Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6127
Peer-review started: December 12, 2014
First decision: February 2, 2015
Revised: February 26, 2015
Accepted: April 16, 2015
Article in press: April 17, 2015
Published online: May 28, 2015
Patients with pancreatic cancer have a poor prognosis with a median survival of 4-6 mo and a 5-year survival of less than 5%. Despite therapy with gemcitabine, patient survival does not exceed 6 mo, likely due to natural resistance to gemcitabine. Therefore, it is hoped that more favorable results can be obtained by using guided immunotherapy against molecular targets. This review summarizes the new leading targeted therapies in pancreatic cancers, focusing on passive and specific immunotherapies. Passive immunotherapy may have a role for treatment in combination with radiochemotherapy, which otherwise destroys the immune system along with tumor cells. It includes mainly therapies targeting against kinases, including epidermal growth factor receptor, Ras/Raf/mitogen-activated protein kinase cascade, human epidermal growth factor receptor 2, insulin growth factor-1 receptor, phosphoinositide 3-kinase/Akt/mTOR and hepatocyte growth factor receptor. Therapies against DNA repair genes, histone deacetylases, microRNA, and pancreatic tumor tissue stromal elements (stromal extracellular matric and stromal pathways) are also discussed. Specific immunotherapies, such as vaccines (whole cell recombinant, peptide, and dendritic cell vaccines), adoptive cell therapy and immunotherapy targeting tumor stem cells, have the role of activating antitumor immune responses. In the future, treatments will likely include personalized medicine, tailored for numerous molecular therapeutic targets of multiple pathogenetic pathways.
Core tip: Adjuvant therapy in pancreatic cancer has limited efficiency, and low survival rates are related to resistance to gemcitabine. New targeted therapies, such as passive immunotherapy, may have a role in combination with radiochemotherapy by targeting various protein kinases, as well as specific immunotherapies, such as vaccines, adoptive cell therapy and immunotherapy targeting tumor stem cells. In the future, treatments will likely include personalized medicine, tailored for numerous molecular therapeutic targets of multiple pathogenetic pathways.
- Citation: Seicean A, Petrusel L, Seicean R. New targeted therapies in pancreatic cancer. World J Gastroenterol 2015; 21(20): 6127-6145
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6127
Patients with pancreatic cancer (PC) have a poor prognosis with a median survival of 4-6 mo and a < 5% five-year survival rate[1]. Over 80% of patients have advanced disease at presentation (metastatis or invasion of the superior mesenteric artery or celiac trunk in case of locally advanced tumors), which does not allow for surgical resection of the tumor[2]. Even if resection can be achieved, the median survival is still only 18 mo[3]. Despite therapy with gemcitabine (GEM), which represents the first-line therapy for advanced tumors, patient survival typically does not exceed 6 mo for metastatic disease and 9-12 mo for locally advanced disease, likely due to natural resistance to GEM[4,5]. FOLFIRINOX represents an alternative to gemcitabine in first line settings, with better survival, but it is suitable only for good performance status patients. As second line treatment, GEM-platinum-based combination provide the best results[6].Therefore, it is hoped that more favorable results can be obtained by using passive and specific immunotherapies against molecular targets.
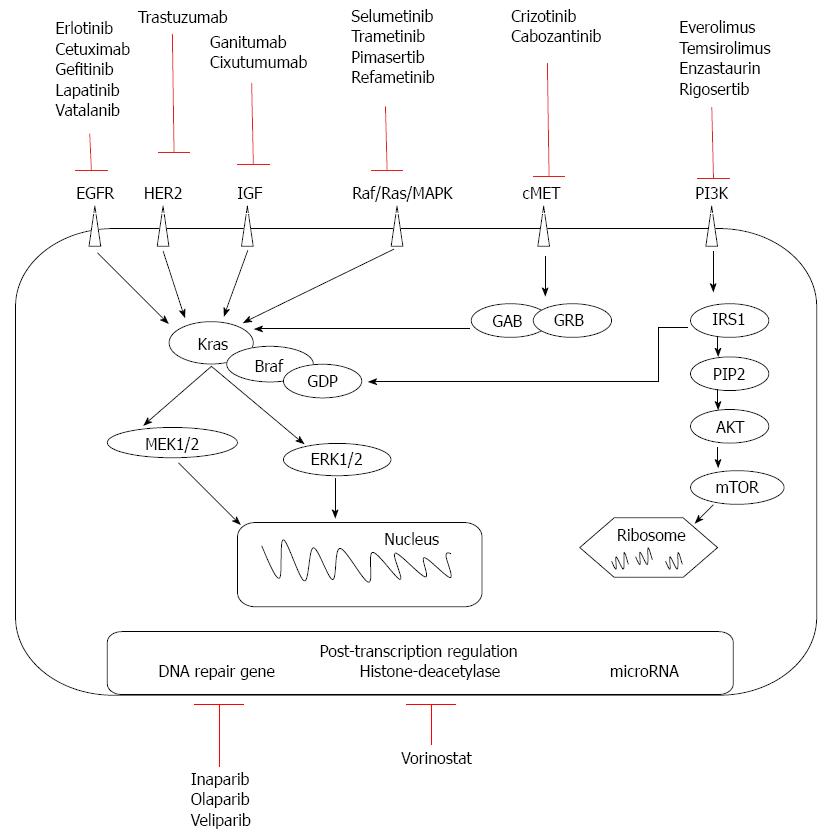
Passive immunotherapy involves in vivo infusion of monoclonal antibodies or in vitro-activated T cells. Monoclonal antibodies have been created to act on molecules at the cell surface of the tumor and on stromal tissue in connection with PC oncogenesis, tumor growth, and chemotherapy-resistant or immune-response regulation. Currently developed therapies target pre-transcriptional kinases, post-transcriptional level (DNA repair genes, histone deacetylases, microRNAs), antipancreatic tumor tissue stromal elements and antiangiogenic factors (Figure 1).
Tyrosine kinases are important in the proliferation, migration, invasion, and resistance to apoptosis of tumor cells, and involve activation of mitogen-activated protein kinase (MAPK; which is responsible for the malignant transformation of pancreatic cells[7]), phosphoinositide 3-kinase (PI3K; which stimulates cell proliferation and chemotherapy resistance[8]), and protein kinase B [Akt; the overexpression of which promotes invasion and expression of insulin growth factor receptor (IGF-1R)[9,10]]. In addition, K-ras is involved in the pathogenesis of PC via tyrosine kinase pathways[11,12]. The expression of two tyrosine kinase receptors, epidermal growth factor receptors (EGFRs) B-1 and B-2, has been found in 90% and 21% of PCs, respectively[13,14]. Increased coexpression of EGFR and its ligand in PC is associated with greater liver metastasis and poorer prognosis[15-17].
Anti-EGFR: Therapies involving anti-EGFR (epidermal growth factor receptor or HER1) monoclonal antibodies include cetuximab, a chimeric IgG1-type, and panitumumab, a humanized IgG2-type antibody. These antibodies reversibly inhibit the tyrosine kinase domain of EGFR by competitive binding of ATP. As a result of antibody binding, the receptor internalizes, complement-mediated cytotoxicity appears, and cell division is stopped. However, the anti-EGFR mechanism may not be effective if there are mutations in the KRAS gene. Cetuximab seems to be more effective than panitumumab, as IgG1 receptors are more effective than IgG2[18]. However, its efficiency was not proved in clinical trials (Table 1).
| Ref. | Patients no./disease stage | Study type | Drugs | OS | PFS | Benefit |
| Burtness et al[19], 2014 | 87/metastatic | II RCT | Docetaxel + Irinotecan ± Cetuximab | 6.5 vs 5.4 | 3.9 vs 4.5 | Negative |
| Fensterer et al[20], 2014 | 73/resected | II | GEM + Cetuximab | 22.4 | NA | Negative |
| Philip et al[21], 2010 | 743/locally advanced or metastatic | III RCT | GEM ± Cetuximab | 5.9 vs 6.3 | 3 vs 3.5 | Negative |
| Munter et al[22], 2008 | 66/locally advanced | II RCT | RT + GEM ± Cetuximab | 15 | - | Negative |
| Lim et al[23], 2014 | 127/locally advanced | Retrospective | GEM + Capecitabine vs GEM + Erlotinib vs GEM | 21 vs 12 vs 15 | 8.9 vs 5.2 vs 3.9 | Negative for Erlotinib |
| Philip et al[24], 2014 | 10/metastatic | I RCT | GEM + Erlotinib + Cixutumumab vs GEM + Erlotinib | 7 vs 6.7 | 3.6 vs 3.6 | Negative |
| Watkins et al[25], 2014 | 44/advanced | II | GEM + Capecitabine + Erlotinib +Bevacizumab | 12.6 | 8.4 | |
| Herman et al[26], 2013 | 48/metastatic | II | Capecitabine + Erlotinib + RT followed by GEM + Erlotinib | 24.4 | 15.6 | |
| Feliu et al[27], 2011 | 42/advanced | II RCT | GEM + Erlotinib | 8 | 5 | Negative |
| Moore et al[28], 2007 | 569/advanced | III RCT | Gem + Erlotinib vs GEM | 6.2 vs 5.9 | 3.7 vs 3.5 | Positive |
| Harder et al[29], 2012 | 17/metastatic HER2+ | II | Capecitabine + Trastuzumab | 6.9 | 12.5 | Negative |
| Safran et al[30], 2004 | 34/metastatic | II | Gemcitabine + Trastuzumab | 7 | Negative | |
| Bodoky et al[31], 2012 | 70/advanced | II | Capecitabine vs Selumetinib | 5 vs 5.4 | 88% vs 84% | Negative |
| Infante et al[32], 2014 | 160/metastatic | II RCT | GEM + Trametinib vs GEM | 8.4 vs 6.7 | - | Negative |
| Fuchs et al[33], 2015 | 322/metastatic | III RCT | GEM + Ganitumab vs GEM | 7.2 vs 7 | 3.7 vs 3.6 | Negative |
| McCaffery et al[34], 2013 | 84/metastatic | IIRCT | GEM+Ganitumab vs GEM | 16 vs 5.9 | Positive | |
| Kindler et al[35], 2012 | 125/metastatic | II RCT | GEM + Ganitumab vs GEM + Conatumumab vs GEM | 8.7 vs 7.5 vs 5.9 | 5.1 vs 4 vs 2 | Positive |
| Bramhall et al[36], 2002 | 239/advanced | RCT | GEM + Marimastat vs GEM | 165.5 d | 92.5 d | Negative |
| De Jesus-Acosta et al[37], 2014 | 17/metastatic second line therapy | I | GEM+ inhibitor γ secretase | 4 | 1.5 | Positive |
| Goldstein et al[38], 2015 | 861/metastatic | III RCT | GEM + Nab-paclitaxel vs GEM | 8.7 vs 6.6 | - | Positive |
| Hosein et al[39], 2013 | 19/advanced second line therapy | II | GEM + Nab-paclitaxel | 7.3 | - | Positive |
| Pant et al[40], 2014 | 30/advanced locally | II | GEM + Capecitabine Bevacizumab | 10.4 | Negative | |
| Kindler et al[41], 2010 | 535/advanced | III RCT | GEM + Bevacizumab vs GEM | 5.8 vs 5.9 | 3.8 vs 2.9 | Negative |
| Crane et al[42], 2009 | 82/advanced | II | RT + capecitabine+bevacizumab, followed by GEM + bevacizumab | 11.9 | Negative | |
| Ko et al[43], 2010 | 36/metastatic GEM refractory | II | Bevacizumab + Erlotinib | 102 d | Negative | |
| Van Cutsem et al[44], 2009 | 607/metastatic | III RCT | GEM + erlotinib + bevacizumab vs GEM + erlotinib | 7.1 vs 6 | 4.6 vs 3.6 | Negative |
| IokaT et al[45], 2015 | 632/advanced | III RCT | GEM + axitinib vs GEM | 5.1 vs 5.4 | - | Negative |
| Spano et al[46], 2008 | 103/advanced and metastatic | II RCT | GEM + axitinib vs GEM | 6.9 vs 5.6 | - | Negative |
| Kindler et al[47], 2011 | 632/advanced or metastatic | III RCT | GEM + axitinib vs GEM | 8.5 vs 8.3 | - | Negative |
| Rougier et al[48], 2013 | 427/metastatic | III RCT | GEM + Aflibercept vs GEM | 6.5 vs 7.8 | 3.7 vs 3.7 | Negative |
| Chiorean et al[49], 2014 | 27/advanced | GEM + Sorafenib followed by RT + GEM | 12.6 | 10.6 | Negative | |
| Cascinu et al[50], 2014 | 144/advanced | II RCT | GEM + Cisplatin + Sorafenib vs GEM + Cisplatin | 7.5 vs 8.3 | 4.3 vs 4.5 | Negative |
| Gonçalves et al[51], 2012 | 104/advanced or metastatic | IIIRCT | GEM + Sorafenib vs GEM | 5.7 vs 3.8 | 9.2 vs 8 | Negative |
Erlotinib is a small inhibitor of EGFR that increases survival by two weeks vs GEM monotherapy[28,52]. However, resistance to erlotinib after an initial response can occur due to EGFR mutations, compensation through hepatocyte growth factor receptor (c-Met), human epidermal growth factor receptor (HER2) or K-ras amplification, EGFR-mediated pathway impairment, and histologic transformation with the addition of a mesenchymal component[53]. Combined with GEM or capecitabine, erlotinib can increase survival approximately one month over conventional monotherapy[54,55], proving its positive role in overall survival and progression disease free[28] Long survival was proved in association with radiotherapy and capecitabine, followed by association with GEM[26]. The dose escalated to rash does not improve the survival rate in gemcitabine refractory patients[56]. As second-line therapy, the erlotinib based-therapy failed to show significant improvement in overall survival compared to other regimens[6]. A phase III study found that the wild-type KRAS genotype is associated with an improved overall survival (OS) in erlotinib-treated PC[57], but it is more of a prognostic than a predictive factor[58]. Other drugs in this class, such as gefitinib, have not been shown to be effective in PC[59]. Lapatinib caused reduction of cell growth and proliferation, but it has only been tested in PC cell lines[60]. Vatalanib is an oral poly-tyrosine kinase inhibitor with strong affinity for platelet-derived growth factor and vascular endothelial growth factor (VEGF) receptors (VEGFRs). In metastatic disease it provided limited survival gain compared to historic controls[61].
Anti-HER2: Trastuzumab, a humanized direct antibody against HER2 (human epidermal growth factor 2) kinase, was used in combination with GEM, but there was no survival benefit in phase II studies[29,30]. As the presence of HER2 is relatively low in PC specimens[62,63], anti-HER2 and anti-EGFR therapies can be combined, producing a synergistic effect in animal models that is independent of EGFR density[64]. The mechanism of this combined action is based either on decreased Akt phosphorylation or on disturbance of EGFR/HER2 heterodimerization[65]. The same mechanism of action occurs with vitamin E isoforms, such as tocotrienols, which inhibit cell proliferation and cell survival in studies on PC cell lines[66].
Anti-MAPK: Inhibitors of the Ras/Raf/MAPK cascade, which represents the effect of K-ras activation, are being tested in clinical trials. In GEM failure therapy, selumetinib had the same efficacy as capecitabine[31], though it seems promising in association with erlotinib[67]. Trametinib inhibits the proliferation of PC cell lines with increased efficiency if EGFR/HER2 inhibitors are added, likely because inhibition of the MAPK pathway leads to activation of the tyrosine kinase pathway through feedback mechanisms[68]. Trials with trametinib and other MAPK cascade inhibitors (pimasertib ClinicalTrial.gov NCT01668017, NCT01390818 and refametinib ClinicalTrial.gov NCT01764828, NCT01392521) are still ongoing.
Anti-IGF-1R: IGF-1R is potentially a predictive marker of resectability in PC. A phase II study for treatment of metastatic PC with monoclonal antibodies against IGF-1R showed that ganitumab resulted in a 10-mo survival benefit[34].However, a phase III study showed no survival improvement[33]. Experimental studies that have associated anti-EGFR therapy with anti-IGF-1R monoclonal antibodies have shown promising results[69], but addition of cixutumumab to erlotinib and GEM did not lead to longer survival in metastatic PC[24].
Anti-c-Met: c-Met and its ligand are overexpressed in PC, but are not sufficient for tumorigenesis in the absence of other pro-oncogenes. Crizotinib is an inhibitor of c-Met that has a role in reducing tumor progression and metastasis, showing efficacy in stimulating apoptosis in combination with GEM[70-73]. Cabozantinib is another inhibitor of c-Met and tumor stem cell markers. Treatment in association with IGF-1R inhibitors may represent a future therapy[74].
Anti-PI3K/Akt/mTOR: The PI3K/Akt/mTOR pathway is one of the major signaling pathways mediating the effect of K-ras. Akt stimulates the phosphorylation of mTOR kinase via activation of cyclin D1 and VEGF. mTOR inhibitors, such as everolimus and temsirolimus, have been tested in a phase II trial in patients with GEM-refractory PC, but with negative results[75,76]. Rapamycin, another mTOR inhibitor, has also failed to demonstrate efficacy in the treatment of PC in humans[76]. Everolimus and enzastaurin had no effect on GEM-resistant tumor therapy or on advanced tumors[75,77]. Rigosertib, a small molecular inhibitor of PI3K, added no survival benefit in a phase III trial[78]. Early phase clinical trials of other inhibitors of the P13K/Akt/mTOR pathway or combining these inhibitors with chemotherapy in PC are ongoing parentheses ClinicalTrials.gov; NCT02294006, NCT01087554, NCT01537107 parentheses.
PC may induce expression of DNA repair genes at post-transcriptional level from BRCA category 1 or 2 in 7%-10% of sporadic tumors[79]. We believe that such tumors are more sensitive to the administration of polymerase inhibitors (iniparib), as verified in vitro[80] and in vivo in a patient who achieved pathologic complete response[81]. Treatments with olaparib or veliparib, in combination with GEM or alone, are currently being assessed in ongoing trials (ClinicalTrials.gov; NCT00515866 and NCT01908478)[82].
Chromatin is formed by the wrapping of DNA around histones, a process that is regulated by histone acetylation status. Epigenetic regulation of tumor suppressor genes via deacetylation of histones is involved in the apoptosis, differentiation and growth of cells, which influence tumor cell survival. Suberoylanilide hydroxamic acid (vorinostat) administered in combination with GEM and bortezomib, a 26S proteasome antagonist, confers a strong apoptotic, especially in association with bortezomib and GEM and radiosensitizing effect through the nuclear factor-κB pathway, which is not activated in normal tissue[83-86].Phase I and II trials using such substances in association with radiotherapy are ongoing(e.g., Clinical Trial.gov. NCT00983268, NCT00243100 and NCT00948688 for nonmetastatic disease).
miRNAs are single-stranded chains of non-coding RNA of 18-24 nucleotides that inhibit gene expression at the post-transcriptional level via triggering complete degradation of the proteins or halting translation. miRNAs can influence the proliferation, apoptosis, and susceptibility of tumors to chemotherapeutic agents. miR-21 regulates the expression of the tumor suppressors CDKN1A, PTEN and PDCD4, and can be stimulated by taking medications that interfere with tyrosine kinase pathways[87]. This miRNA is overexpressed in 79% of evaluated PCs, and represents an unfavorable prognostic factor[88]. miR-21 is also frequently found in chemoresistant pancreatic cells[88-90], and a lower level was associated with better response to GEM[91]. In addition, miR-21 upregulates Bcl-2 and reduces chemosensitivity to GEM, thus increasing cell proliferation[92].
Inhibition of miR-221 in PC cells suppresses proliferation and upregulates the tumor suppressors PTEN, and p27, p57 and PUMA[93]. Introducing anti-sense oligonucleotides targeting miR-221 or miR-21 induces apoptosis and increases cell sensitivity to GEM[94]. Furthermore, miR-181b increases the response of animals and chemoresistant cell lines to chemotherapy[95]. miR-20a targets tumor suppressor gene CDH1 and reduces proliferation and metastasis[96]. miR-96 regulates the expression of KRAS, and shows low expression in PC compared to normal tissues[97]. Administration of a synthetic precursor of this miRNA also decreases cell proliferation and invasion[97]. Therapeutic overexpression of miR-34, which targets the tumor suppressor p53, decreases cell growth, arrests the cell cycle in G1 and G2/M phases, and sensitizes cells to chemotherapy[98]. A summary of cancer-related target genes is presented in Table 2[94-105].
| Ref. | miRNA | Oncogene/tumor suppressor | Target genes | Cellular process affected |
| Moriyama et al[99], 2009 | miR-21 | Oncogene | CDK6, PDCD4, CDKN1A, FAS, IL6R, SOCS5, APAF1, NFlB, TPM1 | Apoptosis, cell proliferation, cell invasion |
| Park et al[94], 2009 | miR-221 | Oncogene | CDKN1B, CDKN1C, KIT | Cell migration, proliferation |
| Habbe et al[100], 2009 | miR-155 | Oncogene | AGTR1, APC, ARID2, BACH1, CEBPB, CYR61, DET1, EDN1, ETS1, FADD, FGF7, FOXO3 | Cell migration |
| Chen et al[101], 2011 | miR-196a | Oncogene | NRAS, HOXB8, HMGA2, ANXA1 | Cell growth and differentiation |
| Cai et al[95], 2013 | miR-181b | Oncogene | BCL2 | Sensitization to gemcitabine |
| Yan et al[96], 2010 | miR-20a | Oncogene | STAT3, CDH1 | Proliferation and invasion |
| Torrisani et al[102], 2009 | Let-7 | Tumor suppressor | KRAS, HMGA2, TRIM71, NF2 | Cell proliferation |
| Ji et al[98], 2009 | miR-34a | Tumor suppressor | NOTCH1, BCL2, E2F3, VEGFA, SIRT1, CCND1, CDK6 | Apoptosis, cell proliferation |
| Zhao et al[103], 2010 | miR-217 | Tumor suppressor | KRAS, SIRT1, PTEN | Cell proliferation, invasion |
| Yu et al[97], 2010 | miR-96 | Tumor suppressor | KRAS | Invasion, cell migration, apoptosis |
| Li et al[104], 2010 | miR-146a | Tumor suppressor | EGFR | Invasion |
| Hou et al[105], 2012 | miR-216a | Tumor suppressor | PTEN, CDC42, CD44, SIRT1 | Tumorigenicity |
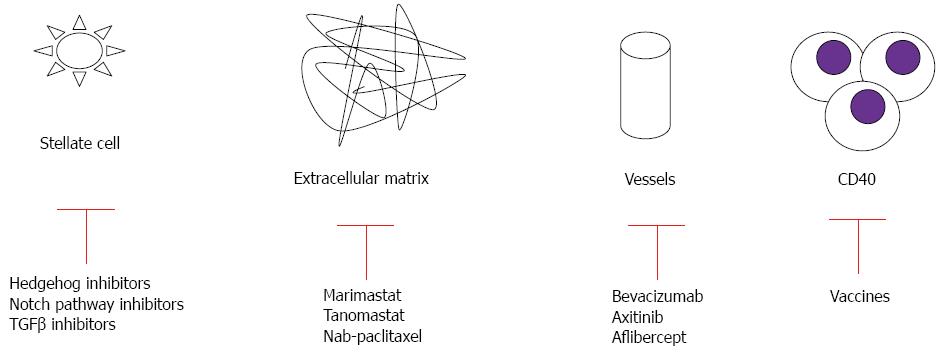
A number of studies have targeted stromal elements of PC, including the extracellular matrix, various intracellular signaling pathways, and immune cells (Figure 2, Table 3)[36,106-114].
| Ref. | Stromal component | Therapeutic target | Treatment |
| Strimpakos et al[106], 2013 | Extracellular matrix | Hyaluronan | PEGPH20 |
| Bramhall et al[36], 2002 | Extracellular matrix | Metalloproteinase | Marimastat |
| Stephenson et al[107], 2011 | Signaling pathways | Hedgehog | Vismodegib (GDC-0449) |
| Oettle et al[108], 2009 | Signaling pathways | Transforming growth factor β receptor | Trabedersen |
| Yabuuchi et al[109], 2013 | Signaling pathways | Notch | PF-03084014 |
| Brahmer et al[106], 2012 | Immune cells | Receiver for programmed cell death | BMS-936559 |
| Le et al[111], 2013 | Immune cells | Cytotoxic T-lymphocyte antigen 4 | Ipilimumab |
| Beatty et al[112], 2013 | Immune cells | CD40 | CP-870893 |
| Lutz et al[113], 2011 | Immune cells | CB8 | GVAX |
| Laheru et al[114], 2008 | Immune cells | CB8 | GVAX |
Therapies against stromal extracellular matrix: In the past few years, scientists have begun to appreciate the importance of the microenvironment in sustaining pancreatic tumor growth. The microenvironment of PC is characterized by an extensive deposition of extracellular matrix components and hypovascularity. These desmoplastic features are believed to prevent drug delivery and contribute to primary resistance of drug therapy. When targeting the stromal tissue, the difference between local tumor and metastasis microenvironments should be considered. Metastasis is characterized by the ability of tumor cells to escape from the primary tumor, survive in circulation, and invade and establish colonies in distant sites, thus warranting special consideration in the design of clinical studies[115].
Matrix metalloproteinases are a family of proteolytic enzymes responsible for the breakdown of connective tissue proteins. These enzymes are crucial in maintaining the growth, differentiation and repair of normal healthy tissue, but aberrant expression is associated with invasive activities of solid tumors[116]. However, inhibition of matrix metalloproteinases by marimastat and tanomastat showed no clinical activity in combination with GEM[36,117]. The extracellular matrix also contains hyaluronan (a nonsulfated glycosaminoglycan), is highly abundant in pancreatic tumors, and has been implicated in angiogenesis, epithelial mesenchymal transition, and chemoresistance[118]. A phase Ib study combining GEM with hyaluronidase demonstrated partial response in 64% of PC patients with high levels of hyaluronan[106]. A phase II study of this combination is currently underway ClinicalTrials.gov; NCT01453153.
Therapies against intracellular signaling pathways: Transforming growth factor (TGF)-β signaling has been implicated in cancer cell proliferation, tumor angiogenesis, metastasis, and suppression of antitumor immunity[119,120]. Its overexpression is associated with disease stage, clinical prognosis, and the immunodeficient state of the patients. TGF-β signaling is mediated by SMAD4, for which 50% of human PCs show allelic deletion[121]. The complex TGFβ-SMAD4 translocate to the nucleus, where they interact at the promoter with other transcription factors at DNA sequence-specific binding sites or with transcriptional coactivators. Thus, aberration of TGFβ-SMAD4 signaling is believed to be an important step in pathogenesis of this cancer[122]. SMAD4 mutation leads to feedback overexpression of TGF-β1. Development of anti-TGF treatment in advanced PC is still in the early clinical stage (ClinicalTrials.gov NCT00844064).
The hedgehog pathway has been shown to be an important signaling system in the microenvironment of PC. The sonic hedgehog ligands are present in the fibroblasts of the PC, but not in the normal pancreatic fibroblasts[123]. Binding of the sonic hedgehog ligand to its patched receptor activates the smoothened and zinc finger proteins, driving the expression of several target genes responsible for desmoplastic reactions and inhibition of pancreatic cell autophagy[124]. Sonic hedgehog is expressed in cancer stem cells (CSCs), rare tumor cells with abilities of self-renewal which are responsible for tumor recurrence and metastasis, as well as resistance to current therapies[125]; thus, this factor represents an attractive target for therapeutic intervention. Saridegib IPI-926 is an inhibitor of this pathway that elevates intratumoral concentrations of GEM, reduces the dense fibrotic reaction, and increases tumor neo-vascularization in an animal model[126]. However, in a double-blind randomized placebo-controlled phase II study, the combination of GEM with Saridegib was associated with shorter survival in PC patients, and the trial was terminated prematurely[127].
The dense fibro-inflammatory microenvironment of PC results in hypoxia, which activates hypoxia-inducible factor-1α and promotes tumor cell secretion of sonic hedgehog. As a result, the epithelial to mesenchymal transition is activated, CSCs are maintained, and resistance to therapy occurs. Moreover, hypoxia-inducible factor-1α activates leptin receptors and influences metastasis and survival[128], and activates actin-related mechanisms as well[129]. Myo-inositol trispyrophosphate can reverse hypoxia and decrease desmoplasia in an animal model, with improved susceptibility to GEM treatment[130,131]. Gene expression of hypoxia-inducible factor 1α was reduced in an animal model by administration of a novel synthetic compound[132], which is currently being tested in an ongoing trial (ClinicalTrials.gov; NCT01248637).
Hypoxic conditions can also trigger Notch signaling, which plays a critical role in organ development and cell differentiation. Notch signaling mediates PC stem cell function, which contributes to chemotherapy resistance, tumor recurrence, and metastasis. Upon receptor activation, Notch is cleaved by a cascade of proteolytic enzymes, including metalloproteinases, tumor necrosis factor-α-converting enzyme, and γ-secretase[133]. The oral γ-secretase inhibitor RO4929097 has completed a phase I trial for treatment of metastatic cancer, and results are promising Recently, preliminary results from two phase I clinical trials testing anti-Notch antibodies (OMP-59R5 and demcizumab) have been presented[134,135]. A phase I study of an oral Notch inhibitor (MK-0752) in combination with GEM is now ongoing (ClinicalTrials.gov; NCT010983440).
Enhanced drug delivery to microenvironment: Inefficient drug delivery might explain the lack of efficacy of systemic treatments. Novel drug delivery vehicles have reformed the clinical use of traditional cytotoxic agents. Nab-paclitaxel is an albumin-bound formulation that increases tumor accumulation of paclitaxel via binding of albumin to the surrounding stroma that is enriched in secreted protein acidic and rich in cysteine (SPARC). Nab-paclitaxel was developed to exploit the ability of SPARC to bind to albumin as a means of increasing drug delivery to the tumor[136]. In an animal model, intratumoral concentration of GEM was increased 2.8-fold in mice receiving nab-paclitaxel in combination with GEM, and treatment of patients with nab-paclitaxel alone was also more effective than GEM alone (aggregate tumor regression rates of 55%, 36% and 24% for nab-paclitaxel plus GEM, nab-paclitaxel alone and GEM alone, respectively)[137]. These findings suggest that nab-paclitaxel is able to destroy or alter the characteristics of the tumor stroma and increase vascularization in order to achieve enhanced delivery of cytotoxic chemotherapy to the tumor. Indeed, in two GEM-resistant xenografts, a profuse desmoplastic stroma remained after treatment with vehicle or GEM alone, whereas the administration of nab-paclitaxel resulted in a significant reduction in stromal content[108]. In another study using the GEM-resistant mouse model, treatment with nab-paclitaxel and GEM also resulted in an increase in intratumoral GEM concentration and a reduction in tumor size compared with treatment with either agent alone[138].
In a recently published phase III study comparing nab-paclitaxel plus GEM vs GEM alone, the addition of nab-paclitaxel significantly prolonged median OS from 6.7 to 8.5 mo, with a corresponding increase in response rate from 7% to 23%[139], and after one year from 22% to 35%[137,140]. The nab-paclitaxel/GEM combination has become the second regimen shown to be superior to GEM alone and has been approved by the FDA for treatment of advanced PC. In a second-line setting, nab-paclitaxel monotherapy demonstrated clinical activity in GEM-refractory advanced PC patients in a phase II trial[39]. In this trial, high expression of stromal protein was used to specifically enrich the concentration of a cytotoxic agent in the tumor. Additionally, Alvarez et al[141] demonstrated that nab-paclitaxel reduces the stiffness and the number of cancer-associated fibroblasts in human tumors treated with nab-paclitaxel. Its combination with different agents is now one of the most popular areas of clinical research in advanced PC. Another innovative approach to improve drug delivery that is under development is the use of nanotechnology and cancer-specific liposomes[142].
Antiangiogenesis is clinically ineffective in treating PC patients. Although most preclinical models of PC have suggested potential activity of many antiangiogenic agents, they failed to simulate human tumor microenvironments where dense stromal tissue with decreased vascular density is now known to be the main obstacle for effective drug delivery. Moreover, the withdrawal of antiangiogenic agents after therapy may be associated with increased tumor aggressiveness and invasion, offsetting the potential therapeutic benefits offered by antiangiogenic agents. It has also been said that angiogenesis inhibition might alter the natural history of tumors by increasing tumor invasion and metastasis[143].
Overexpression of VEGF in PC has been associated with tumor progression and a worse prognosis. Therefore, similar to other cancer therapies, angiogenesis is considered to be a therapeutic target[144,145]. Humanized monoclonal antibodies such as bevacizumab have affinity for circulating VEGF-A, but phase II and III studies showed no survival advantage when bevacizumab was combined with GEM and erlotinib[41-44]. A meta-analysis found that therapy with bevacizumab-GEM was associated with a modest response rate, without survival modifications[52]. Associations of two chemotherapeutic agents (GEM, capecitabine) with two biologic therapies (erlotinib, bevacizumab) provided an additional ten months of survival in metastatic disease[25]. The proposed mechanism for this effect involves the overexpression of platelet-derived growth factor and fibroblast growth factor[146]. The development of bevacizumab-related hypertension is also associated with better survival[147]. Other VEGF inhibitors, such as axitinib and aflibercept, provide no survival advantage[46-48,148]. In addition, sorafenib (an inhibitor of VEGFR and Ras/Raf/MAPK signaling) had no supplementary value for patient survival over GEM[51].
In pancreatic adenocarcinoma, interactions between tumor and host cells are mediated by inflammatory cells, fibroblasts and vascular endothelial cells. Intratumoral desmoplastic tissue is less vascularized, and cytotoxic substances cannot easily penetrate the connective matrix. Therefore, inflammatory cells and macrophages represent potential therapeutic targets. These cells can acquire antitumor properties, which is the main purpose for specific immunotherapies, including vaccines and adoptive cell therapy.
Antitumor vaccines are biologic preparations that involve administering an antigen that is specific for a particular tumor type and stimulating the body’s natural ability to protect itself. There are a number of ways to deliver these vaccines: whole-cell recombinant vaccines, dendritic cell (DC) vaccines that combine antigen with DCs to present to white cells, DNA vaccines (by inserting viral or bacterial DNA into human or animal cells), or T-cell receptor peptide vaccines (by inserting peptides to modulate cell-mediated immunity).
The advantage of using whole-cell recombinant vaccines is that tumor cells express a wide range of tumor-associated antigens. This rich source of antigens contains epitopes of the two types of T cells (CD8+ and CD4+), compared with peptide-based vaccines that contain only one epitope. Autologous tumor cells are the best source of protein for immunization, but only 10%-15% of patients diagnosed with pancreatic tumors are candidates for surgical treatment. In addition, it is difficult to prepare a sufficient quantity of tumor cells required to achieve the vaccine due to prolonged culture periods and possible contamination with bacteria and fungus. To avoid these difficulties, allogeneic tumor cells can be used, which can be produced in larger quantities and do not require determination of the patient’s human leukocyte antigen and cell types. Furthermore, multiple allogeneic tumor antigens can be processed using the mechanism of cross-presentation and simultaneous induction of CD4+ and CD8+ cells[149].
Algenpantucel-L: Algenpantucel-L contains cell lines expressing α-galactosyl epitopes on the surface of proteins and glycolipids. In humans, these epitopes are missing, but there are natural anti-α-gal antibodies that stimulate the immune response, including against tumor cells[150,151]. In a phase II study with this type of immunotherapy in combination with GEM and 5-FU/irradiation, algenpantucel-L was injected intradermally (up to 14 vaccinations)[152]. The adverse reactions were local response and peripheral hypereosinophilia. Survival at 1 year was 86%, better than the 81% reported in the RTOG-9704 trial using the same chemoradiotherapy scheme[153]. Interestingly, the patients who received a higher dose of vaccine in the study (300 vs 100 million cells/dose) had an increase in 12-mo disease-free (81% vs 51%) and overall (96% vs 79%) survivals. Additionally, patients in this trial had a higher percentage of lymph node positivity (stage IIb) in comparison with the RTOG-9704 trial (81% vs 68%)[152]. Phase III studies are ongoing and the results are expected (ClinicalTrials.gov; NCT 01836432).
Granulocyte-macrophage colony-stimulating factor vaccine: Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a potent cytokine that is able to mobilize monocytes, eosinophils and lymphocytes to the tumor sites. GM-CSF vaccine (GVAX) showed tumor-free survival and also caused regression of tumors in mice[154]. In a phase I study, 14 patients were vaccinated with a GVAX made from irradiated cancer cell lines (PANC 6.03 and PANC 10.05) that were engineered to express GM-CSF, with an interval of 8 wk after resection of the pancreas and chemoradiotherapy[155]. Patients who developed delayed hypersensitivity reaction were disease-free at 25 mo from diagnosis. Another phase II study vaccinated 60 patients with surgical treatment of adenocarcinoma and with radiochemotherapy (5-FU-based regimen) with an allogeneic GVAX[113,114]. A total of five immunotherapy treatments were delivered intradermally and the first treatment was given 8-10 wk after surgical resection resulting in an 85% 1-year survival; the effect was attributed to the induction of CD8+ mesothelin-specific T cells. GVAX immunotherapy induces expression of anti-thyroglobulin antibodies that recognize a unique antigenic repertoire associated with prolonged survival[156]. All these trials demonstrate post-vaccination induction of CD8+ T cells to multiple mesothelin-specific epitopes, which correlates with improved survival[113,114,155]. Mesothelin is a tumor-associated antigen that is overexpressed in most ductal adenocarcinomas of the pancreas and is thought to be involved in cell adhesion, and, therefore, to play a role in metastasis[157].
Peptide-based anti-tumor vaccines are prepared from fragments of antigenic proteins, which are the minimal immunogenic region of tumor-associated antigens that are simple, safe, stable and economical for this purpose. Multiple peptides related to major histocompatibility complex class I have been identified and considered as candidates, and vaccination with synthetic peptides has been studied in clinical trials in combination with chemotherapy sessions in order to produce cytotoxic T lymphocytes[158]. The use of peptide vaccines has some limitations: the existence of a limited number of known antigenic peptides; the presence of suppressive immune cells in tumoral microenvironments; the fact that DCs may have poor functionality in patients with advanced pancreatic tumors; the observation that CD8+ cytotoxic T cells are sometimes ineffective in the reaction with pancreatic tumor cells, which is mediated by production of immunosuppressive cytokines such as interleukin-10 and tumor growth factor.
K-ras vaccine: K-ras is thought to be recognized by helper and cytotoxic T cells, and almost 90% of pancreatic tumors involve mutations in the KRAS oncogene. Peptide vaccines against mutated K-ras are safe for administration to humans[159-161], but only one of the nine patients had a cytotoxic T lymphocyte immune response[161]. A study of synthetic vaccine for a K-ras mutation and GM-CSF showed an immune response in 25/48 of the enrolled patients[162]. For these patients, survival was 148 d compared to 61 d for the non-responders. Twenty patients in this study, and another group of 23 patients, have been followed-up for a long time and have shown a median 5-year survival rate of 20% (four patients), while a 29% survival rate was observed in another group of patients with immune response; adverse effects to the vaccine were minimal[163]. Using synthetic K-ras vaccines based on long peptides to induce antigen-specific polyclonal CD8+ and CD4+ T, Weden et al[163] reported a 10-year survival rate of 20% in a group of patients after pancreatic tumor resection. Another recent study showed no effect of a 21-mer peptide vaccine based on a KRAS mutation in 24 patients vaccinated monthly for 3 mo[164]. Administration of Reolysin, an oncolytic virus that replicates and kill cells with a KRAS mutation, was well tolerated by patients with breast tumors[165], but further studies are expected.
Immunotherapy in the form of vaccination against mutant K-ras has been developed as an adjunct to surgical resection and appears as a promising principle of adjuvant therapy. Taking into account that K-ras vaccination is virtually free of side effects, the results should encourage much larger controlled studies.
Telomerase peptide vaccine: Telomerase is a ribonucleotide enzyme that maintains cellular stability and is expressed by almost all cancer cells (85%-90%)[166], including PC[167]. Activation of reverse transcriptase from human telomerase increases cell viability, and is thus an attractive target for an immunotherapy antigen. In a phase I-II study, the administration of a telomerase peptide vaccine (GV1001) and immunogenic response was found to be correlated with prolonged survival (25% at 1 year) and good tolerability[168]. However, a phase III study in unresectable and metastatic pancreatic ductal adenocarcinoma that compared PrimoVax (GV1001 and GVAX) administered sequentially with GEM against GEM alone was closed due to lack of survival (median OS: 5.9 mo vs 7.3 mo)[169,170]. A second GV1001 phase III trial (TeloVac) in unresectable and metastatic PC compared the association between the vaccination and subsequent or concurrent chemotherapy (GEM and capecitabine) vs chemotherapy alone; there were no significant survival differences (median OS: 6.94 and 8.36 mo vs 7.89 mo, respectively)[171]. Furthermore, patients in the sequential arm received only 2 mo of chemotherapy before being taken off an active therapy that has a historical median progression-free survival of 4.3 mo[172]. Despite the disappointing phase III results, the findings have identified biomarkers that may predict response to this vaccine and new research may indicate benefit in a subgroup of patients[173]. In addition, there is another ongoing study in patients with advanced disease that includes radiochemotherapy (ClinicalTrials.gov; NCT01342224).
Survivin-based vaccine: Survivin is an inhibitor of apoptosis and is found in PC. There have been isolated cases of complete remission with a survivin-based vaccine in patients with metastatic disease[158]. This effect was confirmed only in combination with GEM in an experimental study using a modified vaccinia Ankara in a murine pancreatic model, which showed enhanced survivin-specific CD8 interferon-γ immune responses in the vaccinated mice[174].
Mucin 1 vaccine: Mucin (MUC)1 is highly expressed in PC[175], and phase I and II studies of MUC1 antigen-pulsed DC vaccines showed hopeful results in advanced PC[176,177]. A phase I study in advanced PC showed that the vaccinia virus expressing carcinoembryonic antigen (CEA) and MUC1 and co-stimulatory molecules was well tolerated and provided an OS advantage in immune-responsive patients[178]. However, a phase III trial using fowlpox viruses expressing these same molecules failed to show improvement in OS in PC patients when compared to chemotherapy or best supportive care in a palliative setting[179]. Administration of a pox virus-based vaccine targeting MUC-1 and CEA induced a favorable immune response on T cells, but has not been confirmed as beneficial in a phase III study[176,178]. Intratumoral administration of the recombinant fowlpox PANVAC plus subcutaneous recombinant vaccinia and recombinant GM-CSF is currently underway in a phase I study. In another study, 16 patients with advanced PC who were vaccinated with DCs pulsed with MUC1 showed an increase in CD8+ cells in peripheral blood; 2/15 patients with resected PC were alive and disease free at 32 and 61 mo[176].
Anti-VEGFR vaccine: An anti-VEGFR vaccine was given in association with GEM to patients with unresectable or metastatic disease, and produced an OS rate of 8.7 mo; phase II study results are expected[180].
Personalized peptide vaccination: Personalized peptide vaccination was attempted after preparation of pre-vaccination peripheral blood mononuclear cells and plasma as a first-line therapy in association with GEM in unresectable patients. This attempt showed a 1-year survival rate of 38%[181]; however, further evaluations are needed.
Nanoparticles: Nanoparticles are non-specific and are taken-up in the spleen. They can be safely used as a vaccine platform without the risk of prolonged side effects. In animal models, nanoparticulate delivery of diphtheria toxin DNA effectively kills mesothelin-expressing PC cells[182].
Heat shock proteins: Heat shock proteins also play a role in the stabilization and delivery of peptides, and in inducing immunity against autologous tumors[183]. In one study, 3/10 patients treated with an autologous vaccine prepared from resected tumors showed no tumor recurrence at 2.6, 2.7 and 5.0 years of follow-up, though there was no correlation between stimulating immunity and survival[184].
DCs are the most potent antigen-presenting cells, and they can cause a high antigenic response via stimulation of T and B cells. DC vaccines combine tumor antigens with DCs for presentation to effector T cells. Viral or bacterial DNA is inserted into human cells to modulate cell-mediated immunity by the DNA vaccines. It has been shown that DC vaccine plus lymphokine-activated killer cell treatment and chemotherapy prolonged OS compared to effects observed in patients who received only DC vaccine or chemotherapy[158,185]. In a multi-center study of 255 patients who received chemotherapy plus vaccine, the median survival was 16.5 mo, with erythema reaction after vaccination identified as a factor related to better survival[186]. The effects were considered likely due to the enhancement of tumor cell immunogenicity by treatment with GEM, which increases the efficacy of the vaccine[187]. However, tumor-reactive T cells in peripheral blood were decreased and the cytotoxic T cell-mediated killing was normal[188]. The combination of these vaccines with mRNA encoding CEA produced an effective immunization and survival benefit for three patients with resected pancreatic tumors receiving neoadjuvant therapy, each of who survived 30 mo after diagnosis[189]. The combination of DC vaccines with DNA for MUC1 has been found to be beneficial in a small portion of resected patients[176], and as ineffective in metastatic disease[177]. The combination with telomerase reverse transcriptase mRNA demonstrated encouraging results when administrated after radical surgical treatment[190]. Targeting more than one checkpoint pathway at the same time might be another option for obtaining increased efficacy.
Administration of the anti-cytotoxic lymphocyte antibody (ipilimumab) and GVAX increased the survival of 15/30 previously treated patients with metastatic disease compared to GEM alone (5.5 mo vs 3.3 mo), supporting the approach of blocking cytotoxic lymphocytes by promoting the GM-CSF antitumor response[111]. Survival was correlated with CD8+, mesothelin-specific T cell quantity. A phase II study of this protocol is under development due to this promising result. Despite the encouraging findings, however, clinical responses have been seen in only a minority of patients, presumably due to insufficient expansion of antigen-specific cytotoxic T lymphocytes capable of eradicating tumor cells. Interestingly, endoscopic ultrasound-guided fine-needle injection of OK432-pulsed DCs into a tumor followed by intravenous infusion of lymphokine-activated killer cells stimulated with anti-CD3 monoclonal antibody was synergistically effective in a phase I study[191].
CD40 is a potential immunomodulatory target, because it is a co-stimulatory molecule for antigen-presenting cells. GEM with CD40 agonist-activated T cells reduces tumor burden in advanced PC patients in a phase I study[192], by decreasing tumor stroma and increasing infiltration of activated macrophages[112].
Adoptive cell therapy consists of re-transferring autologous cytotoxic T lymphocytes harvested from the patient after in vitro activation of K-ras, telomerase, or mesothelin. This method helps the immune system to recover more quickly after chemotherapy and improves responses to other immunotherapies. However, extensive studies have not been performed[193-195].
Most patients with PC who initially respond to standard chemotherapy relapse because of small populations of tumor cells/tumor stem cells (i.e., CSCs). CSCs are better able than other tumor cells to multiply and to initiate new tumors and sustain tumor growth. It has been shown that pancreatic tumors that are resistant to chemoradiotherapy are rich in CSCs. These tumors are candidates for immunotherapy, and CSC-targeted therapy can be applied to prevent resistance to chemotherapy.
Targeted immunotherapy on tumor stem cells using γδ T cells, natural killer cells, and anti-tumor vaccines based on DCs has been successfully used to activate responses of CSC-specific cytotoxic T lymphocytes, leading to the expression of high levels of interferon-γ and enhanced destruction of CSCs in vitro. Transfer of stem cells may have antitumor effects due to decreased activity of Wnt or Akt pathways[196,197]. Antitumor action will be possible only if three conditions are met: direct tumor migration and intratumoral incorporation, release of the antitumor agent, and generation of a specific organ-vector[197].
The use of immunotherapy for treatment of pancreatic ductal adenocarcinoma is promising, though its immunotolerant environment continues to be a major hurdle. Therapeutic vaccines have the ability to activate antitumor immune responses; however, these strategies need to be combined with immune-modulating agents, chemotherapies or radiation, depending on the patient disease status. There is also a great need to optimize vectors, antigens, and patient selection. Additionally, more preclinical and early-phase clinical trials need to be conducted to determine if and which chemotherapies would complement immunotherapies, and determine how to optimally sequence the administration of immunotherapy with chemotherapy and radiation. Combinations of active and passive immunologic treatments, targeted agents and conventional chemotherapies might be important strategies for increasing efficacy.
The goal of these new treatments is to obtain faster and more stable tumor response. Passive immunotherapy may have a role in combination with radiochemotherapy. Furthermore, vaccines would allow restoration of specific immune responses after adjuvant or palliative treatment, and would continue the fight against residual tumor cells. Knowing the genetic implications in PC, the combination of two or more vaccines would be beneficial.
In the future, treatment will likely include personalized medicine to each patient, tailored for numerous molecular therapeutic targets of multiple pathogenetic pathways in PC, and is expected to occupy a central role in stem cell therapy.
P- Reviewer: Christodoulidis G, Dai ZJ, Du YQ, Izbicki JR, Mizuno N S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10002] [Cited by in F6Publishing: 10353] [Article Influence: 739.5] [Reference Citation Analysis (0)] |
| 2. | Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 609] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1828] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 4. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [Cited in This Article: ] |
| 5. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. [PubMed] [Cited in This Article: ] |
| 6. | Rahma OE, Duffy A, Liewehr DJ, Steinberg SM, Greten TF. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol. 2013;24:1972-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M. Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002;62:5611-5617. [PubMed] [Cited in This Article: ] |
| 8. | Perugini RA, McDade TP, Vittimberga FJ, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39-44. [PubMed] [Cited in This Article: ] |
| 9. | Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636-3641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 550] [Cited by in F6Publishing: 566] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589-593. [PubMed] [Cited in This Article: ] |
| 11. | Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 12. | Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318-330. [PubMed] [Cited in This Article: ] |
| 13. | Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, Berzein K, Moore T, Iannitti D, Reiss P. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Lemoine NR, Hughes CM, Barton CM, Poulsom R, Jeffery RE, Klöppel G, Hall PA, Gullick WJ. The epidermal growth factor receptor in human pancreatic cancer. J Pathol. 1992;166:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565-569. [PubMed] [Cited in This Article: ] |
| 16. | Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res. 1998;18:4613-4619. [PubMed] [Cited in This Article: ] |
| 17. | Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305-309. [PubMed] [Cited in This Article: ] |
| 18. | Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res. 2010;16:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Burtness B, Powell M, Catalano P, Berlin J, Liles DK, Chapman AE, Mitchell E, Benson AB. Randomized Phase II Trial of Irinotecan/Docetaxel or Irinotecan/Docetaxel Plus Cetuximab for Metastatic Pancreatic Cancer: An Eastern Cooperative Oncology Group Study. Am J Clin Oncol. 2014;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 20. | Fensterer H, Schade-Brittinger C, Müller HH, Tebbe S, Fass J, Lindig U, Settmacher U, Schmidt WE, Märten A, Ebert MP. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann Oncol. 2013;24:2576-2581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 478] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 22. | Munter M, Timke C, Abdollahi A, Friess H, Jaeger D, Heeger S, Buchler M, Debus J, Huber P, Krempien R. Final results of a phase II trial [PARC-Study ISRCTN56652283] for patients with primary inoperable locally advanced pancreatic cancer combining intensity modulated radiotherapy (IMRT) with cetuximab and gemcitabine. J Clin Oncol. 2008;26:4613. [Cited in This Article: ] |
| 23. | Lim JY, Cho JH, Lee SJ, Lee DK, Yoon DS, Cho JY. Gemcitabine Combined with Capecitabine Compared to Gemcitabine with or without Erlotinib as First-Line Chemotherapy in Patients with Advanced Pancreatic Cancer. Cancer Res Treat. 2015;47:266-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120:2980-2985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Watkins DJ, Starling N, Cunningham D, Thomas J, Webb J, Brown G, Barbachano Y, Oates J, Chau I. The combination of a chemotherapy doublet (gemcitabine and capecitabine) with a biological doublet (bevacizumab and erlotinib) in patients with advanced pancreatic adenocarcinoma. The results of a phase I/II study. Eur J Cancer. 2014;50:1422-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Herman JM, Fan KY, Wild AT, Hacker-Prietz A, Wood LD, Blackford AL, Ellsworth S, Zheng L, Le DT, De Jesus-Acosta A. Phase 2 study of erlotinib combined with adjuvant chemoradiation and chemotherapy in patients with resectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;86:678-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Feliu J, Borrega P, León A, López-Gómez L, López M, Castro J, Belda-Iniesta C, Barriuso J, Martínez V, González-Barón M. Phase II study of a fixed dose-rate infusion of gemcitabine associated with erlotinib in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2011;67:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [PubMed] [Cited in This Article: ] |
| 29. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 32. | Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072-2081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 33. | Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26:921-927. [PubMed] [Cited in This Article: ] |
| 34. | McCaffery I, Tudor Y, Deng H, Tang R, Suzuki S, Badola S, Kindler HL, Fuchs CS, Loh E, Patterson SD. Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clin Cancer Res. 2013;19:4282-4289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834-2842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A, Blatchford PJ, Quackenbush K, Messersmith W. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs. 2014;32:739-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 418] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 39. | Hosein PJ, de Lima Lopes G, Pastorini VH, Gomez C, Macintyre J, Zayas G, Reis I, Montero AJ, Merchan JR, Rocha Lima CM. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol. 2013;36:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Sahora K, Schindl M, Kuehrer I, Eisenhut A, Werba G, Brostjan C, Telek B, Ba’ssalamah A, Stift J, Schoppmann SF. A phase II trial of two durations of Bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer Res. 2014;34:2377-2384. [PubMed] [Cited in This Article: ] |
| 41. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 42. | Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, Wolff RA, Willett CG. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096-4102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, Schillinger B, Scott J, Hwang J, Tempero MA. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 478] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 45. | Ioka T, Okusaka T, Ohkawa S, Boku N, Sawaki A, Fujii Y, Kamei Y, Takahashi S, Namazu K, Umeyama Y. Efficacy and safety of axitinib in combination with gemcitabine in advanced pancreatic cancer: subgroup analyses by region, including Japan, from the global randomized Phase III trial. Jpn J Clin Oncol. 2015;45:439-448. [PubMed] [Cited in This Article: ] |
| 46. | Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101-2108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 47. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 48. | Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633-2642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 49. | Chiorean EG, Schneider BP, Akisik FM, Perkins SM, Anderson S, Johnson CS, DeWitt J, Helft P, Clark R, Johnston EL. Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2014;89:284-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, Ferrari D, Barni S, Aitini E, Zagonel V. Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: A GISCAD randomized phase II study. Dig Liver Dis. 2014;46:182-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799-2805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 52. | Tian W, Ding W, Kim S, Xu X, Pan M, Chen S. Efficacy and safety profile of combining agents against epidermal growth factor receptor or vascular endothelium growth factor receptor with gemcitabine-based chemotherapy in patients with advanced pancreatic cancer: a meta-analysis. Pancreatology. 2013;13:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 753] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 54. | da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, Squire J, Parulekar W, Moore MJ, Tsao MS. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599-5607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787-4792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Renouf DJ, Tang PA, Hedley D, Chen E, Kamel-Reid S, Tsao MS, Tran-Thanh D, Gill S, Dhani N, Au HJ. A phase II study of erlotinib in gemcitabine refractory advanced pancreatic cancer. Eur J Cancer. 2014;50:1909-1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Boeck S, Jung A, Laubender RP, Neumann J, Egg R, Goritschan C, Vehling-Kaiser U, Winkelmann C, Fischer von Weikersthal L, Clemens MR. EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: translational results from the randomised, crossover phase 3 trial AIO-PK0104. Br J Cancer. 2013;108:469-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Boeck S, Jung A, Laubender RP, Neumann J, Egg R, Goritschan C, Ormanns S, Haas M, Modest DP, Kirchner T. KRAS mutation status is not predictive for objective response to anti-EGFR treatment with erlotinib in patients with advanced pancreatic cancer. J Gastroenterol. 2013;48:544-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Brell JM, Matin K, Evans T, Volkin RL, Kiefer GJ, Schlesselman JJ, Dranko S, Rath L, Schmotzer A, Lenzner D. Phase II study of docetaxel and gefitinib as second-line therapy in gemcitabine pretreated patients with advanced pancreatic cancer. Oncology. 2009;76:270-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Singla S, Pippin JA, Drebin JA. Dual ErbB1 and ErbB2 receptor tyrosine kinase inhibition exerts synergistic effect with conventional chemotherapy in pancreatic cancer. Oncol Rep. 2012;28:2211-2216. [PubMed] [Cited in This Article: ] |
| 61. | Dragovich T, Laheru D, Dayyani F, Bolejack V, Smith L, Seng J, Burris H, Rosen P, Hidalgo M, Ritch P. Phase II trial of vatalanib in patients with advanced or metastatic pancreatic adenocarcinoma after first-line gemcitabine therapy (PCRT O4-001). Cancer Chemother Pharmacol. 2014;74:379-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Saxby AJ, Nielsen A, Scarlett CJ, Clarkson A, Morey A, Gill A, Smith RC. Assessment of HER-2 status in pancreatic adenocarcinoma: correlation of immunohistochemistry, quantitative real-time RT-PCR, and FISH with aneuploidy and survival. Am J Surg Pathol. 2005;29:1125-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Walsh N, Kennedy S, Larkin A, Corkery B, O’Driscoll L, Clynes M, Crown J, O’Donovan N. EGFR and HER2 inhibition in pancreatic cancer. Invest New Drugs. 2013;31:558-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Maron R, Schechter B, Mancini M, Mahlknecht G, Yarden Y, Sela M. Inhibition of pancreatic carcinoma by homo- and heterocombinations of antibodies against EGF-receptor and its kin HER2/ErbB-2. Proc Natl Acad Sci USA. 2013;110:15389-15394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Larbouret C, Gaborit N, Chardès T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pèlegrin A. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14:121-130. [PubMed] [Cited in This Article: ] |
| 66. | Shin-Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, Reddy SA, Krishnan K. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51:1164-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Ko AH, Tempero MA, Bekaii-Saab TB, Kuhn P, Courtin R, Ziyeh S, Tahiri S, Kelley RK, Dito E, Ong A. Dual mek/egfr inhibition for advanced, chemotherapy-refractory pancreatic cancer: A multicenter phase II trial of selumetinib (azd6244; arry-142886) plus erlotinib. J Clin Oncol. 2013;31:4014a. [Cited in This Article: ] |
| 68. | Hirakawa T, Yashiro M, Murata A, Hirata K, Kimura K, Amano R, Yamada N, Nakata B, Hirakawa K. IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC Cancer. 2013;13:392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer. 2013;13:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Avan A, Caretti V, Funel N, Galvani E, Maftouh M, Honeywell RJ, Lagerweij T, Van Tellingen O, Campani D, Fuchs D. Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 2013;73:6745-6756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Avan A, Quint K, Nicolini F, Funel N, Frampton AE, Maftouh M, Pelliccioni S, Schuurhuis GJ, Peters GJ, Giovannetti E. Enhancement of the antiproliferative activity of gemcitabine by modulation of c-Met pathway in pancreatic cancer. Curr Pharm Des. 2013;19:940-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 72. | Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218-2227.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 73. | Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Ucar DA, Magis AT, He DH, Lawrence NJ, Sebti SM, Kurenova E, Zajac-Kaye M, Zhang J, Hochwald SN. Inhibiting the interaction of cMET and IGF-1R with FAK effectively reduces growth of pancreatic cancer cells in vitro and in vivo. Anticancer Agents Med Chem. 2013;13:595-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 76. | Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, Davis D, Zhang Y, Wolff RA, Abbruzzese JL. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Richards DA, Kuefler PR, Becerra C, Wilfong LS, Gersh RH, Boehm KA, Zhan F, Asmar L, Myrand SP, Hozak RR. Gemcitabine plus enzastaurin or single-agent gemcitabine in locally advanced or metastatic pancreatic cancer: results of a phase II, randomized, noncomparative study. Invest New Drugs. 2011;29:144-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Onconova Therapeutics. Onconova Announces Results of Interim Analysis of Metastatic Pancreatic Cancer Study, 2013. Available from: http://investor.onconova.com/releasedetail.cfm? ReleaseID5814465. Accessed July 14, 2014. [Cited in This Article: ] |
| 79. | Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365-374. [PubMed] [Cited in This Article: ] |
| 80. | Jacob DA, Bahra M, Langrehr JM, Boas-Knoop S, Stefaniak R, Davis J, Schumacher G, Lippert S, Neumann UP. Combination therapy of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide and gemcitabine shows strong antitumor activity in pancreatic cancer cells. J Gastroenterol Hepatol. 2007;22:738-748. [PubMed] [Cited in This Article: ] |
| 81. | Fogelman DR, Wolff RA, Kopetz S, Javle M, Bradley C, Mok I, Cabanillas F, Abbruzzese JL. Evidence for the efficacy of Iniparib, a PARP-1 inhibitor, in BRCA2-associated pancreatic cancer. Anticancer Res. 2011;31:1417-1420. [PubMed] [Cited in This Article: ] |
| 82. | Pishvaian MJ, Slack R, Witkiewicz A, He AR He, Hwang JJ, Hankin A, Ley L, Apte SK, Littman SJ, Weiner LM. A phase I/II study of the PARP inhibitor, ABT-888 plus 5-fluorouracil and oxaliplatin (modified FOLFOX-6) in patients with metastatic pancreatic cancer. Proceedings of the ASCO Annual Meeting. 2011;. [Cited in This Article: ] |
| 83. | Lee JK, Ryu JK, Yang KY, Woo SM, Park JK, Yoon WJ, Lee SH, Jeong KS, Kim YT, Yoon YB. Effects and mechanisms of the combination of suberoylanilide hydroxamic acid and bortezomib on the anticancer property of gemcitabine in pancreatic cancer. Pancreas. 2011;40:966-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Deorukhkar A, Shentu S, Park HC, Diagaradjane P, Puduvalli V, Aggarwal B, Guha S, Krishnan S. Inhibition of radiation-induced DNA repair and prosurvival pathways contributes to vorinostat-mediated radiosensitization of pancreatic cancer cells. Pancreas. 2010;39:1277-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Arnold NB, Arkus N, Gunn J, Korc M. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer. Clin Cancer Res. 2007;13:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Sung V, Richard N, Brady H, Maier A, Kelter G, Heise C. Histone deacetylase inhibitor MGCD0103 synergizes with gemcitabine in human pancreatic cells. Cancer Sci. 2011;102:1201-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA. 2009;106:12085-12090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 88. | Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171-2176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 89. | Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28-47. [PubMed] [Cited in This Article: ] |
| 90. | Schultz NA, Andersen KK, Roslind A, Willenbrock H, Wøjdemann M, Johansen JS. Prognostic microRNAs in cancer tissue from patients operated for pancreatic cancer--five microRNAs in a prognostic index. World J Surg. 2012;36:2699-2707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5:e10630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 92. | Dong J, Zhao YP, Zhou L, Zhang TP, Chen G. Bcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cells. Arch Med Res. 2011;42:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 93. | Sarkar S, Dubaybo H, Ali S, Goncalves P, Kollepara SL, Sethi S, Philip PA, Li Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am J Cancer Res. 2013;3:465-477. [PubMed] [Cited in This Article: ] |
| 94. | Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190-e199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 95. | Cai B, An Y, Lv N, Chen J, Tu M, Sun J, Wu P, Wei J, Jiang K, Miao Y. miRNA-181b increases the sensitivity of pancreatic ductal adenocarcinoma cells to gemcitabine in vitro and in nude mice by targeting BCL-2. Oncol Rep. 2013;29:1769-1776. [PubMed] [Cited in This Article: ] |
| 96. | Yan H, Wu J, Liu W, Zuo Y, Chen S, Zhang S, Zeng M, Huang W. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum Gene Ther. 2010;21:1723-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W, Liu J, Yu J, Chen J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015-6025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 98. | Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 522] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 99. | Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, Nabae T, Takahata S, Toma H, Nagai E, Tanaka M. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 100. | Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 101. | Chen C, Zhang Y, Zhang L, Weakley SM, Yao Q. MicroRNA-196: critical roles and clinical applications in development and cancer. J Cell Mol Med. 2011;15:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 102. | Torrisani J, Bournet B, du Rieu MC, Bouisson M, Souque A, Escourrou J, Buscail L, Cordelier P. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther. 2009;20:831-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 103. | Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726-1733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 104. | Li Y, Vandenboom TG, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 105. | Hou B, Jian Z, Chen S, Ou Y, Li S, Ou J. [Expression of miR-216a in pancreatic cancer and its clinical significance]. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1628-1631. [PubMed] [Cited in This Article: ] |
| 106. | Strimpakos AS, Saif MW. Update on phase I studies in advanced pancreatic adenocarcinoma. Hunting in darkness? JOP. 2013;14:354-358. [PubMed] [Cited in This Article: ] |
| 107. | Stephenson J, Richards DA, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, Devens S, Cushing J, Goddard J, Schmalbach T. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2011;29 suppl:Abstr 4114. [Cited in This Article: ] |
| 108. | Oettle H, Hilbig A, Seufferlein T, Schmid RM, Luger T, von Wichert G, Schmaus S, Heinrichs H, Schlingensiepen K. Interim results of the phase I/II study of trabedersen (AP 12009) in patients with pancreatic carcinoma, malignant melanoma, or colorectal carcinoma. J Clin Oncol. 2009;27 suppl:Abstr 4619. [Cited in This Article: ] |
| 109. | Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 110. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5933] [Article Influence: 494.4] [Reference Citation Analysis (0)] |
| 111. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 112. | Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-6295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 113. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 114. | Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 115. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2816] [Cited by in F6Publishing: 2650] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 116. | Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1112] [Cited by in F6Publishing: 1101] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 117. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 118. | Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 119. | Hanks BA, Holtzhausen A, Gimpel P, Jamieson R, Campbell OM, Sun L, Augustine CK, Tyler DS, Osada T, Morse M. Effect of the loss of the type III TGF β receptor during tumor progression on tumor microenvironment: Preclinical development of TGFβ inhibition and TGFβ related biomarkers to enhance immunotherapy efficacy. J Clin Oncol. 2012;30:10563a. [Cited in This Article: ] |
| 120. | Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 843] [Cited by in F6Publishing: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 121. | Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1635] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 122. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3082] [Cited by in F6Publishing: 2902] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 123. | Xu Y, An Y, Wang X, Zha W, Li X. Inhibition of the Hedgehog pathway induces autophagy in pancreatic ductal adenocarcinoma cells. Oncol Rep. 2014;31:707-712. [PubMed] [Cited in This Article: ] |
| 124. | Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1151] [Cited by in F6Publishing: 1138] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 125. | Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094-2096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2272] [Cited by in F6Publishing: 2409] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 127. | Infinity Pharmaceuticals. Infinity Reports Update From Phase 2 Study of Saridegib Plus Gemcitabine in Patients With Metastatic Pancreatic Cancer, 2012. Available from: http://www.businesswire.com/news/home/20120127005146/en/Infinity-Reports-Update-Phase-2-Study-Saridegib#.U8PymNhOV2s. Accessed April 27, 2014. [Cited in This Article: ] |
| 128. | Ren H, Jia L, Zhao T, Zhang H, Chen J, Yang S, Liu J, Yu M, Hao J. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014;354:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 129. | Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun J, Yang S, Hao J. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin. Cancer Res. 2014;74:2455-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 130. | Raykov Z, Grekova SP, Bour G, Lehn JM, Giese NA, Nicolau C, Aprahamian M. Myo-inositol trispyrophosphate-mediated hypoxia reversion controls pancreatic cancer in rodents and enhances gemcitabine efficacy. Int J Cancer. 2014;134:2572-2582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 131. | Kasuya K, Tsuchida A, Nagakawa Y, Suzuki M, Abe Y, Itoi T, Serizawa H, Nagao T, Shimazu M, Aoki T. Hypoxia-inducible factor-1α expression and gemcitabine chemotherapy for pancreatic cancer. Oncol Rep. 2011;26:1399-1406. [PubMed] [Cited in This Article: ] |
| 132. | Bao B, Ali S, Ahmad A, Azmi AS, Li Y, Banerjee S, Kong D, Sethi S, Aboukameel A, Padhye SB. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7:e50165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 133. | Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 555] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 134. | O’Reilly EM, Smith LS, Bendell JC, Strickler JH, Zalupski M, Gluck W, Kapoun A, Yen WC, Xu L, Hill D. Phase 1b of anticancer stem cell antibody omp-59r5 (antinotch 2/3) in combination with nab-paclitaxel and gemcitabine in patients with untreated metastatic pancreatic cancer. J Clin Oncol. 2014;32:220a. [Cited in This Article: ] |
| 135. | Gracian AC, Jameson MB, Grande E, Cooray P, Parnis F, Grimison P, Jeffery M, Stagg RJ, Dupont J, Tebbutt NC. A phase 1b study of the anticancer stem cell agent demcizumab and gemcitabine with or without paclitaxel protein bound particles in patients with pancreatic cancer. J Clin Oncol. 2014;32:279a. [Cited in This Article: ] |
| 136. | Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl Oncol. 2009;2:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 137. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 839] [Article Influence: 64.5] [Reference Citation Analysis (2)] |
| 138. | Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 139. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4035] [Cited by in F6Publishing: 4368] [Article Influence: 397.1] [Reference Citation Analysis (0)] |
| 140. | Von Hoff DD. Final Results of a Randomized Phase III Study of Weekly Nabpaclitaxel Plus Gemcitabine versus Gemcitabine Alone in Patients with Metastatic Adenocarcinoma of the Pancreas. San Francisco, CA: ASCO 2013; . [Cited in This Article: ] |
| 141. | Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 142. | Xie X, Xia W, Li Z, Kuo HP, Liu Y, Li Z, Ding Q, Zhang S, Spohn B, Yang Y. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 143. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1813] [Cited by in F6Publishing: 1857] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 144. | Kuehn R, Lelkes PI, Bloechle C, Niendorf A, Izbicki JR. Angiogenesis, angiogenic growth factors, and cell adhesion molecules are upregulated in chronic pancreatic diseases: angiogenesis in chronic pancreatitis and in pancreatic cancer. Pancreas. 1999;18:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 146. | Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1215] [Cited by in F6Publishing: 1231] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 147. | Pant S, Martin LK, Geyer S, Wei L, Van Loon K, Sommovilla N, Zalupski M, Iyer R, Fogelman D, Ko AH. Treatment-related Hypertension as a Pharmacodynamic Biomarker for the Efficacy of Bevacizumab in Advanced Pancreas Cancer: A Pooled Analysis of 4 Prospective Trials of Gemcitabine-based Therapy With Bevacizumab. Am J Clin Oncol. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 148. | Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, Geissler EK, Stoeltzing O, Lang SA. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10:2157-2167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 149. | Niccolai E, Prisco D, D’Elios MM, Amedei A. What is recent in pancreatic cancer immunotherapy? Biomed Res Int. 2013;2013:492372. [PubMed] [Cited in This Article: ] |
| 150. | Rossi GR, Mautino MR, Unfer RC, Seregina TM, Vahanian N, Link CJ. Effective treatment of preexisting melanoma with whole cell vaccines expressing alpha(1,3)-galactosyl epitopes. Cancer Res. 2005;65:10555-10561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Rossi GR, Unfer RC, Seregina T, Link CJ. Complete protection against melanoma in absence of autoimmune depigmentation after rejection of melanoma cells expressing alpha(1,3)galactosyl epitopes. Cancer Immunol Immunother. 2005;54:999-1009. [PubMed] [Cited in This Article: ] |
| 152. | Hardacre J, Mulcahy M, Small W, Talamonti MS, Obel JC, Rocha Lima CMS, Safran H, Lenz HJ, Chiorean EG, Vahanian NN. Addition of algenpantucel-L immunotherapy to standard of care (SOC) adjuvant therapy for pancreatic cancer. J Clin Oncol. 2012;30 Suppl:Abstract 4049. [Cited in This Article: ] |
| 153. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 154. | Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539-3543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2032] [Cited by in F6Publishing: 1989] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 155. | Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145-156. [PubMed] [Cited in This Article: ] |
| 156. | De Remigis A, de Gruijl TD, Uram JN, Tzou SC, Iwama S, Talor MV, Armstrong TD, Santegoets SJ, Slovin SF, Zheng L. Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int J Cancer. 2015;136:127-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 157. | Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862-3868. [PubMed] [Cited in This Article: ] |
| 158. | Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 159. | Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Gedde-Dahl T, Stokke KT, Sølheim BG, Egge TS, Søreide O, Thorsby E. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer. 1996;65:450-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 160. | Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Søreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet. 1995;346:1399-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 161. | Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D, Maher VE, Stipanov M, Contois D, Johnson BE. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099-5107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 162. | Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 163. | Wedén S, Klemp M, Gladhaug IP, Møller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128:1120-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 164. | Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34:321-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 165. | Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, Coffey M, Gill GM, Mettinger K, Mariadason JM. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 166. | Vasef MA, Ross JS, Cohen MB. Telomerase activity in human solid tumors. Diagnostic utility and clinical applications. Am J Clin Pathol. 1999;112:S68-S75. [PubMed] [Cited in This Article: ] |
| 167. | Suehara N, Mizumoto K, Kusumoto M, Niiyama H, Ogawa T, Yamaguchi K, Yokohata K, Tanaka M. Telomerase activity detected in pancreatic juice 19 months before a tumor is detected in a patient with pancreatic cancer. Am J Gastroenterol. 1998;93:1967-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 168. | Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 169. | Buanes T, Maurel J, Liauw W, Hebbar M, Nemunaitis J. A randomized phase II study of gemcitabine(G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreas cancer. J Clin Oncol. 2009;27:Abstr 4601. [Cited in This Article: ] |
| 170. | Available from: http://clinicaltrials.gov/ct2/show/NCT00358566. [Cited in This Article: ] |
| 171. | Middleton GW, Valle JW, Wadsley J Propper D, Coxon FY, Ross PJ, Madhusudan S, Roques T, Cunningham D, Corrie P, Greenhalf W. A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer. ASCO Meeting Abstracts. 2013;LBA4004. [Cited in This Article: ] |
| 172. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 173. | Final-Results-Phase-III-TeloVac-Trial-Pancreatic , 2013 . Press release. Available from: http://www.businesswire.com/news/home/ 20130608005934 /en/. [Cited in This Article: ] |
| 174. | Ishizaki H, Manuel ER, Song GY, Srivastava T, Sun S, Diamond DJ, Ellenhorn JD. Modified vaccinia Ankara expressing survivin combined with gemcitabine generates specific antitumor effects in a murine pancreatic carcinoma model. Cancer Immunol Immunother. 2011;60:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 175. | Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856-2860. [PubMed] [Cited in This Article: ] |
| 176. | Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 177. | Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, Wu W, Ni X, Mao Z, Kuang T. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med. 2012;12:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 178. | Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 179. | Therion Reports Results of Phase 3 PANVAC-VF Trial and Announces Plans for Company Sale. PR Newswire 28 June. Available from: http://www.prnewswire. com/news-releases/therion -reports-results-of-phase- 3-panvac-vf-trial-and-announces-plans-for-companysale- 56997582.html. [Cited in This Article: ] |
| 180. | Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101:433-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 181. | Yanagimoto H, Shiomi H, Satoi S, Mine T, Toyokawa H, Yamamoto T, Tani T, Yamada A, Kwon AH, Komatsu N. A phase II study of personalized peptide vaccination combined with gemcitabine for non-resectable pancreatic cancer patients. Oncol Rep. 2010;24:795-801. [PubMed] [Cited in This Article: ] |
| 182. | Showalter SL, Huang YH, Witkiewicz A, Costantino CL, Yeo CJ, Green JJ, Langer R, Anderson DG, Sawicki JA, Brody JR. Nanoparticulate delivery of diphtheria toxin DNA effectively kills Mesothelin expressing pancreatic cancer cells. Cancer Biol Ther. 2008;7:1584-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 183. | Oki Y, Younes A. Heat shock protein-based cancer vaccines. Expert Rev Vaccines. 2004;3:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 184. | Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964-1972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 185. | Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 186. | Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S, Yusa S. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother. 2014;63:797-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 187. | Pei Q, Pan J, Zhu H, Ding X, Liu W, Lv Y, Zou X, Luo H. Gemcitabine-treated pancreatic cancer cell medium induces the specific CTL antitumor activity by stimulating the maturation of dendritic cells. Int Immunopharmacol. 2014;19:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 188. | Bauer C, Sterzik A, Bauernfeind F, Duewell P, Conrad C, Kiefl R, Endres S, Eigler A, Schnurr M, Dauer M. Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8(+) T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer Immunol Immunother. 2014;63:321-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, Clay TM, Schlom J, Gilboa E, Lyerly HK. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer. 2002;32:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 190. | Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, Gaudernack G. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother. 2011;60:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 191. | Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69-e74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 192. | Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 1214] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 193. | Yang B, He Y, Sun DL, Zou Y, Qin XH, Huang BH. [Specific immune against pancreatic cancer induced by dendritic cells pulsed with mutant K-ras peptide]. Zhonghua Yi Xue Zazhi. 2008;88:1956-1960. [PubMed] [Cited in This Article: ] |
| 194. | Schmidt J, Ryschich E, Sievers E, Schmidt-Wolf IG, Büchler MW, Märten A. Telomerase-specific T-cells kill pancreatic tumor cells in vitro and in vivo. Cancer. 2006;106:759-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 195. | Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, Schlom J, Tsang KY. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342-6351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 196. | Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 197. | Moniri MR, Dai LJ, Warnock GL. The challenge of pancreatic cancer therapy and novel treatment strategy using engineered mesenchymal stem cells. Cancer Gene Ther. 2014;21:12-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |