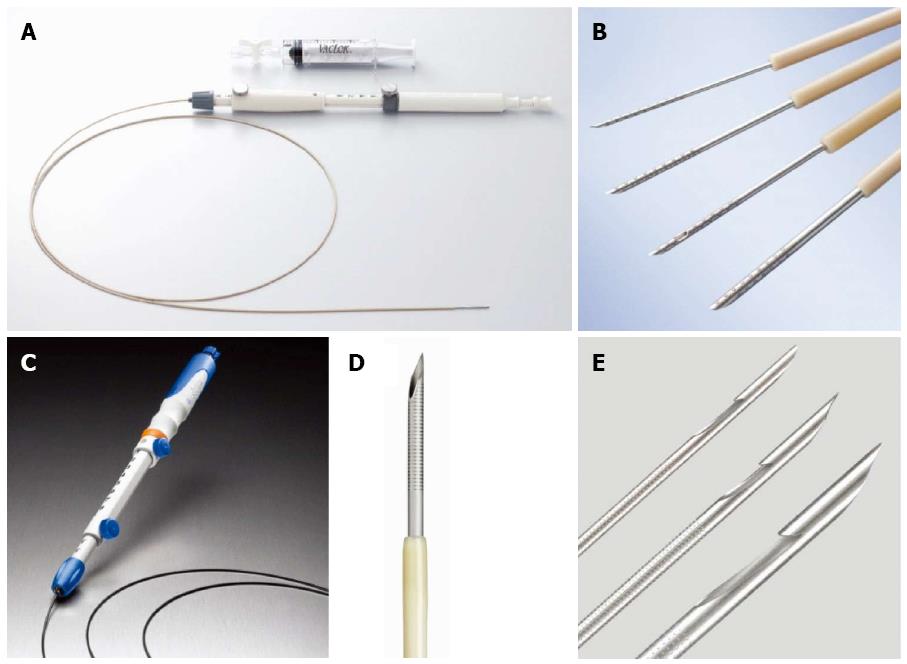
To date, several aspiration biopsy needles and Trucut needles have been used for EUS-FNA or EUS-guided core biopsy (Table 1 and Figure 2). The standard needle for pancreatic EUS-FNA is a 22G, but the needle size is selected by the presumed histological type and location of the targets. In general, a thinner needle (25G) is more flexible and therefore suitable for target lesions that require tight angulation of the scope and/or elevator[18], such as lesions at the pancreatic head. In contrast, a thicker aspiration needle and a Trucut needle (19G) lack flexibility and maneuverability, but can obtain a large piece of tissue, which provides more information for pancreatic pathology. For instance, pancreatic tissues obtained by a 19G FNA needle or core biopsy needle are useful in the diagnosis of pancreatic tumors other than pancreatic adenocarcinoma, tumors surrounded by chronic pancreatitis, lymphoma[61], and autoimmune pancreatitis[28]. However, a flexible 19G needle made of nitinol has recently been used on pancreatic head lesions and enabled satisfactory tissue acquisition from the pancreatic head in 95% of the cases[62]. Another recent advance has been the incorporation of a side port at the needle tip, which promotes efficient tissue acquisition even during the withdrawal manipulation. A comparative study of sampling efficacy from the peripancreatic and gastrointestinal lesions demonstrated that fewer passes were needed for adequate tissue acquisition when using a 22G needle with a side trap than with a 22G standard needle[27].
| Manufacturer | Needle | ||
| Product | Type | Size (gauge) | |
| Boston Scientific | Expect | Aspiration needle | 19, 22, 25 |
| Expect Flex | Aspiration needle | 19 | |
| Beacon Endoscopic | BNX | Aspiration needle | 19, 22, 25 |
| ConMed | Vizeon | Aspiration needle | 19, 22 |
| COOK | EchoBrush | Needle with cytology brush | 19 |
| EchoTip ProCore | Aspiration needle with a core trap | 19, 22, 25 | |
| EchoTip Ultra | Aspiration needle | 19, 22, 25 | |
| QuickCore | Core biopsy needle | 19 | |
| Hakko | Sonopsy CY | Aspiration needle | 21 |
| Medi-globe | Sonotip Pro Control | Aspiration needle | 19, 22, 25 |
| Hancke-Vilmann EUS-FNA System | Aspiration needle | 19, 22 | |
| Olympus | EZ Shot | Aspiration needle | 22 |
| EZ Shot 2 | Aspiration needle | 19, 22, 25 | |
| EZ Shot 2 with sideport | Aspiration needle with a sideport | 22 | |
| Power Shot | Aspiration needle | 22 | |
PC is sometimes accompanied by necrosis, mostly in the central area of the tumor (Figure 1). A previous study using transabdominal ultrasound-guided FNA reported that sampling from the peripheral area of the pancreatic mass improved the diagnostic accuracy[63]. The same is also true with EUS-FNA[60]. In this sense, the fanning method is considered effective, as it collects greater numbers of viable tumor cells. The needle movements within the multiple marginal areas of the mass using the “up-down” dial of the endoscope releases more cells when compared to the standard method that targets one peripheral area of the mass[23] (Figure 1). Bang et al[23] demonstrated the efficacy of a fanning method that targeted four marginal sites of the tumor; they needed significantly fewer passes to establish diagnosis than with the standard method [by randomized control trial (RCT) median 1 (interquartile range: 1-1) vs 1 (1-3), P = 0.02] and found a significantly higher rate of achieving a diagnosis with a single pass (85.7% vs 57.7%; P = 0.02).
The standard EUS-FNA is done with a needle controlled under negative pressure, usually applied with a 10-20 mL syringe[29] (COOK: 10 mL, Boston Scientific and Medi-Globe: 20 mL). However, the suction has been altered to determine its effect on FNA; i.e., by the no suction method[32,36,64,65], slow pull method[22] and high-negative pressure (HNP) method[29]. The no suction method is performed without suction[32,36,64,65] (Figure 1). The slow pull technique applies 10-20 to-and-fro needle movements with simultaneous minimum negative pressure provided by slow and continuous pulling of the stylet from the needle[22]. The HNP method is conducted under a vacuum provided by a 50 mL syringe [in contrast to the normal-negative pressure (NNP) method that uses a 10 mL syringe for vacuum[29].
Recent studies have confirmed that higher amounts of tissue are acquired and that blood contamination increases when the suction level is increased for EUS-FNA of solid pancreatic lesions[22,29,32]. An RCT by Puri et al[32] demonstrated a higher sensitivity by adding suction (86% in suction and 67% in non-suction, P = 0.05), but subsequent studies[22,65] showed no diagnostic superiority for the suction method. Interestingly, a retrospective study by Nakai et al[22] revealed a higher accuracy of the slow-pull method than with the ordinary suction method, but only when using 25G needles (91% vs 70%, P = 0.004); no difference was noted with a 22G needle. A comparison between HNP and NNP for EUS-FNA of a pancreatic mass using 25G needles confirmed that HNP was superior in terms of adequate tissue acquisition and accurate histological diagnosis compared to NNP (adequate tissue: 90% vs 72%, P = 0.0003; diagnostic accuracy: 82% vs 73%, P = 0.06). A high level of blood contamination was recognized in the HNP samples (P = 0.004), but the numbers of blood cells did not affect the histological diagnosis. A concern was noted for highly vascular lesions such as pancreatic neuroendocrine tumors (PNETs), as only limited cases have been examined[29]. For the FNA of lymph nodes, the quantity of tissue acquired is usually good and suction is not recommended, in order to reduce blood contamination[66].
The stylet is believed to prevent a contamination of the sample with tissue that does not originate from the target lesion; however, procedures for pushing out and withdrawing the stylet are time consuming. Three RCTs[67-69] found no superiority arising from the use of a stylet in terms of tissue contamination and diagnostic yield, and conversely found the adequacy of sample acquisition to be inferior (stylet: 75% vs non-stylet: 87%, P = 0.01) and saw an increase in blood contamination (75% vs 52%, P < 0.0001)[68]. However, as mentioned, a slow pull of the stylet during the pass could improve the quality of these FNA samples[22]. A stylet is also useful for pushing the tissues out from the needle onto slides or into a medium (Figure 1).
An on-site pathologist can provide the endosonographer with helpful information; for example, if the samples obtained by EUS-FNA contain tissues from the targets or whether additional procedures are needed[15]. However, this system is not feasible at all institutions and not in every tertiary hospital[22,24,70]. In the absence of rapid on-site evaluation (ROSE), knowing the optimum number of passes is critical information[24,25].
Earlier reports during 2000-2004 recommended 5-7 passes[33,71] for cases with a pancreatic mass. Erickson et al[71] reported in 2002 that cytological diagnosis of malignancy was obtained in 104 (95%) of 110 cases with PC. The average number of needle passes was 3.4 ± 2.2 (range: 1-10) with ROSE, and the number of passes was affected by the differentiation level of the cancer (well differentiated cancer: 5.5 ± 2.7, moderately differentiated: 2.7 ± 1.2, moderately to poorly differentiated: 3.4 ± 2.1, poorly differentiated: 2.3 ± 1.1) (P <0.001)[71]. LeBlanc et al[33] reported in 2004 that 7 passes were needed to achieve a sensitivity of 83% and specificity of 100% from pancreatic and miscellaneous lesions. However, recent studies demonstrated excellent sensitivity (90%-94%)[16,24,25] and specificity (96%-100%)[16,24,25] with fewer numbers of passes. Suzuki et al[25] reported an almost equal sensitivity and specificity of 25G needle EUS-FNA for the solid pancreatic lesion between a fixed number of 4 passes (93% and 100%) and a ROSE dependent procedure with a mean of 2.3 passes (94% and 100%). The sensitivity of the first method was increased by adding passes; 53% by 1 pass, 73% by 2 passes, 87% by 3 passes, and 93% by 4 passes. The most recent RCT by Ramesh et al[20] reported a similar high sensitivity by cytology obtained until the 2nd pass, with either a 19G flexible needle (92%) or a 25G needle (90%). When on-site cytological information is not available, gross inspection of the whitish component in the obtained materials is simple and useful for judgment of good sampling[24] (Figure 1).
The pancreatic neuroendocrine tumor (PNET) accounts for < 3% of all pancreatic neoplasms[72], and is often defined as a well-demarcated, highly vascular tumor based on clinical images. These image characteristics sometimes resemble solid pseudopapillary tumors (SPTs), acinar cell carcinomas (ACCs), and solid-type serous cystic tumors[73]; the required therapy varies among these tumors. Even within the category of PNET, therapeutic strategy varies by the histological grade (G1, G2 and G3), which is defined by the mitotic index and Ki-67 labeling index[74]. Therefore, EUS-FNA must be able to provide a differential diagnosis as well as accurate grading for treatment of PNET.
PNET has been correctly diagnosed by EUS-FNA at a rate of 77%[75]-90%[76]. Chen et al[75] reported that 54 (77%) of 70 histologically confirmed PNETs were preoperatively diagnosed as PNET by EUS-FNA. A cytological diagnosis of the remaining 16 cases of showed suspected PNET (4), no atypical cells (4), atypical cells (3), unsatisfactory material (2), adenocarcinoma (2), and suspected carcinoma (1), suggesting an overall difficulty in the cytological diagnosis of PNET. Chatzipantelis et al[77] reported the most helpful cytological findings of PNETs were a richly cellular sample with a monotonous, poorly cohesive population of small or medium-sized cells with granular chromatin (salt and pepper) and plasmacytoid morphology; helpful immunochemical diagnostic markers were neuron-specific enolase, synaptophysin, and chromogranin A.
Figueiredo et al[76] reported a large discrepancy between FNA and surgical materials (κ: 0.38, P = 0.003) when using a previous grading system of WHO criteria (2004). However, many recent reports demonstrated high agreement with this grading system. Comparison between EUS-FNA cytology and histology of the resected material showed a concordance of WHO grade and percent agreement of the Ki-67 index of 86% (19/22)[78] and 89% (κ: 0.78)[79], respectively. Histology of EUS-FNA samples and surgical materials also showed high agreement (79%[80]-89%[81]) with the WHO grade based on the Ki-67 index. Hasegawa et al[80] analyzed the intratumoral dispersion of the Ki-67 index of PNET and demonstrated a higher level of dispersion in G2 PNET (0.78) than in G1 PNET (0.03) (P < 0.001). In their study, grading concordance increased up to 90% when the FNA sample contained ≥ 2000 tumor cells, suggesting a necessity for a large amount of sample for accurate grading or prediction of patient’s survival[80].
ACC[82-84], mixed acinar carcinoma[83-85], SPT[86,87], and intraductal tubullopapillary neoplasm (ITPN)[88,89] can also be diagnosed using EUS-FNA samples. These tumors are histologically similar and often require additional immunostainings for differentiation markers: BCL10, lipase, and trypsin are useful markers specific to ACC[83,84]; ITPN is often positive for cytokeratin 7 and 19, MUC1, and MUC6[88,89], but negative for MUC2 or MUC5AC, which are usually stained in intraductal papillary mucinous neoplasms (IPMNs)[88]. The cytological features of SPT are papillary structures, cercariform cells, large cytoplasmic vacuoles, foam cells, and giant cells. Immunostaining of SPT is usually positive for nuclear β-catenin, but usually negative for chromogranin or lipase[86].
Mass-forming pancreatitis includes a progressive form of the ordinary chronic pancreatitis or a specific etiology such as focal-type of autoimmune pancreatitis (AIP). The former histologically shows various level of acinar atrophy associated with a progression of the fibrous stroma, sometimes accompanied with ductal dilation, calculi, and squamous metaplasia of the ductal epithelia[72,90]. Autoimmune pancreatitis (AIP) is a unique form of chronic pancreatitis, sometimes resembling pancreatic malignancies on clinical images; it is histologically classified into type 1 (lymphoplasmacytic sclerosing pancreatitis: LPSP) and type 2 (idiopathic duct-centric pancreatitis: IDCP)[91].
Imai et al[16] described that EUS-FNA specimens obtained from 21 cases of AIP using 22G needles showed no histology meeting the criteria of LPSP or IDCP, but was beneficial only for eliminating PC. In contrast, Ishikawa et al[92] performed EUS-FNA using 22G needles on 47 cases of AIP, and obtained level 1 histological findings of LPSP in 9 cases (19%), level 2 LPSP in 5 cases (11%), and level 2 IDCP in 3 cases (6%). Iwashita et al[28] also reported that EUS-FNA using 19G needles supplied adequate tissue for the histological diagnosis of AIP in 43% (19/44) of the cases, suggesting the importance of the amount or size of EUS-FNA samples for proper diagnosis of AIP.
EUS-FNA of a pancreatic lesion rarely yields results of “atypical,”“indeterminate,” or “inconclusive,” but this can happen even with enough passes and even with definitive images for PC. Within the previous studies, inconclusive results were recognized in 4.7%-9.2%[93-95] of EUS-FNA for solid or cystic lesions of the pancreas, even in tertiary hospitals. Repeated EUS-FNA[95-97] or careful follow-up[98] is recommended in these cases. Repeated EUS-FNA has been done within 3-4 wk[96,97] after the initial attempt or upon referral to a tertiary center[95]. More than 80% of cases of initially inconclusive results were diagnostic upon repeated EUS-FNA[95-97]. The following reasons are suspected for the failed initial EUS-FNA: mistargeting by the coexisting pancreatitis, technical difficulty in scope positioning, sedation failure, ascites, or collateral vessels, difficulty in cytology (partially cystic, necrotic, well-differentiated), pathologist’s inter-observer variation[96], and inexperienced endosonographers[95]. Reported predictors of malignancy in negative[98] or indeterminate[94] EUS-FNA are high serum levels of CA19-9 (≥ 40 U/mL)[94], associated lymph node swelling, vascular involvement[98], weight loss, and biliary obstruction[93]. FNA does not provide evidence for the absence of cancer; hence, the clinician must follow and/or reexamine, when EUS-FNA results are inconclusive.
Several studies have been reported the diagnostic ability of EUS-FNA for biliary strictures. Byrne et al[99] reported that EUS-FNA performed using 22G needles in 23 resected cases with biliary stricture or masses revealed 11 cases of confirmed malignancy in the surgically materials. The sensitivity and specificity of EUS-FNA were 100%, although a sensitivity of EUS observation alone was 45%. DeWitt et al[100], Eloubeidi et al[101] and Ohshima et al[102] reported on the effects of EUS-FNA in cases with biliary strictures (22-28 cases); most underwent ERCP but gave negative or non-diagnostic results by brush cytology. In their studies, the sensitivity, specificity, PPV, NPV, and accuracy for detecting malignancy in 71%-82% of the cases were 77%-100%, 100%, 100%, 29%-57%, and 79%-88%, respectively[100-102]. No complications were reported in these studies.
Needle tract seeding is also a concern for the biliary cancers. El Chafic et al[103] analyzed factors associated with survival in 119 patients with biliary cancer that underwent curative-intent surgery with or without preoperative EUS-FNA (EUS-FNA done in 39 cases), and patient’s age, tumor size, and lymph node metastasis were listed as significant prognostic factors but not preoperative EUS-FNA (HR = 1.09, 95%CI: 0.69-1.73, P value = 0.7).
However, indications for EUS-FNA in cases with biliary stricture may be limited, as transpapillary biliary sampling (biopsy and brushing cytology) can be done at the same time as biliary drainage, with safety and high sensitivities [forceps biopsy (77%-92%)[1,6] and brush cytology (75%-79%)[2,7]].
Gallbladder cancer (GBC) is difficult to diagnose with pathological evidence, and sometimes mimics xanthogranulomatous cholecystitis or acute/chronic cholecystitis. Transpapillary approaches such as naso-gallbladder drainage cytology is feasible and highly diagnostic[104,105], but is sometimes technically difficult and is associated with several complications (mild to moderate acute pancreatitis, cholecystitis and cholangitis)[104,106]. EUS-FNA of the gallbladder lesions has been attempted and provided excellent diagnostic rates for detecting GBC (sensitivity: 80%-90% and specificity: 100%)[107-109]. Kim et al[108] reported that all 14 cases of lymphadenopathy accompanied with suspected GBC were confirmed as lymph node metastasis by EUS-FNA. Interestingly, Hijioka et al[109] reported that 40 (87%) out of 46 cases of GBC was accompanied with lymphadenopathy, of which 36 cases (90%) were confirmed as lymph node metastases by EUS-FNA, in contrast to null lymphadenopathy in 5 cases with xanthogranulomatous cholecystitis. EUS-FNA of the gallbladder wall may have a risk for dissemination in cases with GBC; hence, the first attempt is safer on the regional lymph nodes[109], if swollen.
Tumor of the papilla of Vater is usually easy to diagnose if it originates from the duodenal mucosa, as it is exposed to the intestinal lumen and is detectable by the endoscopy. However, some of the ampullary carcinomas originate from ampullary bile duct, ampullary pancreatic duct, or common duct[110] and are not exposed to duodenal lumen at the early stage. In these cases, endoscopic sphincterotomy of the papilla and subsequent forceps biopsy may increase the chance of obtaining cancer tissues[111], but this may not always effective. Ogura et al[112] attempted EUS-FNA in 10 patients and diagnosed 3 intra-ampullary carcinomas without complications, after the diagnostic failure of cytology and/or forceps biopsy under ERCP.
This review focused on the clinical aspects of EUS-FNA for solid pancreaticobiliary lesions. During the past quarter of a century, the instruments, methodology, and environment concerning EUS-FNA of the pancreaticobiliary field have improved for greater safety and efficacy of the procedure and accuracy of diagnosis. The mastery of endoscopic technique, as well as the correct choice of instrument and method for the target lesion, is essential for better outcomes.
P- Reviewer: Biecker E, Lorenzo-Zuniga V S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Kimura H, Matsubayashi H, Sasaki K, Ito H, Hirosawa K, Uesaka K, Kanemoto H, Ono H. Factors affecting the yield of endoscopic transpapillary bile duct biopsy for the diagnosis of pancreatic head cancer. Pancreatology. 2013;13:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Sugimoto S, Matsubayashi H, Kimura H, Sasaki K, Nagata K, Ohno S, Uesaka K, Mori K, Imai K, Hotta K. Diagnosis of bile duct cancer by bile cytology: usefulness of post-brushing biliary lavage fluid. Endosc Int Open. 2015;3:E323-E328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi T, Shirai Y, Nakamura N, Sudo K, Nakamura K, Hironaka S, Hara T, Denda T. Usefulness of brush cytology combined with pancreatic juice cytology in the diagnosis of pancreatic cancer: significance of pancreatic juice cytology after brushing. Pancreas. 2012;41:1225-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Uchida N, Kamada H, Tsutsui K, Ono M, Aritomo Y, Masaki T, Kushida Y, Haba R, Nakatsu T, Kuriyama S. Utility of pancreatic duct brushing for diagnosis of pancreatic carcinoma. J Gastroenterol. 2007;42:657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kubota Y, Takaoka M, Tani K, Ogura M, Kin H, Fujimura K, Mizuno T, Inoue K. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. Am J Gastroenterol. 1993;88:1700-1704. [PubMed] [Cited in This Article: ] |
| 6. | Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, Forsmark CE. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Bang KB, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Comparison of brush and basket cytology in differential diagnosis of bile duct stricture at endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2014;13:622-627. [PubMed] [Cited in This Article: ] |
| 8. | Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Ryozawa S, Kitoh H, Gondo T, Urayama N, Yamashita H, Ozawa H, Yanai H, Okita K. Usefulness of endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of pancreatic cancer. J Gastroenterol. 2005;40:907-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, Raimondo M. The safety of fine-needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy. 2008;40:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Harada N, Kouzu T, Ohshima I, Ichinose M, Arima M, Hishikawa E, Isono K. A trial of endoscopic ultrasound-guided puncturetechnique (in Japanese with English abstract). Gastroenterological endoscopy. 1991;33:1657-1663. [Cited in This Article: ] |
| 12. | Caletti GC, Brocchi E, Ferrari A, Bonora G, Santini D, Mazzoleni G, Barbara L. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy. 1991;23:251-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Yasuda I, Iwashita T, Doi S. Tips for endoscopic ultrasound-guided fine needle aspiration of various pancreatic lesions. J Hepatobiliary Pancreat Sci. 2014;21:E29-E33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, Imaoka H, Niwa Y, Tajika M, Kondo S. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 18. | Itoi T, Itokawa F, Kurihara T, Sofuni A, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Kawai T, Moriyasu F. Experimental endoscopy: objective evaluation of EUS needles. Gastrointest Endosc. 2009;69:509-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Varadarajulu S, Bang JY, Holt BA, Hasan MK, Logue A, Hawes RH, Hebert-Magee S. The 25-gauge EUS-FNA needle: Good for on-site but poor for off-site evaluation? Results of a randomized trial. Gastrointest Endosc. 2014;80:1056-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Ramesh J, Bang JY, Hebert-Magee S, Trevino J, Eltoum I, Frost A, Hasan MK, Logue A, Hawes R, Varadarajulu S. Randomized Trial Comparing the Flexible 19G and 25G Needles for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Solid Pancreatic Mass Lesions. Pancreas. 2015;44:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Iwashita T, Nakai Y, Samarasena JB, Park do H, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Nakai Y, Isayama H, Chang KJ, Yamamoto N, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Yamamoto K, Kawakubo K. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Itoi T, Tsuchiya T, Itokawa F, Sofuni A, Kurihara T, Tsuji S, Ikeuchi N. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc. 2011;23 Suppl 1:34-38. [PubMed] [Cited in This Article: ] |
| 25. | Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Chatterjee S, Oppong KW. Endobronchial ultrasonic videoscope for transgastric/transesophageal fine-needle aspiration in special situations: another tool for the gastrointestinal endosonographer. Endoscopy. 2012;44 Suppl 2 UCTN:E298-E299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Witt BL, Adler DG, Hilden K, Layfield LJ. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030-1037.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854-861. [PubMed] [Cited in This Article: ] |
| 31. | Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: Performance and outcomes. J Gastroenterol Hepatol. 2009;24:90-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [PubMed] [Cited in This Article: ] |
| 34. | Siddiqui AA, Brown LJ, Hong SK, Draganova-Tacheva RA, Korenblit J, Loren DE, Kowalski TE, Solomides C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370-3375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Matsuyama M, Ishii H, Kuraoka K, Yukisawa S, Kasuga A, Ozaka M, Suzuki S, Takano K, Sugiyama Y, Itoi T. Ultrasound-guided vs endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer diagnosis. World J Gastroenterol. 2013;19:2368-2373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Abdelgawwad MS, Alston E, Eltoum IA. The frequency and cancer risk associated with the atypical cytologic diagnostic category in endoscopic ultrasound-guided fine-needle aspiration specimens of solid pancreatic lesions: a meta-analysis and argument for a Bethesda System for Reporting Cytopathology of the Pancreas. Cancer Cytopathol. 2013;121:620-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Gleeson FC, Kipp BR, Caudill JL, Clain JE, Clayton AC, Halling KC, Henry MR, Rajan E, Topazian MD, Wang KK. False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut. 2010;59:586-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Katanuma A, Maguchi H, Yane K, Hashigo S, Kin T, Kaneko M, Kato S, Kato R, Harada R, Osanai M. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013;58:2093-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [PubMed] [Cited in This Article: ] |
| 41. | Hamada T, Yasunaga H, Nakai Y, Isayama H, Horiguchi H, Matsuda S, Fushimi K, Koike K. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: an analysis of 3,090 patients from 212 hospitals. Gut Liver. 2014;8:215-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Matsumoto K, Hara K, Sawaki A, Mizuno N, Hijioka S, Imamura H, Niwa Y, Tajika M, Kawai H, Kondo S. Ruptured pseudoaneurysm of the splenic artery complicating endoscopic ultrasound-guided fine-needle aspiration biopsy for pancreatic cancer. Endoscopy. 2010;42 Suppl 2:E27-E28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Chung KH, Ryu JK, Oh HS, Seo JY, Jin E, Lee DH, Kim YT, Yoon YB. Pancreatic pseudocyst after endoscopic ultrasound-guided fine needle aspiration of pancreatic mass. Clin Endosc. 2012;45:431-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [PubMed] [Cited in This Article: ] |
| 45. | Katanuma A, Maguchi H, Hashigo S, Kaneko M, Kin T, Yane K, Kato R, Kato S, Harada R, Osanai M. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44 Suppl 2 UCTN:E160-E161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Kien-Fong Vu C, Chang F, Doig L, Meenan J. A prospective control study of the safety and cellular yield of EUS-guided FNA or Trucut biopsy in patients taking aspirin, nonsteroidal anti-inflammatory drugs, or prophylactic low molecular weight heparin. Gastrointest Endosc. 2006;63:808-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189-192. [PubMed] [Cited in This Article: ] |
| 48. | Levy MJ, Norton ID, Wiersema MJ, Schwartz DA, Clain JE, Vazquez-Sequeiros E, Wilson WR, Zinsmeister AR, Jondal ML. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [PubMed] [Cited in This Article: ] |
| 50. | Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 51. | Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Kudo T, Kawakami H, Kuwatani M, Eto K, Kawahata S, Abe Y, Onodera M, Ehira N, Yamato H, Haba S. Influence of the safety and diagnostic accuracy of preoperative endoscopic ultrasound-guided fine-needle aspiration for resectable pancreatic cancer on clinical performance. World J Gastroenterol. 2014;20:3620-3627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Ohtsuka T, Tamura K, Ideno N, Aso T, Nagayoshi Y, Kono H, Ueda J, Takahata S, Aso A, Igarashi H. Role of ERCP in the era of EUS-FNA for preoperative cytological confirmation of resectable pancreatic ductal adenocarcinoma. Surg Today. 2014;44:1887-1892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Ikezawa K, Uehara H, Sakai A, Fukutake N, Imanaka K, Ohkawa K, Tanakura R, Ioka T, Tanaka S, Ishikawa O. Risk of peritoneal carcinomatosis by endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. J Gastroenterol. 2013;48:966-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Bang JY, Ramesh J, Trevino J, Eloubeidi MA, Varadarajulu S. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Petersen BT, Raddawi HM, Ryan ME, Vargo JJ, Young HS, Wheeler-Harbaugh J. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811-814. [PubMed] [Cited in This Article: ] |
| 58. | Harewood GC, Wiersema LM, Halling AC, Keeney GL, Salamao DR, Wiersema MJ. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002;55:669-673. [PubMed] [Cited in This Article: ] |
| 59. | Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33-37. [PubMed] [Cited in This Article: ] |
| 60. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | de la Fuente SG, Arnoletti JP. Beyond cytology: why and when does the oncologist require core tissue? Gastrointest Endosc Clin N Am. 2014;24:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Ekberg O, Bergenfeldt M, Aspelin P, Genell S, Lindholm K, Nilsson P, Sigurjónsson S. Reliability of ultrasound-guided fine-needle biopsy of pancreatic masses. Acta Radiol. 1988;29:535-539. [PubMed] [Cited in This Article: ] |
| 64. | Casal RF, Staerkel GA, Ost D, Almeida FA, Uzbeck MH, Eapen GA, Jimenez CA, Nogueras-Gonzalez GM, Sarkiss M, Morice RC. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest. 2012;142:568-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Mohammad Alizadeh AH, Hadizadeh M, Padashi M, Shahbaazi S, Molaee M, Shariatpanahi ZV. Comparison of two techniques for endoscopic ultrasonography fine-needle aspiration in solid pancreatic mass. Endosc Ultrasound. 2014;3:174-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey W, Hawes RH. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441-447. [PubMed] [Cited in This Article: ] |
| 67. | Wani S, Early D, Kunkel J, Leathersich A, Hovis CE, Hollander TG, Kohlmeier C, Zelenka C, Azar R, Edmundowicz S. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 68. | Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, Ulusarac O, Fan F, Romanas M, Dennis KL. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Yasuda I, Goto N, Tsurumi H, Nakashima M, Doi S, Iwashita T, Kanemura N, Kasahara S, Adachi S, Hara T. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248-2254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas, 6th ed. Washington, DC: Armed Forces Institute of Pathology 2007; . [Cited in This Article: ] |
| 73. | Kishida Y, Matsubayashi H, Okamura Y, Uesaka K, Sasaki K, Sawai H, Imai K, Ono H. A case of solid-type serous cystadenoma mimicking neuroendocrine tumor of the pancreas. J Dig Dis. 2014;15:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Bosman FT, Carneiro F, Hruban RH. WHO Classification of Tumours of the Digestive System (World Health Organization Classification of Tumours). Lyon: IARC Press 2010; . [Cited in This Article: ] |
| 75. | Chen S, Lin J, Wang X, Wu HH, Cramer H. EUS-guided FNA cytology of pancreatic neuroendocrine tumour (PanNET): a retrospective study of 132 cases over an 18-year period in a single institution. Cytopathology. 2014;25:396-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Figueiredo FA, Giovannini M, Monges G, Bories E, Pesenti C, Caillol F, Delpero JR. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: a study of 48 cases. Cancer. 2008;114:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Farrell JM, Pang JC, Kim GE, Tabatabai ZL. Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122:770-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Piani C, Franchi GM, Cappelletti C, Scavini M, Albarello L, Zerbi A, Giorgio Arcidiacono P, Bosi E, Manzoni MF. Cytological Ki-67 in pancreatic endocrine tumours: an opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, Imaoka H, Niwa Y, Tajika M, Kondo S. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Unno J, Kanno A, Masamune A, Kasajima A, Fujishima F, Ishida K, Hamada S, Kume K, Kikuta K, Hirota M. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol. 2014;49:1367-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Iwatate M, Matsubayashi H, Sasaki K, Kishida N, Yoshikawa S, Ono H, Maitra A. Functional pancreatic acinar cell carcinoma extending into the main pancreatic duct and splenic vein. J Gastrointest Cancer. 2012;43:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 2013;121:459-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Sullivan PS, Clebanoff JL, Hirschowitz SL. Hints to the diagnosis of mixed acinar-endocrine carcinoma on pancreatic fine-needle aspiration: avoiding a potential diagnostic pitfall. Acta Cytol. 2013;57:296-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Samad A, Shah AA, Stelow EB, Alsharif M, Cameron SE, Pambuccian SE. Cercariform cells: another cytologic feature distinguishing solid pseudopapillary neoplasms from pancreatic endocrine neoplasms and acinar cell carcinomas in endoscopic ultrasound-guided fine-needle aspirates. Cancer Cytopathol. 2013;121:298-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy. 2008;40:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 88. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 89. | Yoshida Y, Matsubayashi H, Sasaki K, Kanemoto H, Uesaka K, Ono H. Intraductal tubulopapillary neoplasm of the pancreatic branch duct showing atypical images. J Dig Dis. 2015;16:357-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Matsubayashi H, Watanabe H, Ajioka Y, Nishikura K, Yamano M, Seki T, Saito T, Matsubayashi T. Different amounts of K-ras mutant epithelial cells in pancreatic carcinoma and mass-forming pancreatitis. Pancreas. 2000;21:77-85. [PubMed] [Cited in This Article: ] |
| 91. | Matsubayashi H, Kakushima N, Takizawa K, Tanaka M, Imai K, Hotta K, Ono H. Diagnosis of autoimmune pancreatitis. World J Gastroenterol. 2014;20:16559-16569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol. 2012;18:3883-3888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 62] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Alston E, Bae S, Eltoum IA. Atypical cytologic diagnostic category in EUS-FNA of the pancreas: follow-up, outcomes, and predictive models. Cancer Cytopathol. 2014;122:428-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Yang D, MoezArdalan K, Collins DP, Chauhan SS, Draganov PV, Forsmark CE, Wagh MS. Predictors of malignancy in patients with suspicious or indeterminate cytology on pancreatic endoscopic ultrasound-guided fine-needle aspiration: a multivariate model. Pancreas. 2014;43:922-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Suzuki R, Lee JH, Krishna SG, Ramireddy S, Qiao W, Weston B, Ross WA, Bhutani MS: Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointest Liver Dis. 2013;22:183-187. [Cited in This Article: ] |
| 96. | Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Tadic M, Kujundzic M, Stoos-Veic T, Kaic G, Vukelic-Markovic M. Role of repeated endoscopic ultrasound-guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig Dis. 2008;26:377-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Spier BJ, Johnson EA, Gopal DV, Frick T, Einstein MM, Byrne S, Koscik RL, Liou JI, Broxmeyer T, Selvaggi SM. Predictors of malignancy and recommended follow-up in patients with negative endoscopic ultrasound-guided fine-needle aspiration of suspected pancreatic lesions. Can J Gastroenterol. 2009;23:279-286. [PubMed] [Cited in This Article: ] |
| 99. | Byrne MF, Gerke H, Mitchell RM, Stiffler HL, McGrath K, Branch MS, Baillie J, Jowell PS. Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy. 2004;36:715-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 101. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. [PubMed] [Cited in This Article: ] |
| 102. | Ohshima Y, Yasuda I, Kawakami H, Kuwatani M, Mukai T, Iwashita T, Doi S, Nakashima M, Hirose Y, Asaka M. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | El Chafic AH, Dewitt J, Leblanc JK, El Hajj II, Cote G, House MG, Sherman S, McHenry L, Pitt HA, Johnson C. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Moriyasu F, Yamagishi T, Serizawa H. Preoperative diagnosis and management of thick-walled gallbladder based on bile cytology obtained by endoscopic transpapillary gallbladder drainage tube. Gastrointest Endosc. 2006;64:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Matsubayashi H, Kiyohara Y, Sasaki K, Kanemoto H, Urikura K, Kawata N, Kimura H, Ono H. Metastatic malignant melanoma of the gallbladder diagnosed by cytology of endoscopic naso-gallbladder drainage fluid. J Dig Dis. 2012;13:190-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Matsubayashi H, Fukutomi A, Kanemoto H, Maeda A, Matsunaga K, Uesaka K, Otake Y, Hasuike N, Yamaguchi Y, Ikehara H. Risk of pancreatitis after endoscopic retrograde cholangiopancreatography and endoscopic biliary drainage. HPB (Oxford). 2009;11:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Meara RS, Jhala D, Eloubeidi MA, Eltoum I, Chhieng DC, Crowe DR, Varadarajulu S, Jhala N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 108. | Kim HJ, Lee SK, Jang JW, Kim TG, Ryu CH, Park do H, Lee SS, Seo DW, Kim MH. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology. 2012;59:1691-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Hijioka S, Hara K, Mizuno N, Imaoka H, Ogura T, Haba S, Mekky MA, Bhatia V, Hosoda W, Yatabe Y. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J Hepatobiliary Pancreat Sci. 2012;19:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Matsubayashi H, Watanabe H, Yamaguchi T, Ajioka Y, Nishikura K, Kijima H, Saito T. Differences in mucus and K-ras mutation in relation to phenotypes of tumors of the papilla of vater. Cancer. 1999;86:596-607. [PubMed] [Cited in This Article: ] |
| 111. | Menzel J, Poremba C, Dietl KH, Böcker W, Domschke W. Tumors of the papilla of Vater--inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol. 1999;10:1227-1231. [PubMed] [Cited in This Article: ] |
| 112. | Ogura T, Hara K, Hijioka S, Mizuno N, Imaoka H, Niwa Y, Tajika M, Kondo S, Tanaka T, Shimizu Y. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for tumors of the ampulla of vater? -an initial study. Endosc Ultrasound. 2012;1:84-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |