Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8282
Revised: February 27, 2014
Accepted: April 21, 2014
Published online: July 7, 2014
AIM: To compare the efficacy and safety of endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) for the treatment of colorectal tumors.
METHODS: Databases, such as PubMed, EMBASE, Cochrane Library and Science Citation Index updated to 2013 were searched to include eligible articles. In the meta-analysis, the main outcome measurements were the en bloc resection rate, the histological resection rate and the local recurrence rate. Meanwhile, we also compared the operation time and the incidence of procedure-related complications.
RESULTS: Six trials were identified and a total of 1642 lesions were included. The en bloc resection rate was higher and the local recurrence rate was lower in the ESD group compared with the EMR group (OR = 7.94; 95%CI: 3.96-15.91; OR = 0.09; 95%CI: 0.04-0.19). There was no significant difference in histological resection rate(OR = 1.65; 95%CI: 0.29-9.30) and procedure-related complication rate between the two groups (OR = 1.59; 95%CI: 0.92-2.73). The meta-analysis also showed that ESD was more time consuming than EMR.
CONCLUSION: Compared with EMR, ESD results in higher en bloc resection rate and lower local recurrence rate for the treatment of colorectal tumors, without increasing the procedure-related complications.
Core tip: Endoscopic submucosal dissection (ESD) was originally developed for en bloc resection of lager, flat gastrointestinal tumors. Compared with endoscopic mucosal resection (EMR), ESD was considered to be more time consuming and have more complications for the treatment of colorectal tumors. This meta-analysis of six trials shows that compared with EMR, ESD gives higher en bloc resection rate and lower local recurrence rate for the treatment of colorectal tumors, without increasing the procedure-related complications. ESD should be considered in the endoscopic treatment of colorectal tumors.
- Citation: Wang J, Zhang XH, Ge J, Yang CM, Liu JY, Zhao SL. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J Gastroenterol 2014; 20(25): 8282-8287
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8282
Endoscopic mucosal resection (EMR) is widely accepted for the treatment of superficial colorectal tumors, because of its minimal invasion, low cost, good patients’ tolerance, and better patient quality of life after operation, but large lesions (≥ 2 cm) are hard to be resected completely[1,2]. Endoscopic submucosal dissection (ESD), which had a significantly higher rate of en bloc and histological resection, was developed to solve these problems[3].
However, compared with conventional EMR, ESD has several disadvantages, such as long operation time, high procedure-related complication rate, and technical difficulty in the resection of colorectal tumors[4-8].
There is no current consensus on the optimal endoscopic method for the treatment of colorectal tumors. We conducted a systematic review to compare the efficacy and safety of EMR and ESD for the treatment of colorectal tumors.
We searched databases including PubMed, EMBASE, the Cochrane Library, and Science Citation Index updated to August 2013 to identify related articles in English language that compared EMR and ESD. All bibliographies were indentified in the reference lists. The searching terms used were “EMR or endoscopic mucosal resection” and “ESD or endoscopic submucosal dissection”. Major proceedings of international conferences (such as Digestive Disease Week, Asian Pacific Digestive Week, and so on) were also hand-searched.
The inclusion and exclusion criteria are shown in Table 1.
| Inclusion criteria | Exclusion criteria |
| Colorectal tumors diagnosis for every patient has been confirmed by histology | Case report |
| Comparison of EMR and ESD for the treatment of colorectal tumors | Comment |
| Written in English | Review |
| Letter to editor | |
| Insufficient data | |
| Guidelines |
Data were extracted by one investigator and confirmed by the other according to a predefined data extraction form. Disagreements were resolved by consultation with a third investigator. The following data were collected: year of publication, first author, country, duration, number of participants and lesions in each group, age, tumor size and endpoints (en bloc resection rate, histological resection rate, local recurrence rate, operation time and complications). The definitions of the endpoints were: (1) en bloc resection rate - en bloc removal of tumors in one piece without fragmentation; (2) histological resection rate - complete resection of tumors with no local tumor residue in both margin and basal sites; (3) local recurrence rate - a histological diagnosis of tumor at the resected site during follow-up; (4) operation time - from marking to resection of the lesions; and (5) rate of complications - procedure related bleeding or perforation incidence during or after the operation. The Newcastle-Ottawa Scale was used to assess the quality of the included non-randomized studies.
All data extracted were entered in the freeware program Review Manager (Version 5.0 for Windows, Cochrane Collaboration). The weighted mean difference was calculted for continuous data, and the odds ratio (OR) with 95%CI was used for dichotomous data. Statistical heterogeneity between trials was evaluated by the χ2 test and was considered to be present at P < 0.1. We also used I2 to assess the heterogeneity. I2 > 50% was considered statistically significant. In the presence of statistical heterogeneity, heterogeneity was explored by subgroup analysis or a random-effects model. Publication bias was detected by a funnel plot, and then the symmetry of the funnel plot was confirmed by the Egger’s test, with a P value of 0.05.
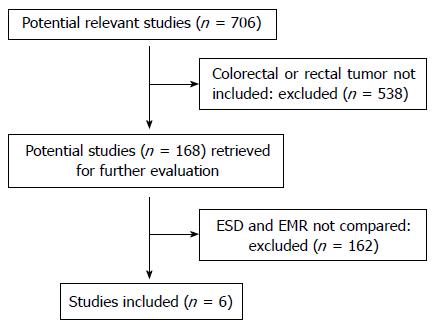
A total of 706 potential studies were retrieved for the meta-analysis, and 538 were excluded for not including the colorectal or rectal tumors, and 162 were excluded because EMR and ESD were not compared. The remaining six eligible studies[9-14] were chosen for further analysis (Figure 1). A total of 1642 lesions were included in the meta-analysis, including 776 lesions in the ESD group and 866 lesions in the EMR group. All of the studies were respective case-control studies, not randomized controlled trials (RCTs). The key characteristics of the studies are listed in Table 2.
| Country | Duration | Patients | Lesions | Mean age (yr) | Tumor size(mm) | Score | Ref. |
| Japan | 2003.1-2006.12 | Not mentioned | ESD 145 | ESD 64 ± 4 | ESD 28 ± 8 | ****** | [9] |
| EPM 228 | EMR 64 ± 11 | EMR 37 ± 14 | |||||
| Japan | 2006.4-2009.12 | Not mentioned | ESD 89 | ESD 66.7 ± 10.7 | ESD 38.8 ± 17.3 | ****** | [10] |
| EMR 178 | EMR 67.9 ± 11.3 | EMR 32.2 ± 15.5 | |||||
| Japan | 1995.1-2009.12 | ESD 85 | ESD 85 | ESD 64.3 ± 9.2 | ESD 31.6 ± 9.0 | ****** | [11] |
| EMR 100 | EMR 104 | EMR599 ± 10.6 | EMR 25.5 ± 6.8 | ||||
| Japan | 2000.1-2009.2 | Not mentioned | ESD 28 | ESD 65.1 | ESD 27.1 | ****** | [12] |
| EMR 56 | EMR 65.9 | EMR 25 | |||||
| South Korea | 2004.1-2009.11 | ESD 303; | ESD 314; | ESD 61 | ESD 28.9 ± 12.7 | ****** | [13] |
| EPMR 67; | EPMR 69; | EMRP 62 | EPMR 23.5 ± 5.6 | ||||
| EMR 135 | EMR 140 | EMR 63 | EMR 21.7 ± 3.5 | ||||
| South Korea | 2002.1-2007.12 | A total of 203 | ESD 58 | ESD 63.8 ± 11.6 | ESD 30.6 ± 10.6 | ****** | [14] |
| ESD-S 57 | ESD-S 63.2 ± 10.7 | ESD-S 26.4 ± 9.9 | |||||
| EMR 91 | EMR 60.1 ± 10.8 | EMR 20.9 ± 7.9 |
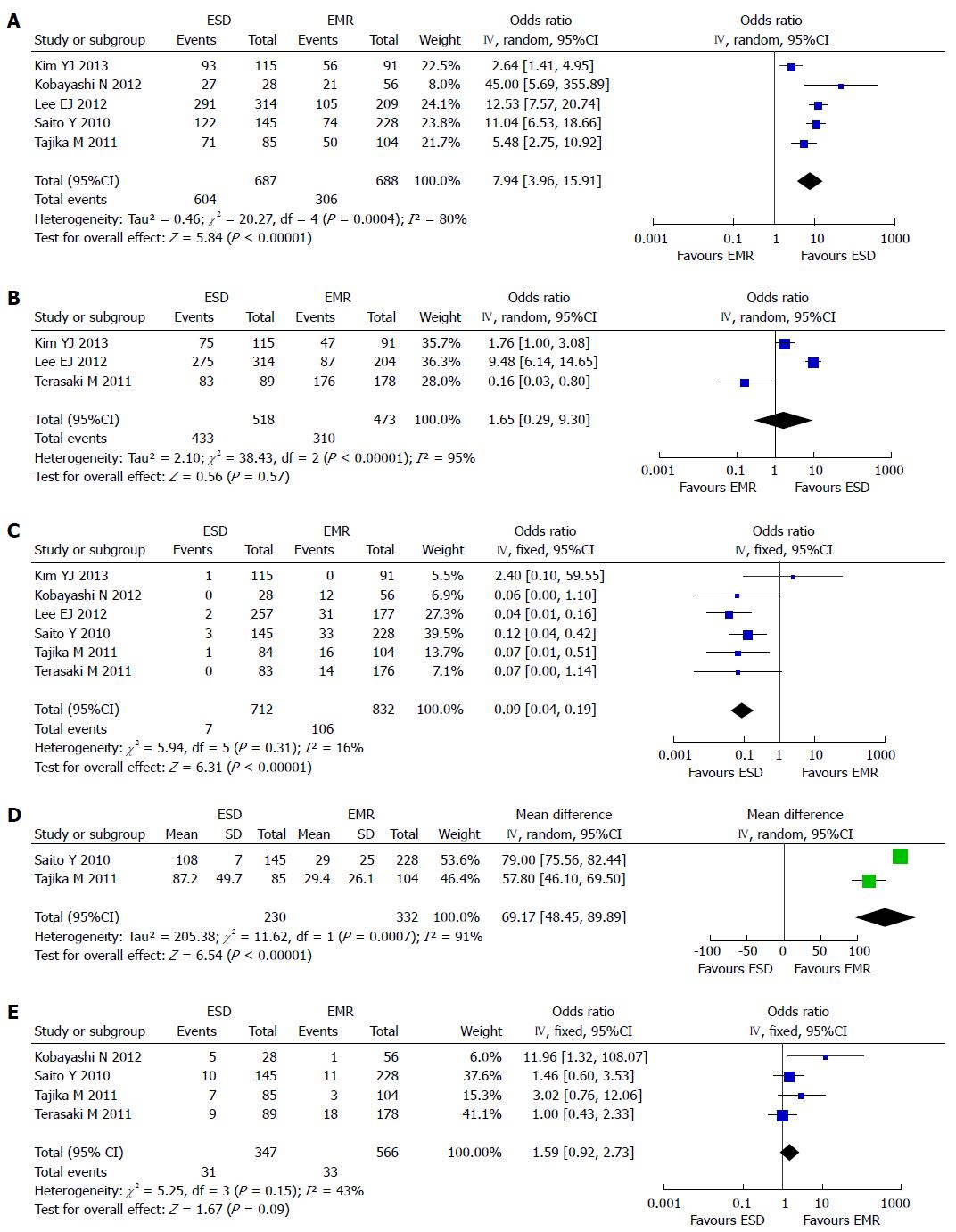
The en bloc resection rate was reported in five studies[9,11-14]. Because heterogeneity was detected (P = 0.0004; I2 = 80%), a random effect model was applied. The analysis showed a higher en bloc resection rate in the ESD group (604/687) than in the EMR group (306/688) (OR = 7.94; 95%CI: 3.96-15.91) (Figure 2A). Based on the result of the sensitivity analysis, we excluded the study with smallest samples[12], but the heterogeneity could not be eliminated (P = 0.0006; I2 = 83% ).
The histological resection rate was reported in three studies[10,13,14]. A random effect model was applied because of the heterogeneity (P < 0.00001, I2 = 94%). The analysis showed no significant difference in histological resection rate between the ESD group (434/518) and the EMR group (310/473) (OR = 1.65; 95%CI: 0.29-9.30) (Figure 2B). We ruled out the study from Japan[10], and heterogeneity still existed (P < 0.00001, I2 = 95%).
All of the studies reported the local recurrence after operation. There was no heterogeneity in the studies (P = 0.31, I2 = 16%), and a fixed effect model was applied. The local recurrence rate was higher in the EMR group (106/832) than in the ESD group (7/712) (OR = 0.09; 95%CI: 0.04-0.19) (Figure 2C).
The operation time for ESD vs EMR was reported in four studies. Only two[9,11] were included in the meta-analysis for the reason that one study provided only median operation time and the operation time of ESD group was divided into two parts in the other study. A random effect model was applied because of the heterogeneity (P = 0.0007, I2 = 91%). Longer time was needed in the ESD group than in the EMR group (Figure 2D).
Data for procedure-related complications were reported in all of the studies included. There was heterogeneity among the studies (P = 0.04, I2 = 58%). We excluded two studies from Korea[13,14], and eliminated the heterogeneity (P = 0.15, I2 = 43%). A fixed effect model was applied, the subsequent analysis showed that there was no statistical difference between the two groups (OR = 1.59; 95%CI: 0.92-2.73) (Figure 2E).
We used the en bloc resection rate as the outcome, and no publication bias was detected by funnel plot and the Egger’s test (P = 0.217).
To our knowledge, this meta-analysis is the first to compare the clinical outcomes of ESD vs EMR for the treatment of colorectal tumors. In the present analysis, six retrospective studies were included, the results confirmed that, compared with EMR, ESD showed higher en bloc resection rate and lower local recurrence rate. The curative histological resection rate was similar between the two groups. Meanwhile, the procedure-related complication rate was higher in the ESD group. On the other hand, ESD is more time-consuming because of the complex procedure and more time is needed to stop the bleeding.
EMR, first developed in Japan, is an endoscopic technique designed for the removal of sessile or flat neoplasms confined to the superficial layers (mucosa and submucosa) of the gastrointestinal tract. For many years, conventional EMR and surgery have been the only available therapy for large colorectal tumors. EMR is typically used for removal of lesions smaller than 2 cm or piecemeal removal of larger lesions[15]. But for large lesions, incomplete resection is common, which can lead to local recurrence. ESD is a newly developed and epoch-making method, which has been developed for en bloc removal of large (usually larger than 2 cm), flat gastrointestinal tract lesions. The shortcomings of ESD were: more time-consuming, higher rate of procedure related complications and more costly[16-20].
The comparison between ESD and EMR in the treatment of colorectal tumor is still controversial. Therefore, we designed the meta-analysis to systematically evaluate the two techniques, providing evidence for endoscopic treatment of colorectal tumors. Actually, the meta analysis by Cao et al[21] compared the clinical outcomes of ESD vs EMR for the treatment of tumors of the gastrointestinal tract, they found that ESD showed better en bloc and curative resection rates and local recurrence, but was more time-consuming and had higher rates of bleeding and perforation complications. Lian et al[22] demonstrated that ESD is more promising in the treatment of early gastric carcinoma, but it had the disadvantages of higher complication rates with perforation and bleeding. In view of the present meta-analysis and all available trials, we suggest that ESD is appropriate to most of the colorectal lesions, especially the large lesions, as ESD has a higher en bloc resection rate and lower local recurrence rate. When we excluded the two studies from Korea, the heterogeneity was eliminated, the result showed no significant difference between the two groups in complication rate.
There were certain limitations in our analysis. Firstly, none of the included studies were randomized. This certainly attenuates the evidence level and value of this meta-analysis. Secondly, all included studies were from only two countries, Japan and Korea, so the results need further confirmation by studies from other countries. Thirdly, the diameters of colorectal tumors were not uniform across the studies.
In conclusion, based on the findings of our meta-analysis, ESD showed considerable advantages over EMR for colorectal tumors regarding en bloc resection rate and local recurrence rate, without increasing the procedure-related complication rate. The disadvantage of ESD for the treatment of colorectal tumors was the prolonged operation time. Yet, more high quality randomized controlled clinical trials in colorectal tumors are needed to validate the effectiveness of ESD.
Endoscopic submucosal dissection (ESD) was originally developed for en bloc resection of lager, flat gastrointestinal tumors. Compared with endoscopic mucosal resection (EMR), ESD was considered to be more time consuming and have more complications for the treatment of colorectal tumors.
There is no current consensus on the optimal endoscopic method for the treatment of colorectal tumors. Authors conducted a systematic review to compare the efficacy and safety of EMR and ESD for the treatment of colorectal tumors.
Authors designed the meta-analysis to systematically evaluate the two techniques, ESD showed considerable advantages over EMR for colorectal tumors regarding en bloc resection rate and local recurrence rate, without increasing the procedure-related complication rate. They provide evidence for endoscopic treatment of colorectal tumors.
The conclusions of this meta-analysis can help the endoscopists to select the right tool to treat colorectal tumors.
EMR is an endoscopic technique developed for removal of sessile or flat neoplasms confined to the superficial layers (mucosa and submucosa) of the gastrointestinal (GI) tract. A principle EMR consists of three steps: marking, lifting, and cutting. ESD is a newly developed technique in which submucosal dissection is carried out using an electrocautery knife to acquire a single-piece specimen, it is developed for en bloc removal of large (usually more than 2 cm), flat GI tract lesions.
This article of a meta-analysis of ESD vs EMR for the treatment of colorectal tumors with interest. ESD for colorectal lesions is still a developing area with a short history compared to ESD for gastric tumors. Since there is no prospective study assessing the efficacy of colorectal ESD, a meta-analysis of retrospective data of EMR and ESD is thus helpful in comparing the two procedures.
P- Reviewers: Kakushima N, Lee YY S- Editor: Gou SX L- Editor: Ma JY E- Editor: Zhang DN
| 1. | Tamura S, Nakajo K, Yokoyama Y, Ohkawauchi K, Yamada T, Higashidani Y, Miyamoto T, Ueta H, Onishi S. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors. Endoscopy. 2004;36:306-312. [PubMed] [Cited in This Article: ] |
| 2. | Saito Y, Fujii T, Kondo H, Mukai H, Yokota T, Kozu T, Saito D. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy. 2001;33:682-686. [PubMed] [Cited in This Article: ] |
| 3. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Karpińska K, Marlicz W, Milkiewicz P, Starzyńska T. Endoscopic submucosal dissection for the treatment of neoplastic lesions in the gastrointestinal tract. World J Gastroenterol. 2013;19:1953-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. [PubMed] [Cited in This Article: ] |
| 5. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [PubMed] [Cited in This Article: ] |
| 6. | Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [PubMed] [Cited in This Article: ] |
| 8. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 535] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 9. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 10. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Kim YJ, Kim ES, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS. Comparison of clinical outcomes among different endoscopic resection methods for treating colorectal neoplasia. Dig Dis Sci. 2013;58:1727-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 15. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [PubMed] [Cited in This Article: ] |
| 17. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [PubMed] [Cited in This Article: ] |
| 18. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [Cited in This Article: ] |
| 19. | Yamamoto H, Koiwai H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Utsunomiya K, Ido K, Sugano K. A successful single-step endoscopic resection of a 40 millimeter flat-elevated tumor in the rectum: endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:701-704. [PubMed] [Cited in This Article: ] |
| 20. | Kato M. Endoscopic submucosal dissection (ESD) is being accepted as a new procedure of endoscopic treatment of early gastric cancer. Intern Med. 2005;44:85-86. [PubMed] [Cited in This Article: ] |
| 21. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 298] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 22. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |