Published online Jan 14, 2013. doi: 10.3748/wjg.v19.i2.219
Revised: October 24, 2012
Accepted: November 14, 2012
Published online: January 14, 2013
AIM: To investigate the diagnostic performance of acoustic radiation force impulse (ARFI) elastography for characterizing focal liver mass by quantifying their stiffness.
METHODS: This prospective study included 62 patients with a focal liver mass that was well visualized on conventional ultrasonography performed in our institution from February 2011 to November 2011. Among them, 12 patients were excluded for ARFI measurement failure due to a lesion that was smaller than the region of the interest and at an inaccessible location (deeper than 8 cm) (n = 7) or poor compliance to hold their breath as required (n = 5). Finally, 50 patients with valid ARFI measurements were enrolled. If a patient had multiple liver masses, only one mass of interest was chosen. The masses were diagnosed by histological examination or clinical diagnostic criteria. During ultrasonographic evaluation, stiffness, expressed as velocity, was checked 10 times per focal liver mass and the surrounding liver parenchyma.
RESULTS: After further excluding three masses that were non-diagnostic on biopsy, a total of 47 focal mass lesions were tested, including 39 (83.0%) malignant masses [24 hepatocellular carcinomas (HCC), seven cholangiocellular carcinomas (CCC), and eight liver metastases] and eight (17.0%) benign masses (five hemangiomas and three focal nodular hyperplasias, FNH). Thirty-seven (74.0%) masses were confirmed by histological examination. The mean velocity was 2.48 m/s in HCCs, 1.65 m/s in CCCs, 2.35 m/s in metastases, 1.83 m/s in hemangiomas, and 0.97 m/s in FNHs. Although considerable overlap was still noted between malignant and benign masses, significant differences in ARFI values were observed between malignant and benign masses (mean 2.31 m/s vs 1.51 m/s, P = 0.047), as well as between HCCs and benign masses (mean 2.48 m/s vs 1.51 m/s, P = 0.006). The areas under the receiver operating characteristics curves (AUROC) for discriminating the malignant masses from benign masses was 0.724 (95%CI, 0.566-0.883, P = 0.048), and the AUROC for discriminating HCCs from benign masses was 0.813 (95%CI, 0.649-0.976, P = 0.008). To maximize the sum of sensitivity and specificity, an ARFI value of 1.82 m/s was selected as the cutoff value to differentiate malignant from benign liver masses. Furthermore, the cutoff value for distinguishing HCCs from benign masses was also determined to be 1.82 m/s. The diagnostic performance of the sum of the ARFI values for focal liver masses and the surrounding liver parenchyma to differentiate liver masses improved (AUROC = 0.853; 95%CI, 0.745-0.960; P = 0.002 in malignant liver masses vs benign ones and AUROC = 0.948; 95%CI, 0.896-0.992, P < 0.001 in HCCs vs benign masses).
CONCLUSION: ARFI elastography provides additional information for the differential diagnosis of liver masses. However, our results should be interpreted in clinical context, because considerable overlap in ARFI values existed among liver masses.
- Citation: Park H, Park JY, Kim DY, Ahn SH, Chon CY, Han KH, Kim SU. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol 2013; 19(2): 219-226
- URL: https://www.wjgnet.com/1007-9327/full/v19/i2/219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i2.219
Focal liver masses are being discovered at increasing rates due to the wide accessibility of modern high resolution imaging procedures. Conventional ultrasonography (US) is typically used as a first imaging modality to evaluate a focal liver mass. The differential diagnosis of a focal liver mass using imaging studies is based on the characteristics of the surrounding liver parenchyma and underlying clinical conditions such as cirrhosis and the characteristics of the mass itself. Although contrast-enhanced US, computed tomography (CT), and magnetic resonance imaging (MRI) can assess the morphology of a focal liver mass and its vasculature with a high level of diagnostic accuracy[1-5], patients are exposed to potential risks, including contrast medium-induced side-effects and irradiation hazards[6], particularly when repeated examinations are required.
Acoustic radiation force impulse (ARFI) elastography has been introduced as a new ultrasound imaging modality to evaluate tissue stiffness using the radiation forced-based imaging method. The tissue response to the radiation force is observed using conventional B-mode imaging pulses, and it is possible to display the quantitative shear wave velocity (m/s) of the ARFI image[7,8]. Because velocity is directly related to tissue stiffness, ARFI imaging can be applied to evaluate tissue elasticity. Generally, the stiffer the tissue, the faster the shear wave propagates[6,9-13]. Although several reports have indicated a good correlation of ARFI elastography with the other elastography systems such as transient elastography[14,15] and with histological fibrosis grade[14,16,17], ARFI elastography differs from other elastography systems which apply pressure manually to the surface of the organs or a mechanical vibration to induce an elastic shear wave with only M-mode US imaging. Instead, ARFI uses short-duration acoustic pulses generated from a probe under real-time B-mode imaging to produce localized displacements in tissue[18-20]. Furthermore, because ARFI elastography uses elastography with a flexible metering box of the region of the interest (ROI), it is the only elastography method suitable for quantifying focal liver mass stiffness.
Few reports have investigated the applicability of ARFI elastography to evaluate focal liver masses[6,20-25]. Thus, we prospectively recruited patients with a focal liver mass and investigated the diagnostic performance of ARFI elastography to discriminate malignant liver masses and hepatocellular carcinoma (HCC) from benign masses by quantifying their stiffness.
From February 2011 to November 2011, a total of 62 patients with a focal liver mass that was well visualized on conventional US were prospectively recruited for this study. The subjects were referred to our institute for further evaluation of a focal liver mass from primary or secondary clinics, or had been diagnosed with a focal liver mass during a surveillance examination at our institute. Of these, 12 patients were excluded for the following reasons: (1) ARFI measurement failure due to a lesion that was smaller than the ROI and at an inaccessible location (deeper than 8 cm) (n = 7) or (2) poor compliance or inability to hold their breath as required (n = 5). Finally, 50 patients with valid ARFI measurements were enrolled. If a patient had multiple liver masses, only one mass of interest was chosen.
This study was approved by the institutional review board at Severance Hospital in Seoul, South Korea, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants.
We performed a targeted biopsy on masses when a confirmatory histological diagnosis was needed or the radiological diagnosis was not confirmative. Among the 50 masses in our study, 37 (74.0%) received targeted biopsy for histological confirmation. Masses that had any component of cholangiocellular carcinomas (CCC) or combined HCC (CHCC) were classified as CCC and the others as HCC.
A clinical diagnosis of HCC was made according to the American Association for the Study of Liver Disease recommendation[26]. Briefly, patients were diagnosed with HCC if they had a tumor with a maximum diameter > 2 cm and exhibited typical features of HCC on dynamic CT, defined as enhancement in the arterial phase, early washout on the portal phase, and an α-fetoprotein level > 200 ng/mL. Overall, nine patients satisfied these criteria sufficiently to be diagnosed with HCC without a histological examination.
The presence of a hemangioma was diagnosed clinically in four patients, based on a combination of typical findings, determined using CT or MRI, and a lack of growth for at least 12 mo[22]. A hemangioma appears as a mass with hypoattenuation on unenhanced CT, very high signal intensity on T2-weighted images, peripheral nodular enhancement in the arterial phase, and progressive filling-in of enhancement with no washout in later phases[20].
ARFI elastography was performed with an Acuson S2000 ultrasound system (Siemens, Erlanger, Germany), using a 4-1 MHz curved array probe. ARFI elastography was performed by a single physician in all patients (2 years of experience with US and more than 100 examinations with ARFI elastography) who was blinded with regard to the clinical and biochemical data. For patients who underwent liver biopsy, ARFI elastography was performed just before the biopsy, on the same day. For patients with a solitary liver mass that was clinically diagnosed, ARFI elastography was performed at the time of enrollment in this study.
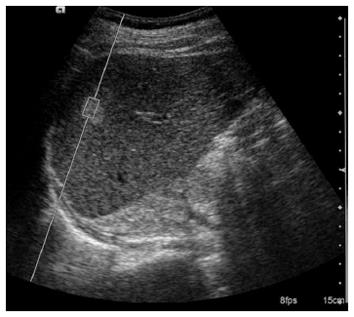
Details of the technical background and examination procedure have been described previously[27]. Briefly, a B-mode US image of the lesion was identified utilizing a ROI, characterized by a box with a fixed dimension of 1 cm × 0.5 cm and a maximum depth of 8 cm. The ROI was entirely located in the lesion, and the ROI location was changed to cover large masses as much as possible without including any vascular or biliary structures (Figure 1). The potential presence of degeneration, such as necrotic, cystic, or calcified portions, was not included in the ROI. To evaluate background liver status of the focal mass, measurements were also taken in the surrounding liver parenchyma with the ROI within 2-3 cm from the target mass, taking care not to comprise any vascular or biliary structure. To ensure quality of the ARFI measurement, 10 measurements were performed for each mass and surrounding liver parenchyma.
Continuous variables are expressed as medians and ranges. The χ2 test or Fisher’s exact test and the Mann-Whitney test were used to compare categorical and continuous variables, respectively. Receiver operating characteristics (ROC) curves and areas under the ROC curves (AUROC) were used to estimate diagnostic performance. The cutoff ARFI value for maximal diagnostic accuracy was selected by considering the highest sum of sensitivity and specificity. A P-value < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS software ver. 18.0.0 (Chicago, IL, United States).
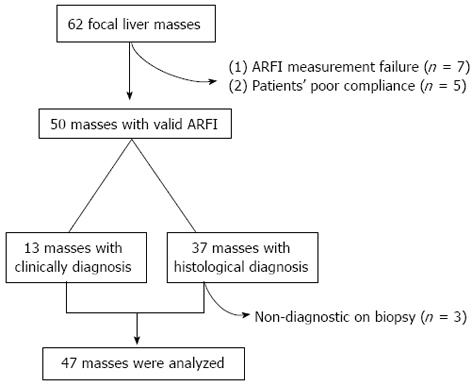
We further excluded three masses that were non-diagnostic on biopsy due to a lack of sufficient tissue or ineffective targeting of the mass. Thus, 47 masses (34 histologically confirmed and 13 clinically diagnosed) were evaluated (Figure 2).
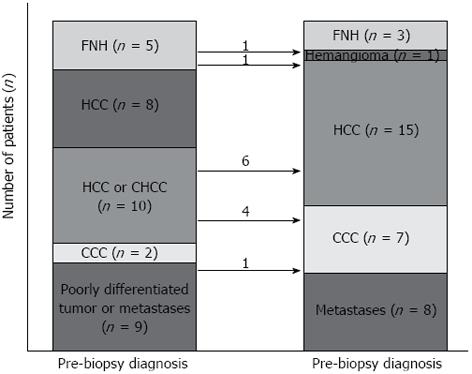
Overall, 15 HCCs, eight metastases (three from colorectal cancers, two from gallbladder cancer, one from pancreatic cancer, one from a gastrointestinal stromal tumor, and one from cervical cancer), seven CCCs, three focal nodular hyperplasias (FNH), and one hemangioma were diagnosed. Figure 3 describes pre-biopsy and post-biopsy diagnoses: 21 (61.8%) masses were consistent with the pre-biopsy diagnosis, and 10 masses diagnosed as HCC or CHCC at the time of pre-biopsy were finally confirmed as six HCCs and four CCCs through the biopsy. The histological diagnosis of the other three masses changed (two FNHs to one hemangioma and one HCC; one poorly-differentiated malignancy or metastatic mass to CCC).
The depth, location (right vs left lobe), and size of each focal liver mass are described in Table 1. ARFI values of HCCs and metastases were higher (2.48 and 2.35 m/s) than those of hemangiomas (1.83 m/s), CCCs (1.65 m/s), and FNH (0.97 m/s). ARFI values for CCCs, hemangiomas, and metastases were significantly higher than those for their surrounding liver parenchyma (mean 1.65 vs 1.07 m/s in CCCs; 1.83 m/s vs 1.10 m/s in hemangiomas; 2.35 m/s vs 1.45 m/s in metastases; all P < 0.05), whereas ARFI values for HCCs were similar to those of surrounding liver parenchyma (2.48 m/s vs 2.14 m/s, P = 0.134).
| Variables | Total masses (n = 47) | HCC (n = 24) | CCC (n = 7) | Metastases (n = 8) | Hemangioma (n = 5) | FNH (n = 3) |
| Histologic confirmation | 34 (72.3) | 15 (62.5) | 7 (100) | 8 (100) | 1 (20.0) | 3 (100) |
| Size, cm | 5.0 (1.4-20.5) | 4.9 (1.4-18.3) | 9.4 (7.4-18.8) | 8.0 (1.6-20.5) | 1.9 (1.5-4.7) | 2.7 (2.0-3.0) |
| Depth, cm | 5.6 (2.7-7.8) | 5.7 (2.7-7.8) | 6.4 (4.8-7.2) | 4.9 (3.5-7.6) | 5.3 (4.5-6.5) | 5.6 (2.8-7.8) |
| Right/left lobe | 34 (72.3)/13 (27.7) | 19 (79.2)/5 (20.8) | 5 (71.4)/2 (28.6) | 5 (62.5)/3 (37.5) | 4 (80.0)/1 (20.0) | 1 (33.3)/2 (66.7) |
| ARFI value, m/s | ||||||
| Masses | 2.23 ± 0.98 | 2.48 ± 0.84 | 1.65 ± 1.43 | 2.35 ± 1.18 | 1.83 ± 0.62 | 0.97 ± 0.48 |
| Surrounding parenchyma | 1.83 ± 0.73 | 2.14 ± 0.59 | 1.07 ± 0.49 | 1.45 ± 0.51 | 1.10 ± 0.14 | 1.63 ± 0.40 |
| P value | 0.029 | 0.134 | 0.015 | 0.043 | 0.013 | 0.581 |
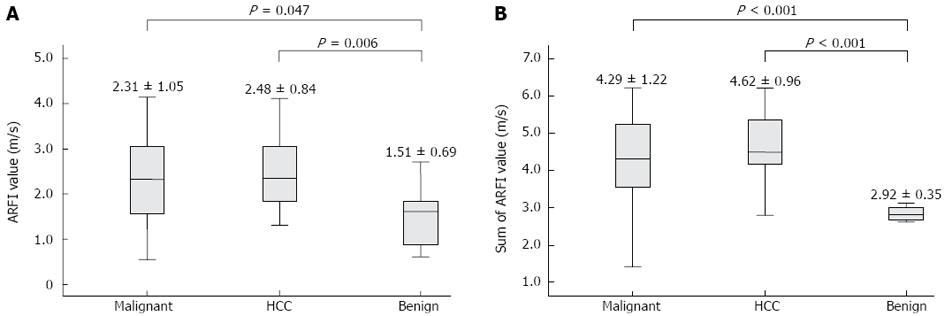
When HCCs, CCCs, and metastases were categorized into a malignant liver mass group (n = 39) and the others were stratified into a benign mass group (n = 8), a significant difference appeared in the between the malignant and benign liver masses (2.31 ± 1.05 m/s vs 1.51 ± 0.69 m/s, P = 0.047) (Figure 4A). However, considerable overlap in ARFI values was noted between malignant and benign masses. Additionally, a significant difference in the ARFI values was observed between the HCCs and the benign masses (2.48 ± 0.84 m/s vs 1.51 ± 0.69 m/s, P = 0.006) (Figure 4A). To address this overlap, we attempted further analysis taking into consideration the ARFI values of surrounding liver parenchyma as well as those of focal liver masses. When ARFI values of focal liver masses and their surrounding liver parenchyma were combined, the differences in ARFI values for each tumor type became more prominent (4.29 ± 1.22 m/s vs 2.92 ± 0.35 m/s for malignant masses vs benign masses, P < 0.001; 4.62 ± 0.96 m/s vs 2.92 ± 0.35 m/s for HCCs vs benign masses, P < 0.001) (Figure 4B).
The AUROC for discriminating the malignant masses from benign masses was 0.724 (95%CI, 0.566-0.883, P = 0.048), and the AUROC for discriminating HCCs from benign masses was 0.813 (95%CI, 0.649-0.976, P = 0.008) (Table 2). To maximize the sum of sensitivity and specificity, an ARFI value of 1.82 m/s was selected as the cutoff value to differentiate malignant from benign liver masses. Furthermore, the cutoff value for distinguishing HCCs from benign masses was also determined to be 1.82 m/s (Table 2). Additionally, the diagnostic performance of the sum of the ARFI values for focal liver masses and the surrounding liver parenchyma to differentiate liver masses improved (AUROC = 0.853; 95%CI, 0.745-0.960; P = 0.002 in malignant liver masses vs benign ones and AUROC = 0.948; 95%CI, 0.896-0.992, P < 0.001 in HCCs vs benign masses) (Table 2).
| Malignant vs benign | HCC vs benign | |||
| Masses | Masses with surrounding liver parenchyma | Masses | Masses with surrounding liver parenchyma | |
| Cutoff ARFI value, m/s | 1.821 | 3.722 | 1.821 | 3.792 |
| AUROC (95%CI) | 0.724 (0.566-0.883) | 0.853 (0.745-0.960) | 0.813 (0.649-0.976) | 0.948 (0.896-0.992) |
| Sensitivity, % | 71.8 | 71.8 | 79.2 | 87.5 |
| Specificity, % | 75.0 | 100 | 75.0 | 100 |
| Positive predictive value, % | 93.3 | 100 | 90.5 | 100 |
| Negative predictive value, % | 35.3 | 42.1 | 54.5 | 72.9 |
ARFI elastography has been proposed as a new method for assessing liver stiffness[7-9]. Although ARFI elastography uses shear wave velocity (m/s) to assess liver stiffness, which is similar to FibroScan®, it exhibits several unique properties. First, ARFI elastography allows for the evaluation of deep tissue by generating a shear wave without the need for exertional compression[7,28]. Second, ARFI elastography has the distinct advantage of being integrated into a conventional US system and can provide additional real-time information during a conventional US study[14,15]. Third, ARFI elastography can be performed regardless of the presence of impediments such as ascites, although the reproducibility in this setting should be further investigated[19,29]. Finally, ARFI elastography offers a flexible metering box at variable depths, allowing the examination of specific liver areas[21,30]. Due to these characteristics, the clinical applicability of ARFI elastography has expanded to characterize and distinguish focal liver masses beyond a simple assessment of liver fibrosis[6,20-25].
According to previous studies[22,24,25], malignant liver masses are stiffer than benign masses, as reflected by higher ARFI values. Consistent with this finding, the ARFI values of malignant liver masses (2.31 m/s) or HCCs (2.48 m/s) in our cohort were significantly higher than those of benign masses (1.51 m/s) (all P < 0.05). However, when we consider that two types of malignant liver masses (HCCs and CCCs) with different ARFI values were stratified into one malignant mass group in our study, the accuracy of ARFI elastography for identifying malignant liver masses would change according to the proportion of HCCs and CCCs. Thus, an exact comparison between the ARFI values of HCCs and CCCs as well as those of benign masses should be performed in future, larger-scale studies. Additionally, the respective ARFI values of HCCs and metastatic masses in our study were 2.48 m/s and 2.35 m/s, which were similar to data from previous studies (2.45 to 2.63 m/s in HCC and 2.18 to 2.88 m/s in metastasis)[22,23].
ARFI values for hemangiomas vary among reports[6,20,22,23]. The ARFI values of hemangiomas reported in two previous studies (1.51 and 1.75 m/s, respectively) were comparable to our data (1.83 m/s)[20,22,23], whereas the ARFI value for a hemangioma in another study was rather higher (2.36 m/s)[22,23]. The variability in hemangioma ARFI values has been explained based on the amount of fibrotic septa that divide the dilated vascular spaces[22]. That is, hemangiomas are composed of multiple vascular channels filled with blood so they would not be expected to be stiff, and therefore would have low ARFI values; in contrast, those including pathological patterns such as sclerosis, thrombosis of the vessels, or calcification, would be stiff and have high ARFI values[20]. Because relatively few hemangioma cases were analyzed in these studies, this discrepancy will need to be investigated via further study including a larger number of cases.
Some researchers have attempted to suggest a cutoff ARFI value for distinguishing malignant liver masses from benign masses. We obtained a high PPV (malignant masses vs benign masses, PPV 93.3%; HCC vs benign masses, 90.5%) using the cutoff ARFI value of 1.82 m/s, which maximized the sum of the sensitivity and specificity for distinguishing masses. Our cutoff value of 1.82 m/s for identifying malignant liver masses was slightly lower than those in previous studies (2.0 to 2.5 m/s)[22,24,25], which can be partially explained by the different methods used to categorize the masses. In one study, which reported a rather high cutoff value of 2.5 m/s, only metastases that had a high ARFI value of 4.18 m/s were categorized into a malignant group and compared with benign masses[24], whereas another study that reported 2.22 m/s as a cutoff value for discriminating malignant masses from benign masses included FNHs, adenomas, and focal fatty change lesions in the benign mass group as well as hemangiomas[25]. Thus, in this regard, further study is needed to prevent potential bias due to heterogeneity of the masses and to confirm the clinically applicable cutoff values for ARFI elastography. Although our cutoff value may be useful to physicians who encounter focal liver masses during routine US evaluation, it should be interpreted cautiously in the clinical context for several reasons. First, although our cutoff value would correctly characterize HCC and metastasis, it would mischaracterize CCC with a relatively low ARFI value and hemangioma with a relatively high ARFI value. Second, there was considerable overlap of ARFI values between malignant and benign masses.
Underlying fibrosis of the liver is a consideration for the differential diagnosis of a focal liver mass. Several previous studies have proposed that HCCs generally appear softer (lower ARFI values) than the surrounding liver[21], whereas metastases and hemangiomas generally appear harder than that of the surrounding liver despite some controversies among studies[21]. Similarly, we found that CCCs, hemangiomas, and metastases had higher ARFI values than those of surrounding liver parenchyma, whereas ARFI values of HCCs were statistically equivalent to those of the surrounding liver parenchyma. Because hemangiomas and metastases were evaluated in patients without chronic liver disease in most studies[6,25], ARFI values seemed consistently higher than those of a background liver. In contrast, because HCCs were evaluated in patients with chronic liver disease and diverse degrees of background liver fibrosis, the comparative results between ARFI values of HCCs and those of background liver differed among studies based on the characteristics of each study cohort[6,21,25]. That is, simultaneously measuring ARFI values of focal liver mass and the surrounding liver should focus on assessing the respective characteristics of the hepatic mass and surrounding fibrosis to prevent a misdiagnosis of the hepatic mass using the correlation of ARFI values between the liver mass and background liver parenchyma. However, when we used the sum of ARFI values of focal liver mass and the surrounding liver parenchyma, the diagnostic performance in terms of distinguishing liver mass improved. Thus, these controversial findings concerning the simultaneous measurement of liver masses and their surrounding liver parenchyma should be further investigated in future larger-scale studies.
Although most cases (72.3%) were histologically confirmed in our study, the relative small sample size of our cohort and inclusion of patients with high ALT, which has the potential of overestimating influences on ARFI values[30,31], are potential limitations. Although ARFI elastography can freely locate the ROI box in a specific area within a mass and measure its stiffness, morphological characteristics of liver masses including heterogeneous components such as HCC with hemorrhage and fatty metamorphosis[6,20,32] and lesion shapes were not considered in our study, which is a limitation of this study. Further investigation of how to evaluate such heterogeneous or morphologically varying liver masses using ARFI elastography and their influences on ARFI values of liver masses should be conducted.
In our study, we demonstrated the potential clinical utility of ARFI elastography for characterization of focal liver masses. Although this study had limitations and should be interpreted cautiously, our findings provide a useful reference for the differential diagnosis of a focal liver mass and will provide additional information to clinicians who are confronted with a need for an immediate diagnosis of a focal liver mass during a routine US examination before a further diagnostic imaging study such as contrast-enhanced US, CT or MRI. However, further studies with larger numbers of cases are warranted to assess the utility of ARFI elastography in the clinic.
The authors are grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, South Korea) for his help with the figures.
Acoustic radiation force impulse (ARFI) elastography can quantify tissue elasticity. Compared to previous elastography techniques, one of the distinguishing advantages of ARFI elastography is that it offers a flexible metering box at variable depths, which allows an examination of specific liver areas. With using this characteristic of ARFI elastography, authors investigated its applicability to evaluate focal liver masses.
According to previous studies, malignant liver masses are stiffer than benign masses, as reflected by higher ARFI values. However, previous studies have showed somewhat controversial results with various median ARFI values for each focal liver mass and they have had limitations, such as most subjects not being supported by adequate histological confirmation.
Authors prospectively investigated 50 focal liver masses found during routine ultrasonography (US) study, and measured the stiffness of each focal liver mass using ARFI elastography. Focal liver masses included hepatocellular carcinomas (HCCs), cholangiocellular carcinomas, metastases, hemangiomas, and focal nodular hyperplasia. The advantage of our study over other previous studies is that majority of our subjects (72.3%) are confirmed with histological examinations.
Using ARFI elastography, physicians who are confronted with a need for an immediate diagnosis of a focal liver mass during a routine US examination can get additional information for the differential diagnosis of liver masses in clinical practice. Further future studies with larger numbers of cases are warranted to assess the utility of ARFI elastography.
ARFI elastography is an emerging examination which can quantify tissue elasticity. ARFI elastography has the distinct advantage of being integrated into a conventional US system and can be checked simultaneously during a conventional US study. Furthermore, because ARFI elastography uses elastography with a flexible metering box of the region of the interest, it is suitable for quantifying focal liver mass stiffness.
Although this study had limitations and should be interpreted cautiously, their results show the clinical applicability of ARFI elastography as a complementary diagnostic tool for the differential diagnosis of liver masses.
P- Reviewers Di Carlo I, Montet X S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009;15:3217-3227. [PubMed] [Cited in This Article: ] |
| 2. | Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748-3756. [PubMed] [Cited in This Article: ] |
| 3. | Vilgrain V. Advancement in HCC imaging: diagnosis, staging and treatment efficacy assessments: hepatocellular carcinoma: imaging in assessing treatment efficacy. J Hepatobiliary Pancreat Sci. 2010;17:374-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Hohmann J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147-159. [PubMed] [Cited in This Article: ] |
| 5. | Numminen K, Isoniemi H, Halavaara J, Tervahartiala P, Makisalo H, Laasonen L, Hockerstedt K. Preoperative assessment of focal liver lesions: multidetector computed tomography challenges magnetic resonance imaging. Acta Radiol. 2005;46:9-15. [PubMed] [Cited in This Article: ] |
| 6. | Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Fahey BJ, Nightingale KR, Nelson RC, Palmeri ML, Trahey GE. Acoustic radiation force impulse imaging of the abdomen: demonstration of feasibility and utility. Ultrasound Med Biol. 2005;31:1185-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Nightingale K, Palmeri M, Trahey G. Analysis of contrast in images generated with transient acoustic radiation force. Ultrasound Med Biol. 2006;32:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Kim SU, Jang HW, Cheong JY, Kim JK, Lee MH, Kim DJ, Yang JM, Cho SW, Lee KS, Choi EH. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol. 2011;26:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] [Cited in This Article: ] |
| 13. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 15. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] [Cited in This Article: ] |
| 16. | Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J, Bertrais S, Gallois Y, Calès P, Aubé C. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Yu H, Wilson SR. Differentiation of benign from malignant liver masses with Acoustic Radiation Force Impulse technique. Ultrasound Q. 2011;27:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE. In vivo visualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol. 2008;53:279-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Heide R, Strobel D, Bernatik T, Goertz RS. Characterization of focal liver lesions (FLL) with acoustic radiation force impulse (ARFI) elastometry. Ultraschall Med. 2010;31:405-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Davies G, Koenen M. Acoustic radiation force impulse elastography in distinguishing hepatic haemangiomata from metastases: preliminary observations. Br J Radiol. 2011;84:939-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Shuang-Ming T, Ping Z, Ying Q, Li-Rong C, Ping Z, Rui-Zhen L. Usefulness of acoustic radiation force impulse imaging in the differential diagnosis of benign and malignant liver lesions. Acad Radiol. 2011;18:810-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4333] [Cited by in F6Publishing: 4404] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 27. | Son CY, Kim SU, Han WK, Choi GH, Park H, Yang SC, Choi JS, Park JY, Kim do Y, Ahn SH. Normal liver elasticity values using acoustic radiation force impulse imaging: a prospective study in healthy living liver and kidney donors. J Gastroenterol Hepatol. 2012;27:130-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | Lim SM, Kim SU. Acoustic radiation force impulse elastography is useful to exclude nonliver-related ascites. Eur J Gastroenterol Hepatol. 2011;23:1080-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 30. | Yoon KT, Lim SM, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Cho M, Lee JW, Kim SU. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci. 2012;57:1682-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Lim SM, Chung MJ, Han KH, Kim SU. Acoustic radiation force impulse elastography: a better option for patients with extrahepatic cholestasis. Eur J Gastroenterol Hepatol. 2012;24:215-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Kwon HJ, Kang MJ, Cho JH, Oh JY, Nam KJ, Han SY, Lee SW. Acoustic radiation force impulse elastography for hepatocellular carcinoma-associated radiofrequency ablation. World J Gastroenterol. 2011;17:1874-1878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |