Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17448
Revised: March 13, 2014
Accepted: July 16, 2014
Published online: December 14, 2014
AIM: To analyze whether pancreaticoduodenectomy with simultaneous resection of tumor-involved vessels is a safe approach with acceptable patient survival.
METHODS: Between January 2001 and March 2012, 136 patients received pancreaticoduodenectomy for adenocarcinoma at our hospital. Seventy-eight patients diagnosed with pancreatic head carcinoma were included in this study. Among them, 46 patients received standard pancreaticoduodenectomy (group 1) and 32 patients received pancreaticoduodenectomy with simultaneous resection of the portal vein or the superior mesenteric vein or artery (group 2) followed by reconstruction. The immediate surgical outcomes and survivals were compared between the groups. Fifty-five patients with unresectable adenocarcinoma of the pancreas without liver metastasis who received only bypass operations (group 3) were selected for additional survival comparison.
RESULTS: The median ages of patients were 67 years (range: 37-82 years) in group 1, and 63 years (range: 35-86 years) in group 2. All group 2 patients had resection of the portal vein or the superior mesenteric vein and three patients had resection of the superior mesenteric artery. The pancreatic fistula formation rate was 21.7% (10/46) in group 1 and 15.6% (5/32) in group 2 (P = 0.662). Two hospital deaths (4.3%) occurred in group 1 and one hospital death (3.1%) occurred in group 2 (P = 0.641). The one-year, three-year and five-year overall survival rates in group 1 were 71.1%, 23.6% and 13.5%, respectively. The corresponding rates in group 2 were 70.6%, 33.3% and 22.2% (P = 0.815). The one-year survival rate in group 3 was 13.8%. Pancreaticoduodenectomy with simultaneous vascular resection was safe for pancreatic head adenocarcinoma.
CONCLUSION: The short-term and survival outcomes with simultaneous resection were not compromised when compared with that of standard pancreaticoduodenectomy.
Core tip: Whipple operation with vascular reconstruction is considered one of the most difficult operations with high morbidity. In this paper, we demonstrate that this complicated surgery can be performed in low-volume centers where a high volume of other complicated liver surgeries, including liver transplant, are performed.
- Citation: Cheung TT, Poon RT, Chok KS, Chan AC, Tsang SH, Dai WC, Chan SC, Fan ST, Lo CM. Pancreaticoduodenectomy with vascular reconstruction for adenocarcinoma of the pancreas with borderline resectability. World J Gastroenterol 2014; 20(46): 17448-17455
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17448.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17448
Carcinoma of the pancreas is one of the leading causes of death, with 43140 new cases reported in the United States in 2010, which will lead to an estimated 36800 deaths at a five-year survival of 6%[1]. Amongst different treatment options, surgical resection offers the best survival outcome to patients with carcinoma of the head of the pancreas[2]. With the advancement in technology and experience sharing, the hospital mortality and morbidity for pancreaticoduodenectomy, also known as the Whipple operation, have improved when compared with the first report in 1935[3]. However, the surgery remains a challenging operation, with hospital mortality rates ranging from 1% to 6% even at experienced centers[4,5]. The issue is even more complicated if the tumor involves major vessels around the pancreatic region. The definition of borderline resectability is controversial. Although many centers have advocated resection of the tumor together with the superior mesenteric vein (SMV) or the portal vein (PV), many other centers simply do not consider operation for this group of patients after balancing the risk of surgery and predicted survival outcomes[6,7].
The aim of this study was to compare standard pancreaticoduodenectomy and pancreaticoduodenectomy with simultaneous vascular resection with or without vascular reconstruction in terms of survival outcomes in patients who had adenocarcinoma of the pancreas with borderline resectability.
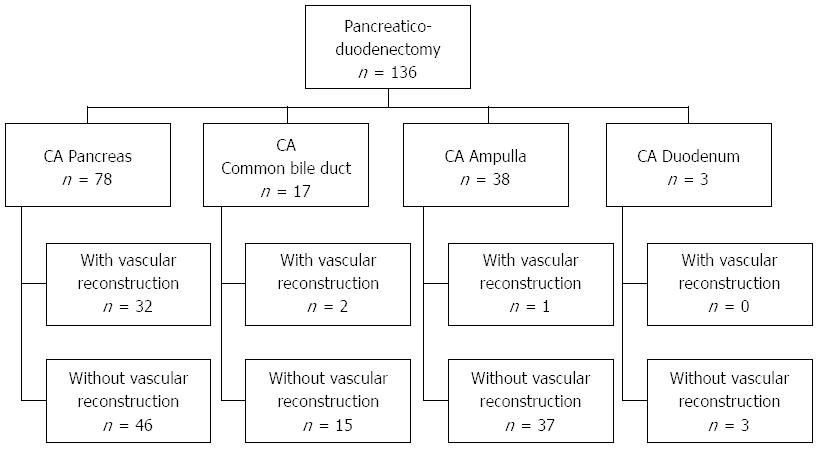
From January 2001 to March 2012, 136 patients received pancreaticoduodenectomy at Queen Mary Hospital of the University of Hong Kong, China. Of these, 2.2% (3/136) had adenocarcinoma of the duodenum, 12.5% (17/136) had adenocarcinoma of the common bile duct, 27.9% (38/136) had adenocarcinoma of the ampulla of Vater, and 57.4% (78/136) had adenocarcinoma of the head of the pancreas (Figure 1). None of the patients received neoadjuvant chemotherapy before operation.
The study population included the 78 patients with adenocarcinoma of the pancreas. Forty-six of these patients underwent standard pancreaticoduodenectomy (group 1), and the remaining 32 patients underwent pancreaticoduodenectomy together with vascular resection (group 2). The patients’ preoperative data including their clinical presentations are listed in Table 1.
| Variables | Group 1 | Group 2 | P value |
| (n = 46) | (n = 32) | ||
| Age (yr) | 67 (37-82) | 63 (35-86) | 0.452 |
| Male:female, n | 25:21 | 20:12 | 0.473 |
| Presence of comorbid illness | 25 (54.3) | 16 (50.0) | 0.705 |
| Cardiovascular disease | 15 (32.6) | 9 (28.1) | 0.673 |
| Pulmonary disease | 5 (10.9) | 1 (3.1) | 0.406 |
| Renal disease | 2 (4.3) | 1 (3.1) | 1.000 |
| Diabetes Mellitus | 9 (19.6) | 11 (34.4) | 0.141 |
| Hemoglobin (g/dL) | 12.5 (9.0-15.6) | 12.7 (9.5-15.6) | 0.614 |
| White blood cell count (× 109/L) | 6.9 (3.6-21.2) | 6.7 (3.9-13.1) | 0.763 |
| Prothrombin time (s) | 11.4 (9.8-40.8) | 11.3 (9.0-14.4) | 0.421 |
| International normalized ratio | 1.0 (0.8-3.6) | 1.0 (0.8-1.2) | 0.938 |
| Serum creatinine (mmol/L) | 75 (52-212) | 84 (47-143) | 0.607 |
| Serum bilirubin ( mmol/L) | 102 (5-533) | 38 (5-342) | 0.020 |
| Albumin (g/L) | 39 (27-46) | 40.5 (8-47) | 0.041 |
Fifty-five patients with unresectable adenocarcinoma of the pancreas without liver metastasis who received only bypass surgery (group 3) were selected for additional survival comparison. These were patients who were physically unfit for major pancreaticoduodenectomy or who had long-segment arterial encasement by tumors.
Contrast computed tomography (CT) of the abdomen was performed for all the patients. A tumor was considered unresectable if the contrast CT scan showed obvious extrapancreatic metastasis. Pancreaticoduodenectomy was offered to patients with a patent SMV-PV confluence despite suspected tumor involvement of the PV or SMV. Operation was also offered to patients with suspected tumor involvement of the short segment of the superior mesenteric artery (SMA). Tumors involving the celiac artery were considered not suitable for surgical resection.
All operations were performed by a team of surgeons specialized in hepatobiliary and pancreatic surgery. Conventional or pylorus-preserving pancreaticoduodenectomy was performed according to the decision of the responsible surgeon. Lymph nodes around the head of the pancreas, the common hepatic artery and the hepatoduodenal ligament were dissected. Dissection of lymph nodes around the celiac and SMA regions was performed in patients who showed evidence of lymph node enlargement. Wedge or segmental resection of the PV or SMV was performed if a pancreatic head mass was inseparable from the vein. The pancreas was dissected from the splenic vein to the left of the mesenteric-portal junction and then transected at this level. All tissue around the PV and SMV was circumferentially cleared to free the veins up to the bifurcation of the PV and down to the branches of the SMV. The PV was then sectioned between the vascular clamp, and the surgical specimen was removed. For segmental resections of the PV or the SMV shorter than 3 cm, end-to-end anastomosis without the use of a graft was possible in all patients in group 2 after adequate mobilization of the SMV and the PV[8]. For segmental resections of the PV or the SMV longer than 3 cm, end-to-end anastomosis was performed with the use of a vascular conduit. Conduit selections included autologous vein, cryo-preserved vein and gortex grafts. The anastomosis was constructed continuously in a single layer with 6/0 Prolene suture. One third of the circumference or one diameter of the PV was allowed in the final knotting in order to avoid narrowing of the anastomosis[9]. For resection of the SMA or hepatic artery, the anastomosis was performed with the microvascular technique with 9/0 nylon suture[10].
Pancreaticoduodenectomy anastomosis was performed by an end-to-side, duct-to-mucosa, two-layer anastomosis using interrupted fine Prolene sutures. The diameter of the pancreatic duct was measured in every case. If the pancreatic duct was thinner than 2 mm or if the pancreas was considered soft in consistency by the operating surgeon, the dunking method without duct-to-mucosa anastomosis or the double-loop technique was employed[11].
Pancreatic stenting was employed if the pancreatic duct was small. For external drainage, depending on the size of the pancreatic duct, an Fr 3-8 polyvinyl catheter with multiple side holes was inserted into the pancreatic duct. A stent with the largest size that could be put into the pancreatic duct was used. Catheter migration was prevented by an anchoring stitch that secured the catheter onto the mucosa of the jejunal side of the pancreaticoduodenectomy anastomosis with a single absorbable suture. For internal drainage, an internal drain was put across the pancreaticoduodenectomy anastomosis, with the tip of the catheter distal to the anastomosis[12].
After performing the anastomosis, an end-to-side, single-layer, interrupted hepaticojejunostomy without stenting was performed using the same jejunal loop. A single-layer, continuous, hand-sewn antecolic gastrojejunostomy or duodenojejunostomy was then performed, with a nasogastric tube placed in the afferent jejunal limb of the anastomosis. No vagotomy, gastrostomy or feeding jejunostomy was performed. A drain was placed anterior to the pancreaticoduodenectomy anastomosis, and another drain was placed posterior to the anastomosis. The vascular reconstruction technique and methods of pancreaticoduodenectomy anastomosis are shown in Table 2.
| Variables | Group 1 | Group 2 | P value |
| (n = 46) | (n = 32) | ||
| Portal vein resection | - | 18 (56.3) | - |
| Reconstruction method | - | - | |
| Primary anastomosis | 14 (77.8) | ||
| Cadaveric vein graft | 3 (16.7) | ||
| Gortex graft | 1 (5.6) | ||
| Superior mesenteric vein resection | - | 22 (68.8) | - |
| Reconstruction method | - | - | |
| Primary anastomosis | 18 (81.8) | ||
| Cadaveric vein graft | 3 (13.6) | ||
| Gortex graft | 1 (4.5) | ||
| Superior mesenteric artery resection | - | 3 (9.4) | - |
| Reconstruction method | - | - | |
| Primary anastomosis | 3 (100) | ||
| Pancreaticojejunostomy construction method | 0.358 | ||
| Dunking | 1 (2.2) | 3 (9.4) | |
| Duct to mucosa | 43 (93.5) | 28 (87.5) | |
| Double loop | 2 (4.3) | 1 (3.1) | |
| Size of pancreatic duct | 0.239 | ||
| < 2 mm | 1 (2.4) | 3 (13.0) | |
| 2-4 mm | 15 (36.6) | 8 (34.8) | |
| < 4 mm | 25 (61.0) | 12 (52.2) | |
| Pancreatic stent | 0.882 | ||
| No | 32 (69.6) | 21 (65.6) | |
| Internal | 3 (6.5) | 3 (9.4) | |
| External | 11 (23.9) | 8 (25.0) |
All patients were followed up monthly in the first year, and then quarterly if no recurrence was detected. CT was performed one month after the operation, quarterly in the first year, and half-yearly subsequently. Pancreatic fistula was classified into type A, B and C according to the International Study Group of Pancreatic Fistula[13]. Recurrence was defined as the presence of typical features appearing on CT or magnetic resonance imaging scans on follow-up. If necessary, recurrence was confirmed by cytology. Patients were referred to medical oncologists for consideration for adjuvant chemotherapy after the surgery.
The baseline characteristics of patients are expressed as median and range or n and percent. The Mann-Whitney U test was used to compare continuous variables, and Pearson’s χ2 test was used to compare discrete variables. Survival analysis was performed on the time of disease-free survival vs tumor recurrence or death. Survival curves were computed with the Kaplan-Meier method and compared between groups by the log-rank test. Significance was defined as P < 0.05. All statistical calculations were made with the computer software SPSS/PC+ (SPSS Inc., Chicago, IL, United States).
The medians for volume of blood loss (1200 mL vs 800 mL; P < 0.05) and operation time (715 min vs 580 min; P < 0.05) were significantly greater in group 2 compared to group 1 (Table 3). Both groups had an intensive care unit stay of 2 d and a median hospital stay of 17 d, with no differences observed in postoperative complications.
| Variables | Group 1 | Group 2 | P value |
| (n= 46) | (n = 32) | ||
| Blood loss (mL) | 800 (250-1600) | 1200 (100-5000) | 0.007 |
| Operation time (min) | 580 (378-855) | 715 (487-992) | < 0.0001 |
| Hospital stay (d) | 17 (9-120) | 17 (11-89) | 0.316 |
| Intensive care unit stay (d) | 2 (1-10) | 2 (0-24) | 0.847 |
| Hospital death | 2 (4.3) | 1 (3.1) | 1.000 |
| Patients with complication | 20 (43.5) | 10 (31.3) | 0.275 |
| Chest infection | 3 (6.5) | 2 (6.3) | 1.000 |
| Pleural effusion | 1 (2.2) | 1 (3.1) | 1.000 |
| Wound infection | 7 (15.2) | 1 (3.1) | 0.176 |
| Subphrenic abscess or collection | 5 (10.9) | 1 (3.1) | 0.406 |
| Intra-abdominal bleeding | 1 (2.2) | 0 (0.0) | 1.000 |
| Gastrointestinal bleeding | 1 (2.2) | 1 (3.1) | 1.000 |
| Cardiac arrhythmia | 4 (8.7) | 2 (6.3) | 1.000 |
| Acute coronary syndrome | 2 (4.3) | 1 (3.1) | 1.000 |
| Deep vein thrombosis | 2 (4.3) | 0 (0.0) | 0.641 |
| Delayed gastric emptying (> 7 d) | 3 (6.5) | 5 (15.6) | 0.355 |
| Types of pancreatic fistula according to ISGPF | 0.662 | ||
| All types | 10 (21.7) | 5 (15.6) | |
| Type A | 6 (13.0) | 4 (12.5) | |
| Type B | 3 (6.5) | 1 (3.13) | |
| Type C | 1 (2.2) | 0 (0.0) |
The median tumor size for both groups was 3 cm, and the majority of patients had fewer than five lymph node metastases (Table 4). There were no differences between the groups regarding the method of pancreaticojejunostomy, type of resection, or disease stage as classified the American Joint Committee on Cancer (AJCC) classification (7th edition)[14].
| Variables | Group 1 | Group 2 | P value |
| (n = 46) | (n = 32) | ||
| Tumor size (cm) | 3 (1.0-8.0) | 3 (1.6-6.0) | 0.315 |
| Lymph node metastases | 0.299 | ||
| No | 21 (45.7) | 12 (37.5) | |
| < 5 | 22 (47.8) | 15 (46.9) | |
| 5-10 | 2 (4.3) | 5 (15.6) | |
| > 10 | 1 (2.2) | 0 (0.0) | |
| Pancreaticojejunostomy construction method | 0.613 | ||
| Dunking or other | 4 (12.5) | 3 (6.5) | |
| Duct to mucosa | 28 (87.5) | 43 (93.5) | |
| R0 resection | 35 (75.6) | 25 (78.1) | 0.793 |
| R1 resection | 11 (24.4) | 7 (21.9) | |
| AJCC staging (7th edition) | 0.981 | ||
| Stage IA | 2 (4.3) | 2 (6.3) | |
| Stage IB | 4 (8.7) | 3 (9.4) | |
| Stage IIA | 14 (30.4) | 8 (25.0) | |
| Stage IIB | 25 (54.3) | 18 (56.3) | |
| Stage III | 1 (2.2) | 1 (3.1) | |
| Stage IA/IB/IIA (no lymph node involvement) | 20 (43.5) | 13 (40.6) | 0.802 |
| Stage IIB/III (advanced) | 26 (56.5) | 19 (59.4) |
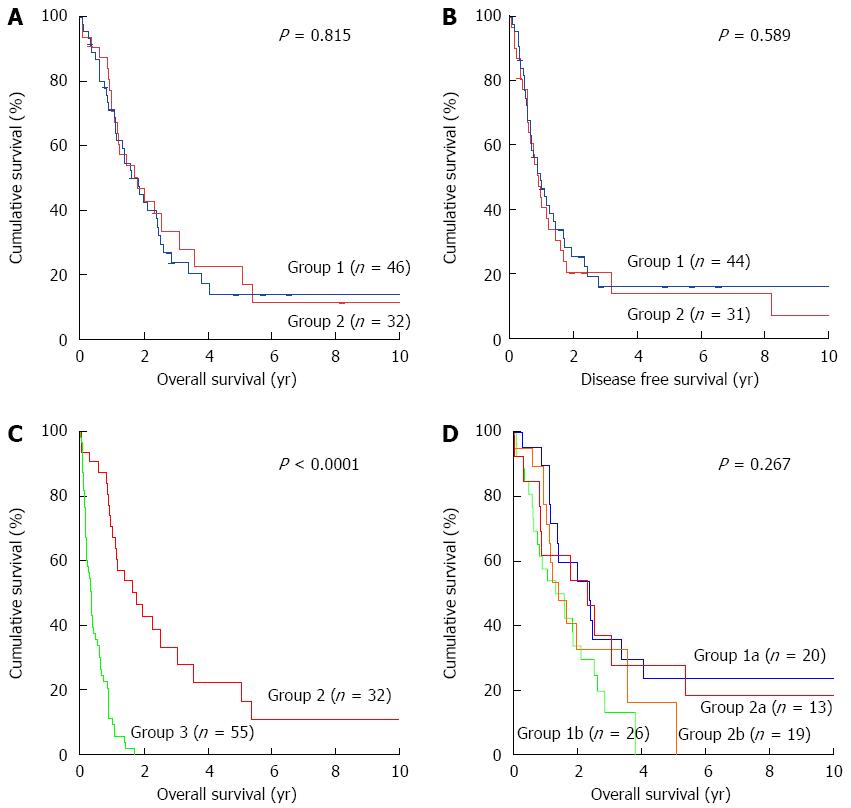
The one-, three-, and five-year survival rates in group 1 were 71.1%, 23.6% and 13.5%, respectively (Figure 2A). These were not different from group 2, who showed corresponding rates of 70.6%, 33.3% and 22.2%. The one-, three-, and five-year disease-free survival rates in group 1 were 48.7%, 15.7% and 15.7%, respectively (Figure 2B), which corresponded to 40.3%, 20.2% and 13.4% disease-free survivals in group 2.
The one-year survival of patients in group 3 (stage IIB/III) who had only undergone bypass surgery was 13.8%, which was significantly lower than in group 2 (P < 0.05) (Figure 2C). These patients were either considered physically unfit for pancreaticoduodenectomy or found to have an SMA encasement larger than 2 cm during laparotomy. Patients with liver metastasis and distant metastasis were not included in this group.
Patients were classified into early and advanced groups for survival comparison according to the presence of lymph node metastasis. The early group was comprised of patients with stage IA, IB or IIA disease. The advanced group was comprised of patients with disease stage IIB or III. The median survival durations for group 1 and group 2 patients with early disease were similar at 28.8 and 27.5 mo, respectively, and 15.8 and 17.1 mo for advanced disease stages (Figure 2D).
Sixteen factors that might affect patient survival after pancreaticoduodenectomy were identified (Table 5). Multivariate analysis showed that only lymph node metastasis was significant for poorer survival (P = 0.021), whereas a univariate analysis identified AJCC disease stage as the only significant factor.
| Factor | Median survival (mo) | P value |
| Median age (yr) | 0.634 | |
| ≤ 64 (n = 40) | 22.35 ± 3.73 | |
| > 64 (n = 38) | 15.84 ± 4.40 | |
| Sex | 0.445 | |
| Male (n = 45) | 22.35 ± 6.32 | |
| Female (n = 33) | 19.26 ± 3.74 | |
| Comorbidity | 0.890 | |
| No (n = 37) | 20.05 ± 4.08 | |
| Yes (n = 41) | 21.36 ± 4.34 | |
| Preoperative cholangitis | 0.487 | |
| No (n = 76) | 19.26 ± 3.54 | |
| Yes (n = 2) | 21.36 | |
| Postoperative complication | 0.204 | |
| No (n = 48) | 23.79 ± 5.26 | |
| Yes (n = 30) | 16.76 ± 4.18 | |
| International normalized ratio | 0.924 | |
| ≤ 1 (n = 55) | 21.36 ± 2.79 | |
| > 1 (n = 15) | 27.51 ± 14.53 | |
| Creatinine level (mmol/L) | 0.652 | |
| ≤ 79 (n = 37) | 23.79 ± 4.42 | |
| > 79 (n = 36) | 13.90 ± 2.98 | |
| Total bilirubin level ( mmol/L) | 0.581 | |
| ≤ 72 (n = 39) | 22.35 ± 3.16 | |
| > 72 (n = 38) | 16.66 ± 5.01 | |
| Serum albumin level (g/L) | 0.419 | |
| ≤ 40 (n = 44) | 28.79 ± 8.12 | |
| > 40 (n = 29) | 19.09 ± 3.77 | |
| Blood loss (mL) | 0.284 | |
| ≤ 850 (n = 36) | 28.79 ± 5.84 | |
| > 850 (n = 33) | 21.36 ± 4.89 | |
| Tumor Size (cm) | 0.630 | |
| ≤ 3 (n = 43) | 16.76 ± 3.22 | |
| > 3 (n = 28) | 19.09 ± 7.97 | |
| R1 resection | 0.055 | |
| No (n = 59) | 25.34 ± 5.32 | |
| Yes (n = 18) | 13.44 ± 3.87 | |
| Lymph node metastases | 0.113 | |
| No (n = 33) | 28.79 ± 3.82 | |
| < 5 (n = 37) | 17.12 ± 3.05 | |
| 5-10 (n = 7) | 11.40 ± 5.68 | |
| > 10 (n = 1) | 22.18 | |
| Disease stage (AJCC staging, 7th edition) | 0.036 | |
| Early (IA + IB + IC) (n = 33) | 28.79 ± 5.00 | |
| Advanced (IIB + III + IV) (n = 45) | 17.12 ± 3.41 | |
| Simultaneous vascular resection | 0.815 | |
| Yes (n = 32) | 21.36 ± 5.93 | |
| No (n = 46) | 19.26 ± 3.58 | |
| Pancreatic fistula type (according to ISGPF) | 0.488 | |
| No fistula + type A (n = 73) | 20.05 ± 3.43 | |
| Type B + type C (n = 5) | 13.90 ± 6.61 |
Pancreaticoduodenectomy is a technically challenging procedure, and the rate of morbidities (including pancreatic fistula) ranges between 15% and 50%[15,16]. A gradual decrease in mortality has been observed in recent years, though the patient age and the incidence of comorbid illness have increased. It is generally agreed that a hospital mortality rate of 3%-5% is acceptable for pancreaticoduodenectomy without vascular resection at high-volume centers[4]. However, it is believed that the risk of this operation is high if simultaneous vascular resection with reconstruction is required. Many surgeons have taken a more conservative approach when the tumor is considered barely resectable[17,18].
Tumor invasion of the PV or SMV is considered a sign of advanced disease, which is likely to result in poor surgical outcomes. Thus, many centers adopt more conservative approaches for patients with vascular invasion[19]. In this study, we found that tumor invasion or dense adhesion to the PV or SMV did not correlate with the stage of disease. The median tumor size was 3 cm in both groups, but tumors as small as 1.6 cm could have invaded the PV or SMV, necessitating vascular resection. It is tumor location rather than tumor size that affects surgical planning. Fuhrman et al[20] described a similar observation in 23 patients whose tumors with a median size of 3 cm led to major vessel involvement.
Due to the retrospective design of this study, we did not present the degree of invasion of the PV or SMV found by histopathologic examinations. Since histologic information can only be obtained after surgery, an operating surgeon has to judge during laparotomy whether there is genuine vascular invasion and whether simultaneous vascular resection is required. At centers experienced in vascular reconstruction, simultaneous resection for suspected venous invasion should be performed. The AJCC has not considered tumor invasion of the PV or SMV a factor affecting tumor staging, and therefore a tumor with PV or SMV involvement is classified stage IIA if there is no lymph node metastasis. In accordance with this, patients with tumor invasion of the PV or SMV are still considered to have a relatively early-stage cancer. This might be quite contradictory to many clinicians’ concepts.
The definition of borderline resectability for pancreatic cancer remains controversial. Varadhachary et al[21] proposed three categories of patients with tumors with borderline resectability: types A, B and C. Type-A patients have tumors with one or more of the following three findings on CT images: (1) tumor abutment (≤ 180° of the circumference of the vessel) of the SMA or celiac axis; (2) tumor abutment or encasement (> 180° of the circumference of the vessel) of a short segment of the hepatic artery, typically at the origin of the gastroduodenal artery; and (3) tumor-related occlusion of a short segment of the SMV, PV, or SMV-PV confluence that is amenable to vascular resection and reconstruction because of a patent SMV and PV below and above the area of occlusion. Type-B patients have tumors with extrapancreatic metastasis. Type-C patients are patients who have marginal physical fitness for major operations.
In fact, many patients with adenocarcinoma of the pancreas can have a relatively early cancer stage even if they are classified as a type-A patient. The decision on treatment strategy for these patients depends on the risk and benefit of surgery and whether or not there is a good alternative treatment. Neoadjuvant chemotherapy has been suggested to increase the resection rate. Chemotherapy or chemoradiation have a partial tumor response rate of 56%[22]. Although it might be effective for some patients, subjecting every patient with SMV or PV involvement to neoadjuvant therapy without considering upfront surgery would allow progression of cancer in 40% of patients who would not respond well.
This study shows that pancreaticoduodenectomy with vascular resection can be performed safely at centers with experience and expertise. The complication and pancreatic fistula rates in group 2 were not inferior to those in group 1. No SMV or PV thrombosis was found in the study. The experience and techniques in vessel reconstruction we have learned from our liver transplant program can be transferred to many complicated hepatobiliary and pancreatic surgeries[10,23,24]. Techniques used in reconstruction of the PV, SMV or SMA are identical to those used in liver transplantation. Most of the time, PV or SMV reconstruction is possible without generating tension on the venous anastomosis after a Cattell-Braasch maneuver[25]. When simultaneous resection of a PV longer than 5 cm is required during pancreaticoduodenectomy, cadaveric vein grafts available from the liver transplant program give extra flexibility for the vascular reconstruction.
Contrast CT is one of the most sensitive ways for preoperative staging of pancreatic cancer[26,27]. It has been suggested that the morphologic features of portal vein invasion could predict the survival outcomes of patients. Patients with extensive tumor involvement of the vessel would have poorer survival[27,28]. Likewise, possible regional lymph node metastases can now be revealed by fine-cut CT scans. Regional lymph node metastases are associated with poor patient survival. However, these so-called poor prognostic indicators should only be regarded as a prognostic suggestion before surgery, and should not become an absolute contraindication to surgery, as surgical resection provides the best survival outcomes for patients with barely resectable diseases. Pancreatic cancer with arterial invasion is also associated with poor patient survival. In a previous study, patients who had pancreaticoduodenectomy with simultaneous arterial resection had a median overall survival duration of only 15.8 mo[29].
In our present study, patients having only bypass surgery with palliative intention (group 3) were included for comparison. Many of these patients were not subjected to pancreaticoduodenectomy because they were not physically fit for major surgery. In terms of survival, these patients fared far worse than group 2 patients. Approximately 23% of the patients in the study had R1 resection as shown by final pathologic examination. Many of these patients had posterior margin involvement. No difference in the distribution of margin involvement was found between group 1 and group 2. As there is no association between SMV or PV invasion and R1 resection, and the degree of posterior margin involvement cannot be known before laparotomy, patients should not be denied pancreaticoduodenectomy on the basis of CT images of vascular invasion.
Adenocarcinoma of the pancreas is a cancer with poor patient survival. Surgical resection provides the best chance of cure. Pancreaticoduodenectomy with simultaneous vascular resection is a safe and effective treatment option. The postoperative morbidity and pancreatic fistula rates are not inferior at centers with expertise. In patients who suffer from adenocarcinoma of the pancreas with portal venous invasion, survival after this complicated procedure is not compromised when compared with that after standard pancreaticoduodenectomy.
Carcinoma of the pancreas is one of the leading causes of death. With advancement in technology and experience sharing, the hospital mortality and morbidity for pancreaticoduodenectomy have improved. However, pancreaticoduodenectomy remains a challenging operation with possible hospital mortality. The issue becomes even more complicated if the major vessels around the pancreatic region are involved. Although many centers advocate en bloc resection of the tumor and the portal vein or superior mesenteric vein, many others do not consider operation for this group of patients after balancing predicted survival outcomes against the risk of surgery.
Pancreaticoduodenectomy with vascular resection is considered a high-risk operation. It also requires experience in vascular reconstruction. This study shows that it can achieve excellent survival outcomes for patients with pancreatic adenocarcinoma at centers with expertise.
This study shows that skills honed in complicated hepatobiliary operations can be transferred to pancreatic surgery. Approximately 300 partial hepatectomies and 100 liver transplants are conducted at Queen Mary Hospital every year, but there are only about 30 pancreaticoduodenectomies a year. This is partly because pancreaticoduodenectomy is not a centralized operation in Hong Kong; many other hospitals also perform this operation. However, this study shows that with expertise, pancreaticoduodenectomy with vascular resection can achieve good patient survival with low morbidity.
At centers with experience in vascular reconstruction, en bloc resection of tumor and vessels with suspected invasion should be performed. The American Joint Committee on Cancer does not consider tumor invasion of the portal or superior mesenteric vein a factor affecting staging, and thus a tumor involving the portal or superior mesenteric vein without any lymph node metastasis is classified as stage IIA. Patients who have tumor invasion of the portal or superior mesenteric vein can still be considered as having a relatively early-stage cancer.
Pancreaticoduodenectomy is also known as the Whipple procedure. It is a major surgical operation that involves resection of the pancreas, duodenum and other organs. It is considered a definitive treatment for malignant tumors at the head of the pancreas or involving the common bile duct, duodenal papilla or duodenum near the pancreas. The portal vein and the superior mesenteric vein are major vessels that connect the bowel to the liver. Reconstruction of these vessels is a crucial step if they are also resected in the Whipple procedure. Catastrophic consequences could arise if leakage or stricture occurs to the anastomosis.
This manuscript is well written and documented. I think that this manuscript is suitable and worth publishing.
P- Reviewer: Boin IFSF, Chiaro M, Coskun A, ElGeidie AAR S- Editor: Gou SX L- Editor: AmEditor E- Editor: Ma S
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10002] [Cited by in F6Publishing: 10353] [Article Influence: 739.5] [Reference Citation Analysis (0)] |
| 2. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 431] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 3. | Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102:763-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 882] [Cited by in F6Publishing: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 4. | Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, Choti MA, Schulick RD, Wolfgang CL, Pawlik TM. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210; discussion 1210-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1087] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 6. | Nakagohri T, Kinoshita T, Konishi M, Inoue K, Takahashi S. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg. 2003;186:149-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Shibata C, Kobari M, Tsuchiya T, Arai K, Anzai R, Takahashi M, Uzuki M, Sawai T, Yamazaki T. Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg. 2001;25:1002-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg. 2004;28:602-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Starzl TE, Iwatsuki S, Shaw BW. A growth factor in fine vascular anastomoses. Surg Gynecol Obstet. 1984;159:164-165. [PubMed] [Cited in This Article: ] |
| 10. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, Salvia R, Pederzoli P. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, Wong J. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;246:425-433; discussion 433-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3282] [Cited by in F6Publishing: 3400] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 14. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6152] [Article Influence: 439.4] [Reference Citation Analysis (0)] |
| 15. | Fischer M, Matsuo K, Gonen M, Grant F, Dematteo RP, D’Angelica MI, Mascarenhas J, Brennan MF, Allen PJ, Blumgart LH. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010;252:952-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1097] [Cited by in F6Publishing: 1094] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 17. | Andersen HB, Baden H, Brahe NE, Burcharth F. Pancreaticoduodenectomy for periampullary adenocarcinoma. J Am Coll Surg. 1994;179:545-552. [PubMed] [Cited in This Article: ] |
| 18. | Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Makowiec F, Post S, Saeger HD, Senninger N, Becker H, Betzler M, Buhr HJ, Hopt UT; German Advanced Surgical Treatment Study Group. Current practice patterns in pancreatic surgery: results of a multi-institutional analysis of seven large surgical departments in Germany with 1454 pancreatic head resections, 1999 to 2004 (German Advanced Surgical Treatment study group). J Gastrointest Surg. 2005;9:1080-1086; discussion 1086-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 248] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846; discussion 846-848. [PubMed] [Cited in This Article: ] |
| 23. | Chan SC, Fan ST, Lo CM, Liu CL, Wong J. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg. 2007;245:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Chan SC, Lo CM, Fan ST. Simplifying living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:9-14. [PubMed] [Cited in This Article: ] |
| 25. | Bachmann J, Michalski CW, Martignoni ME, Büchler MW, Friess H. Pancreatic resection for pancreatic cancer. HPB (Oxford). 2006;8:346-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Tamm EP, Balachandran A, Bhosale PR, Katz MH, Fleming JB, Lee JH, Varadhachary GR. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Nakao A, Kanzaki A, Fujii T, Kodera Y, Yamada S, Sugimoto H, Nomoto S, Nakamura S, Morita S, Takeda S. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012;255:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Ishikawa O, Ohigashi H, Imaoka S, Furukawa H, Sasaki Y, Fujita M, Kuroda C, Iwanaga T. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg. 1992;215:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Bockhorn M, Burdelski C, Bogoevski D, Sgourakis G, Yekebas EF, Izbicki JR. Arterial en bloc resection for pancreatic carcinoma. Br J Surg. 2011;98:86-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |