Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3089
Revised: March 20, 2012
Accepted: April 9, 2012
Published online: June 28, 2012
AIM: To investigate the impact of different anesthetic techniques on T-helper (Th) cell subsets in hepatocellular carcinoma (HCC) patients undergoing hepatectomy.
METHODS: Sixty-one HCC patients who received hepatectomies were randomized into an epidural combined general anesthesia (G + E; n = 31) or a general anesthesia (G; n = 30) group. Blood samples were obtained the morning before the operation (d0), and on the second (d2) and seventh (d7) day after the operation. Th cell contents were evaluated using flow cytometry, real-time reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay.
RESULTS: In all 61 patients, Th1 and Th2 cell frequencies, and interferon-γ (IFN-γ) mRNA expression markedly increased on d2, compared to d0. They recovered slightly on d7, and the Th1/Th2 ratio increased markedly on d7, compared with d2. In contrast, Th17, regulatory T cell (Treg), and interleukin-17 (IL-17) levels and FOXP3 mRNA expression showed no significant change on d2, and then markedly decreased on d7. Similarly, plasma IFN-γ concentration on d2 was much higher than that on d0, and then partly recovered on d7. As compared with the G group, in the G + E group, Th1 cell frequencies and the Th1/Th2 ratio were slightly higher on d2 and significantly higher on d7, while Th2, Th17, and Treg cell frequencies were slightly lower on d2, and significantly lower on d7. Consistently, on d7, IFN-γ mRNA and protein levels and the IFN-γ/IL-4 ratio in the G + E group were higher than those in the G group. In contrast, the IL-17 mRNA level, and IL-17 and transforming growth factor-β1 concentrations in the G + E group were lower than those in the G group.
CONCLUSION: G + E is superior to G in shifting the Th1/Th2 balance towards Th1, while decreasing Th17 and Treg, potentially benefiting HCC patients by promoting anti-tumor Th polarization.
- Citation: Zhou D, Gu FM, Gao Q, Li QL, Zhou J, Miao CH. Effects of anesthetic methods on preserving anti-tumor T-helper polarization following hepatectomy. World J Gastroenterol 2012; 18(24): 3089-3098
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3089
The overall survival of patients with hepatocellular carcinoma (HCC) remains poor despite improved diagnostic and treatment strategies[1]. Although surgical resection is still one of the first priorities[1], surgery may inevitably induce profound systemic neuroendocrine, metabolic, inflammatory, and immunological stress[2,3]. In particular, alterations occur in every component of the immune response, including T-helper (Th) cells[4]. As a consequence, the stress response could lead to significant changes in post-operative recurrence rates and patient survival[5,6].
Th cells are sub-groups of lymphocytes that play a central role in orchestrating host immune responses through their capacity to help other cells in the immune system[7]. In the scenario of cancer, Th1 cells mediate anti-tumor reactivity, by producing interferon-γ (IFN-γ), resulting in tumor regression[8]. In contrast, most studies have shown that Th2, Th17, and regulatory T cell (Treg) cells may inhibit anti-tumor efficacy[9,10]. The Th subsets are known for their altered frequencies, distribution and balance in cancer-bearing patients. More importantly, recent research has revealed that the balance of Th subsets determines the direction of anti-tumor immune responses and hence patient clinical outcomes[11,12]. Proper peri-operative management, including selection of suitable anesthetic methods, may help recover the disturbed balances of Th subsets or even maintain the balance of anti-tumor responses.
Epidural anesthesia is known to prevent or attenuate an excessive stress response during or after surgery, which prevents noxious afferent input from reaching the central nervous system[13]. Both preclinical and clinical studies have suggested that the addition of spinal blockade to general anesthesia attenuates the metastasis-promoting effect of surgery in the tumor-bearing host[14,15]. Currently, general anesthesia alone, and epidural blockade combined with general anesthesia are the two most commonly used methods in hepatectomy. However, there is a lack of clinical studies evaluating the effects of hepatectomy itself, as well as the choice of anesthetic technique on peri-operative Th cell subset balances.
In this study, we investigate Th cell subset balances in the peripheral blood of HCC patients before surgery, and on 2 and 7 d after hepatectomy, to elucidate the changes in Th frequencies induced by surgery. In particular, we compared the differences in Th cell subsets between the epidural anesthesia combined with general anesthesia (G + E) group and the general anesthesia (G) group at multiple peri-operative time points to determine whether anesthetic methods have an impact on postoperative Th subset balances.
Sixty-one patients with HCC who had undergone hepatectomies between October 2009 and April 2010 at the Liver Cancer Institute, Zhongshan Hospital of Fudan University (Shanghai, China) were enrolled. We randomized the patients between 18-65 years old, American Society of Anesthesiologists (ASA) scores I-II, with normal leukocyte and lymphocyte counts, who had an imaging diagnosis of HCC with the intention of curative surgery and without distant metastasis or any prior anti-cancer treatments into two groups, that is, the G + E group and G group. We then excluded patients who were not pathologically diagnosed as HCC or who were given a blood transfusion during or after surgery. We finally chose 61 out of 70 patients, 31 in the G + E group and 30 in the G group. Fourteen cases received right lobectomy, 11 cases left hemihepatectomy and 36 cases segmentectomy. The study was approved by the Research Ethics Committee of Zhongshan Hospital, and informed consent was obtained from all patients.
All patients were free of preoperative medication and had fasted for over 8 h. Epidural puncture for patients in the G + E group was carried out at T8 and T9 into the epidural space, with patients in a left-lateral position. After successful puncture, epidural catheters were placed toward the head for 3-4 cm. We fixed the catheters and gave 2% lidocaine (3 mL) through the catheters. After 5 min, upon confirming no spinal analgesia and that the epidural block was successful, we gave the patients 0.375% bupivacaine liquor (8 mL), then 4 mL at each 50-min interval.
Induction and maintenance of general anesthesia: All patients were induced with 5 μg/kg fentanyl, 2.0 mg/kg propofol and 0.9 mg/kg rocuronium and then incubated for approximately 90 s. Anesthesia was maintained with sevoflurane (1.5%-3.5%) based on a bispectral index monitor.
Postoperative analgesia: Patients in the G + E group used postoperative patient-controlled epidural analgesia, made up of 0.125% bupivacaine + morphine (30 μg/mL). The patients in the G group used postoperative patient-controlled intravenous analgesia containing morphine (0.4 mg/mL) administered via a jugular vein catheter. Patient-controlled analgesia was administered via an ambulatory infusion pump (AutoMed, Acemedical AM3400) and lasted for about 48 h. All patients were followed up at the end of analgesia using a visual analogue scale (VAS) for evaluation of their pain levels, and the corresponding drug dosage used was recorded.
Blood samples (10 mL) were obtained from all patients in the recumbent position with a 20-gauge needle for a clean veni-puncture of an antecubital vein at three time points: early morning on the operation day (d0), and the second (d2) and seventh day (d7) after the operation. The samples were collected into tubes containing 0.2 mL sodium heparin. Peripheral blood mononuclear cells (PBMCs) were prepared using a Ficoll density gradient, for flow cytometry and real-time polymerase-chain-reaction analyses. Plasma was obtained after centrifugation and stored at -80 °C for measurement of cytokines.
For analyses of Th1, Th2 and Th17, PBMCs were suspended at a density of 2 × 106 cells/mL in complete culture medium (RPMI 1640 supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol glutamine and 10% heat-inactivated fetal calf serum). The cell suspension was transferred to individual wells of 24-well plates. Cultures were stimulated with phorbol myristate acetate (50 ng/mL), ionomycin (1 μmol) and Brefeldin A (10 μg/mL, all from Sigma-Aldrich, St. Louis, MO, United States) for 4 h. The cultures were incubated at 37 °C, in a 5% CO2 environment. After 4 h of culture, the contents of the well were transferred to 1.5 mL sterile centrifuge tubes. To analyze Tregs, PBMCs were aliquoted into tubes for further staining.
For Th1, Th2 and Th17 analyses, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 at 4 °C for 20 min. For Treg analysis, the cells were incubated with FITC-conjugated anti-human CD4 and allophycocyanin (APC)-conjugated anti-human CD25 monoclonal antibodies (mAbs). After surface staining, the cells were stained with R-phycoerythrin (PE)-conjugated anti-human IFN-γ, APC-conjugated anti-human interleukin (IL)-4 and IL-17 mAbs for detection of Th1, Th2 and Th17, respectively; or PE-conjugated anti-human FOXP3 mAb for Treg detection after fixation and permeabilization, according to the manufacturer’s instructions. Isotype controls were given to enable correct compensation and confirm antibody specificity. All antibodies were purchased from eBioscience (San Diego, CA, United States). Stained cells were analyzed using a FACScan cytometer equipped with CellQuest software (BD Bioscience, San Jose, CA, United States).
Total RNA was extracted with TRIzol regent (Invitrogen, Carlsbad, CA, United States) according to the manufac-turer’s instructions. First-strand cDNA was synthesized using random hexamer primers and RNase H-reverse transcriptase (Fermentas, Glen Burnie, MD, United States). TaqMan primers and probes for human IFN-γ, IL-4, IL-17 and FoxP3 were purchased from TAKARA Bio Inc. (Osaka, Japan), and samples were analyzed utilizing the Opticon Monitor 3 System (Bio-Rad, Hercules, CA, United States, formerly MJ Research). The following primer sets were used: IFN-γ: F: 5’-TGCAGGACCCATATGTAAAAGA-3’, R: 5’-TCAAAATGCCTAAGAAAAG-3’; IL-4: F: 5’-TACAGCCACCATGAGAAGGACA-3’, R: 5’-GCCAGGCCCCAGAGGTT-3’; IL-17: F: 5’-CGCTGATGGGAACGTGGACTAC-3’, R: 5’-GGTGGACAATCGGGGTGACA-3’; FoxP3: F: 5’-CGCTGATGGGAACGTGGACTAC-3', R: 5’-GGTGGACAATCGGGGTGACA-3’; and β-actin: F: 5’-CAACTGGGACGACATGGAGAAAAT-3’, R: 5’-CCAGAGGCGTACAGGGATAGCAC-3’. For each sample, the mRNA expression level was normalized to the level of β-actin.
The plasma levels of IFN-γ, IL-4, IL-17, IL-10 and transforming growth factor-β1 (TGF-β1) were measured by enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s instructions (R and D Systems, Minneapolis, MN, United States). Intra-assay and inter-assay coefficients of variation for all ELISAs were < 5% and < 10%, respectively. All samples were measured using three independent experiments, in duplicate.
Statistical analyses were performed with SPSS 16.0 software (SPSS, Chicago, IL, United States). Values are expressed as the mean ± SE of the mean. Student’s t tests were used to compare quantitative variables, and Fisher’s exact tests were used for categorical variables. Two-tailed P < 0.05 was judged to be significant.
There were no significant differences in age, gender, clinicopathologic factors, blood loss, anesthesia time and VAS scores between patients in the G + E group and G group. Because of the different anesthetic methods, the dosage of opioids (fentanyl and morphine) and inhalation anesthetics (sevoflurane) were obviously different between the two groups (Table 1).
| Characteristics | G + E group (n = 31) | G group (n = 30) | P |
| Age (yr) | 50.7 ± 1.8 | 46.9 ± 1.5 | 0.4161 |
| Gender (male/female) | 4/27 | 8/22 | 0.2112 |
| Hepatitis history (yes/no) | 5/26 | 6/24 | 0.7492 |
| AFP ( ≤ 20 ng/mL/> 20 ng/mL) | 13/18 | 14/16 | 0.7992 |
| Liver cirrhosis (Yes/no) | 10/21 | 7/23 | 0.5702 |
| ALT ( ≤ 40 U/L/> 40 U/L) | 18/13 | 11/19 | 0.7952 |
| Child-Pugh Score (A/B) | 2/29 | 2/28 | 1.0002 |
| Tumor differentiation (I-II/III-IV) | 7/24 | 9/21 | 0.5702 |
| Tumor size ( ≤ 5 cm/> 5 cm) | 8/23 | 7/23 | 1.0002 |
| Tumor number (single/multiple) | 8/23 | 5/25 | 0.5342 |
| Tumor encapsulation (complete/none) | 12/19 | 15/15 | 0.4442 |
| Vascular invasion (no/yes) | 6/25 | 8/22 | 0.5542 |
| Blood loss ( ≤ 200 mL/> 200 mL) | 1/30 | 4/26 | 0.1952 |
| Anesthesia time ( ≤ 3 h/> 3 h) | 18/13 | 9/21 | 0.4262 |
| VAS score | 3.58 ± 0.32 | 3.67 ± 0.38 | 0.8631 |
| Dose of fentanyl (μg) | 0.30 ± 0.01 | 0.51 ± 0.01 | < 0.0011 |
| Dose of morphine (mg) | 2.26 ± 0.08 | 95.30 ± 1.17 | < 0.0011 |
| Dose of sevoflurane (mL) | 21.74 ± 0.70 | 37.90 ± 1.60 | < 0.0011 |
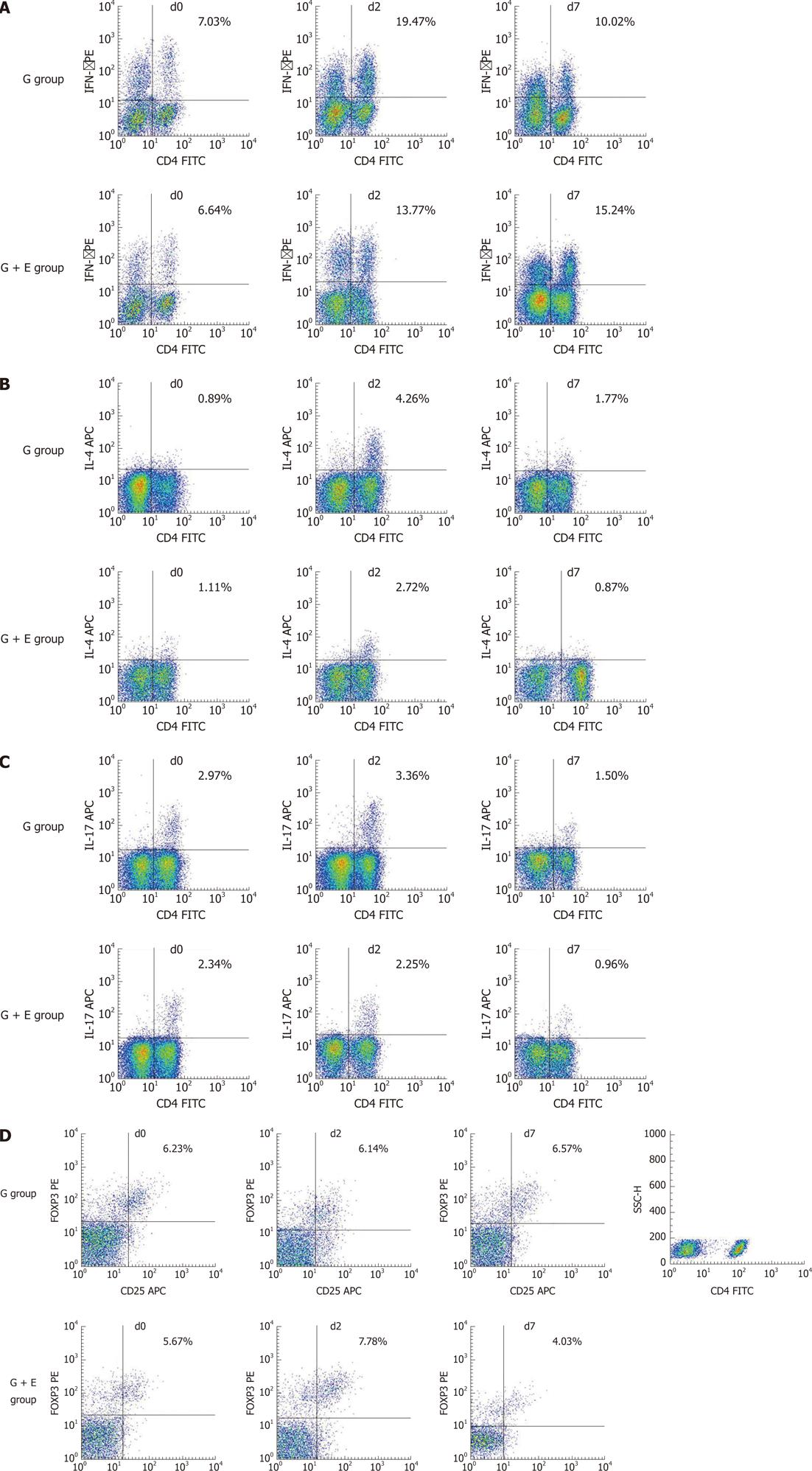
In all 61 patients, the frequencies of Th1 (CD4+IFN-γ+CD4+ T cells) and Th2 (CD4+IL-4+CD4+ T cells) were markedly increased on d2 (P < 0.001 and P < 0.001, respectively), as compared with d0. The frequencies partly recovered on d7 (P = 0.015 and P < 0.001, respectively), while the ratio of Th1/Th2 showed no differences between d0 and d2, but increased significantly on d7 (P < 0.001, Figure 1 and Table 2). In contrast, the frequencies of Th17 (CD4+IL-17+CD4+ T cells) and Treg (CD4+CD25+FoxP3+CD4+ T cells) on d2 showed no significant differences compared to those on d0 (P = 0.269 and P = 0.06, respectively), but markedly decreased on d7 (P < 0.001 and P = 0.021, respectively, Figure 1 and Table 2).
| Variables | d0 (%) | d2 (%) | d7 (%) | P (d0/d2) | P (d0/d7) | P (d2/d7) |
| Th1 | 7.21 ± 1.01 | 18.82 ± 1.93 | 13.40 ± 0.79 | < 0.001 | < 0.001 | 0.015 |
| Th2 | 1.12 ± 0.09 | 3.32 ± 0.31 | 0.93 ± 0.10 | < 0.001 | 0.180 | < 0.001 |
| Th17 | 2.57 ± 0.22 | 2.95 ± 0.26 | 1.23 ± 0.10 | 0.269 | < 0.001 | < 0.001 |
| Treg | 6.02 ± 0.27 | 6.93 ± 0.38 | 5.45 ± 0.52 | 0.160 | 0.329 | 0.021 |
| Th1/Th2 | 8.12 ± 1.24 | 8.50 ± 1.14 | 14.87 ± 1.26 | 0.823 | < 0.001 | < 0.001 |
Further comparisons were made between the G + E group and G group. As expected, Th1, Th2, Th17 and Treg cell frequencies showed no differences on d0 between the G + E group and G group (Table 3). On d2, only Th2 cell frequencies were lower in patients in the G + E group, compared with patients in the G group (P = 0.028), while Th1, Th17, and Treg frequencies, as well as the Th1/Th2 ratio showed no significant differences (Figure 2 and Table 3). Notably, circulating Th1 cell frequencies were considerably higher, but Th2 frequencies showed no significant change in patients in the G + E group when compared with patients in the G group on d7 (P < 0.01 and P = 0.231, respectively). Consequently, on d7, the Th1/Th2 ratio of the G + E group was significantly higher than that in the G group (P < 0.001). Conversely, Th17 and Treg cell frequencies were significantly lower in patients in the G + E group than in patients in the G group on d7 (P = 0.047 and P = 0.003, respectively, Figure 2 and Table 3).
| Variables | G + E group (%) | G group (%) | P |
| Th1 | |||
| d0 | 7.44 ± 1.54 | 7.38 ± 1.35 | 0.87 |
| d2 | 14.66 ± 1.96 | 20.42 ± 2.77 | 0.098 |
| d7 | 16.57 ± 0.96 | 10.37 ± 0.84 | < 0.001 |
| Th2 | |||
| d0 | 1.08 ± 0.14 | 1.14 ± 0.12 | 0.745 |
| d2 | 2.64 ± 0.30 | 3.98 ± 0.49 | 0.028 |
| d7 | 0.73 ± 0.07 | 1.14 ± 0.17 | 0.231 |
| Th17 | |||
| d0 | 2.80 ± 0.26 | 2.65 ± 0.36 | 0.424 |
| d2 | 2.56 ± 0.30 | 3.01 ± 0.38 | 0.374 |
| d7 | 1.08 ± 0.10 | 1.45 ± 0.15 | 0.047 |
| Treg | |||
| d0 | 5.44 ± 0.26 | 6.62 ± 0.45 | 0.372 |
| d2 | 7.42 ± 0.60 | 6.43 ± 0.46 | 0.194 |
| d7 | 4.51 ± 0.56 | 6.19 ± 0.80 | 0.003 |
| Th1/Th2 ratio | |||
| d0 | 8.63 ± 1.76 | 7.62 ± 1.32 | 0.688 |
| d2 | 9.67 ± 1.94 | 7.40 ± 1.26 | 0.253 |
| d7 | 14.64 ± 0.56 | 6.03 ± 0.83 | < 0.001 |
mRNA expression of IFN-γ, IL-4, IL-17 and FoxP3 in PBMCs
IFN-γ, IL-4, and IL-17 are the cytokines typically produced by Th1, Th2 and Th17 cells, respectively[16]. FOXP3 is the master transcription factor in Treg cells[9]. We thus investigated the mRNA expression profile of IFN-γ, IL-4, IL-17 and FOXP3 in PBMCs of HCC patients.
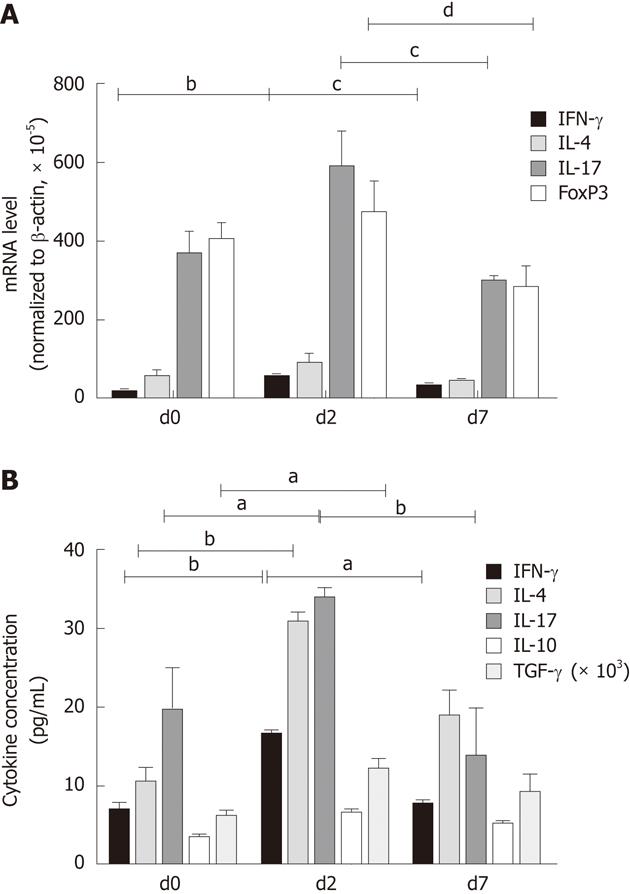
In all 61 patients, the mRNA expression levels of IFN-γ on d2 were increased compared to that on d0 (P < 0.01), then partly recovered on d7 (P = 0.042). IL-4, IL-17 and FOXP3 mRNA expression levels showed no significant differences from d0 to d2, whereas mRNA levels of IL-17 and FOXP3 clearly decreased from d2 to d7 (P = 0.032 and P < 0.01, respectively, Figure 3A).
Similar to Th cell frequencies, IFN-γ, IL-4, IL-17 and FOXP3 mRNA expression levels on d0 showed no significant differences between the two groups (Table 4). On d2, the IL-4 mRNA level in the G + E group was significantly lower as compared with that in the G group (P < 0.01). On d7, the IFN-γ mRNA level in the G + E group and the IFN-γ/IL-4 ratio were significantly higher than that in the G group (P = 0.029 and P = 0.003, respectively); IL-17 mRNA level in the G + E group was significantly lower than that in the G group (P = 0.042); FOXP3 mRNA expression level in the G + E group was relatively lower than that in the G group, but this did not reach statistical significance (P = 0.109, Table 4). The results derived from mRNA expression analyses of Th-cell markers were generally in line with the results of flow cytometry analyses of Th-cell frequencies.
| Variables | G + E group (×105) | G group (×105) | P |
| IFN-γ | |||
| d0 | 21.84 ± 2.99 | 16.49 ± 0.84 | 0.585 |
| d2 | 50.91 ± 1.46 | 62.87 ± 2.71 | 0.096 |
| d7 | 41.05 ± 1.05 | 29.36 ± 0.73 | 0.029 |
| IL-4 | |||
| d0 | 47.12 ± 28.65 | 59.97 ± 15.17 | 0.651 |
| d2 | 66.63 ± 18.45 | 110.12 ± 24.11 | < 0.001 |
| d7 | 31.80 ± 14.59 | 58.21 ± 5.74 | 0.224 |
| IL-17 | |||
| d0 | 351.29 ± 55.29 | 386.64 ± 61.88 | 0.768 |
| d2 | 406.68 ± 68.21 | 677.80 ± 133.07 | 0.171 |
| d7 | 226.20 ± 18.37 | 429.94 ± 11.90 | 0.042 |
| FoxP3 | |||
| d0 | 430.38 ± 48.95 | 384.52 ± 42.19 | 0.408 |
| d2 | 468.85 ± 89.81 | 432.97 ± 44.45 | 0.338 |
| d7 | 248.68 ± 58.71 | 361.01 ± 46.75 | 0.109 |
| IFN-γ/IL-4 ratio | |||
| d0 | 0.44 ± 0.03 | 0.39 ± 0.04 | 0.883 |
| d2 | 0.80 ± 0.10 | 0.58 ± 0.03 | 0.198 |
| d7 | 1.14 ± 0.08 | 0.56 ± 0.01 | 0.003 |
Plasma concentrations of IFN-γ, IL-4, IL-17, IL-10 and TGF-β1
IFN-γ, IL-4 and IL-17 are the cytokines typically produced by Th1, Th2 and Th17 cells, respectively. IL-10 and TGF-β1 are the cytokines produced by Treg cells. In all 61 patients, the plasma IFN-γ, IL-4, IL-17 and TGF-β1 concentrations on d2 were higher than on d0 (P < 0.01, P < 0.01, P = 0.011 and P = 0.021, respectively). Subsequently, IFN-γ and IL-17 levels recovered toward baseline levels on d7 (P = 0.008, P < 0.001, respectively), while IL-4, IL-10 and TGF-β1 showed no obvious decrease (Figure 3B).
The levels of all plasma cytokines on d0 showed no significant differences between the G + E and G groups. On d2, IFN-γ and IL-4 concentrations in the G + E group were significantly lower than those in the G group (P = 0.018 and P = 0.013, respectively). On d7, the IFN-γ/IL-4 ratio in the G + E group was obviously higher than that in the G group (P < 0.001). However, IL-17 and TGF-β1 concentrations in the G + E group were significantly lower than those in the G group (P = 0.009 and P = 0.031, respectively, Table 5). These data were highly consistent with the conclusions from flow cytometry and mRNA expression analyses.
| Variables | G + E group (pg/mL) | G group (pg/mL) | P |
| IFN-γ | |||
| d0 | 7.56 ± 0.44 | 6.67 ± 0.70 | 0.653 |
| d2 | 11.17 ± 0.97 | 19.94 ± 1.37 | 0.018 |
| d7 | 8.67 ± 0.74 | 5.76 ± 0.53 | 0.165 |
| IL-4 | |||
| d0 | 10.31 ± 1.78 | 10.53 ± 1.28 | 0.892 |
| d2 | 21.11 ± 3.76 | 36.69 ± 6.97 | 0.013 |
| d7 | 13.00 ± 1.88 | 25.50 ± 2.98 | 0.065 |
| IL-17 | |||
| d0 | 20.26 ± 5.82 | 18.83 ± 3.95 | 0.304 |
| d2 | 27.26 ± 10.30 | 40.10 ± 10.56 | 0.239 |
| d7 | 9.12 ± 1.67 | 16.66 ± 2.38 | 0.009 |
| IL-10 | |||
| d0 | 3.67 ± 0.35 | 3.37 ± 0.31 | 0.528 |
| d2 | 7.17 ± 0.86 | 5.97 ± 0.48 | 0.218 |
| d7 | 4.09 ± 0.29 | 5.65 ± 0.72 | 0.06 |
| TGF-β1 | |||
| d0 | 6565 ± 1667 | 5312 ± 1102 | 0.539 |
| d2 | 15680 ± 4254 | 8375 ± 1395 | 0.12 |
| d7 | 7555 ± 1555 | 10691 ± 2571 | 0.311 |
| IFN-γ/ IL-4 | |||
| d0 | 0.74 ± 0.10 | 0.65 ± 0.13 | 0.618 |
| d2 | 0.67 ± 0.08 | 0.56 ± 0.05 | 0.309 |
| d7 | 1.10 ± 0.14 | 0.35 ± 0.08 | < 0.001 |
The Th1/Th2 balance, as well as Th17 and Treg levels are key indices of immune function, playing an important role in HCC metastasis and recurrence[10,17]. To assess whether this balance was disturbed during anesthesia and to determine which anesthetic method was better at restoring the balance toward anti-tumor responses in HCC patients after hepatectomy, we examined Th1, Th2, Th17 and Treg levels through the analyses of cell frequencies, mRNA expression of Th-cell markers, and protein levels of typical Th-cell cytokines.
Surgical trauma is generally considered to have a major role in altering the immune response[3,6]. We had thus expected that surgical trauma would have a significant impact on the Th1/Th2 ratio, as well as Th17 and Treg levels after hepatectomy. In this study, we found that, on d2 after hepatectomy, all Th cells showed an increased trend, to varying degrees. By d7 after surgery, all Th cells recovered towards the baseline. These results revealed that surgical trauma increased Th cell numbers, but the stimulation did not last a significantly long time. There was no obvious change in the Th1/Th2 balance on d2 after surgery. However, there was a detectable shift towards Th1 dominance on d7 after surgery. In addition, Treg and Th17 frequencies decreased significantly compared with d0. These results support the conclusion that removal of the tumor leads to the relief of systemic immune suppression. As a result, host responses are reprogrammed towards a direction that benefits patient outcome. Therefore, it is likely that the change in Th1/Th2 balance, as well as Th17 and Treg frequencies in patients undergoing hepatectomy are not only a consequence, but may also play an important role in the defense mechanism against tumor cells. Supporting this idea, it has been reported that surgical removal of primary tumors could reverse tumor-induced immunosuppression, even in the presence of metastatic disease[18,19]. On the other hand, the increase in Th cell numbers after hepatectomy may be due to stress caused by surgery. It is well recognized that inflammation typically peaks at d2 after surgery, and gradually dissipates by d7 after surgery. Consistently, Th cell frequencies reach their plateau on d2 and then decrease significantly on d7.
Anesthetics per se are associated with suppressed immunity during perioperative periods because of their direct suppressive effects on cellular and humoral immunity[4]. As such, anesthetics and anesthetic methods should be selected with careful consideration. In the current study, on d7, the Th1/Th2 balance in the G + E group was profoundly shifted towards Th1 dominance, compared to the G group. In contrast, Th17 and Treg frequencies in the G + E group decreased significantly, indicating the benefit of combined G + E anesthesia against G anesthesia alone in HCC patients. In addition, on d2, Th1, Th2 and Th17 frequencies in the G + E group were relatively lower than those in the G group. This could also be attributed to the hypothesis that epidural anesthesia combined with general anesthesia was better than general anesthesia in reducing or eliminating surgery-related stress responses, which peaked on d2 after surgery.
Several major differences between the two anesthetic methods may be responsible for the distinct patterns in Th1/Th2 balance, as well as Th17 and Treg frequencies between the two groups. First, regional anesthesia substantially attenuates surgery-induced stress responses, including increases in levels of corticosteroid hormone and catecholamine[13]. Second, opioids inhibit both cellular and humoral immune function in humans[20]. In the G + E group, opioids were administered epidurally, whereas morphine was administered systemically in the G group. Animal experiments have shown that morphine suppresses the lymphocyte proliferative response to mitogens when given systemically, but not when given intrathecally[21]. Similarly, patients receiving an epidural mixture of opioids and local anesthetics exhibited better preservation of lymphocyte proliferation and cytokine production than those receiving intravenous opioids alone. Third, intravenous and inhalation anesthetics are associated with elevated serum concentrations of catecholamines and cortisol[22,23]. Glucocorticoids and catecholamines can heavily influence immunomodulation, including decreases in Th1/Th2 cytokine production and an increase in FOXP3 mRNA expression[24]. Consequently, it is not surprising that G anesthesia used alone suppressed the surgical stress-induced immune response more profoundly than combined G + E anesthesia.
Apart from the surgery and anesthesia, there are still many factors that could influence the Th1/Th2 balance, as well as Th17, and Treg frequencies, including HCC itself, hepatitis virus infection, liver cirrhosis, blood transfusion, and pain levels[25,26]. To eliminate these confounding factors, we prospectively and randomly assigned patients into the G + E or G group, and hence there were no significant differences in these factors. In addition, the results from flow cytometry and cytokine expression analyses were highly consistent with each other, indicating the reproducibility and reliability of the current study.
Oncologic prognosis has been reported to be associated with tumor bionomics, as well as trauma related to surgery, but not anesthesia. Recently however, the effect of anesthetic technique on tumor prognosis has received widespread attention[27]. In rat models, the addition of a spinal block to halothane general anesthesia markedly attenuated the promotion of pulmonary metastasis induced by surgery[28]. Furthermore, the reduction in tumor metastasis by the addition of a spinal block to sevoflurane general anesthesia accompanying surgery was ascribed to preserving the Th1/Th2 balance in mice[29]. Importantly, there are several retrospective clinical studies that are consistent with this hypothesis. Paravertebral anesthesia and analgesia for breast cancer surgery have been associated with an approximately four-fold reduced risk of recurrence or metastasis[30]. Similar results were observed in melanoma, prostate and colon cancer, where epidural anesthesia or epidural supplementation was associated with enhanced survival and reduced tumor recurrence[14,15,31]. The above results indicate that a regional block combined with general anesthesia is a better choice for cancer patients, compared to general anesthesia alone. Considering that there was postoperative Th1 dominance, and observed decreases in Th17 and Treg were more prominent in the G + E group than the G group, we assume that G + E may be superior to G alone for HCC patients in terms of achieving better patient clinical outcomes.
Hepatectomy resulted in the shifting of the Th1/Th2 balance towards Th1, with concomitant decreases in Th17 and Treg frequencies. Epidural anesthesia combined with general anesthesia is superior to general anesthesia alone in preserving the Th1/Th2 balance, and reducing Th17 and Treg numbers after surgery. We thus propose that epidural anesthesia combined with general anesthesia might be an optimal choice for HCC patients undergoing hepatectomies. However, animal experiments and prospective trials evaluating the effects of the Th1/Th2 balance, as well as Th17 and Treg on tumor prognosis are warranted.
Surgical resection is one of the first priorities for hepatocellular carcinoma (HCC), but may inevitably induce immunological stress. T-helper (Th) cells are sub-groups of lymphocytes that play a central role in orchestrating host immune responses through their capacity to help other cells in the immune system. Whether proper peri-operative management, including selection of suitable anesthetic methods, may help to recover the disturbed balances of Th subsets is still questionable. In particular, they compared the differences in Th cell subsets between an epidural anesthesia combined with general anesthesia (G + E) group and a general anesthesia (G) group at multiple peri-operative time points to determine whether anesthetic methods have an impact on postoperative Th subset balances.
Epidural anesthesia is known to prevent or attenuate an excessive stress response during or after surgery, which prevents noxious afferent input from reaching the central nervous system. Both preclinical and clinical studies have suggested that the addition of spinal blockade to general anesthesia attenuated the metastasis-promoting effect of surgery in a tumor-bearing host. The research hotspot is how epidural anesthesia affects peri-operative Th cell subset balances.
The Th1/Th2 balance, as well as Th17, and Treg numbers are key indices of immune function in many types of carcinoma. They also play an important role in HCC metastasis and recurrence. There are rare reports of surgery or anesthetic methods affecting the Th subset balance. The innovative approach in this study was to assess whether the balance was disturbed and which anesthetic method was better at restoring the balance in anti-tumor responses in HCC patients after hepatectomy.
Epidural anesthesia combined with general anesthesia is superior to general anesthesia alone in preserving the Th1/Th2 balance, and reducing Th17 and Treg numbers after surgery. They thus propose that epidural anesthesia combined with general anesthesia might be an optimal choice for HCC patients undergoing hepatectomy.
Epidural anesthesia is most commonly placed in the lower back (lumbar region). This technique may also be accomplished in the mid-back (thoracic region) for surgery in the chest area. Th cells are a sub-group of lymphocytes that play an important role in the immune system, particularly in the adaptive immune system. They are essential in B cell antibody class switching, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages.
This is a study in which authors investigated the influence of the different anesthesia modalities on Th cells. They randomized 61 HCC patients into G + E group and G group. The cell frequency of Th1, Th2, Th17, Treg and mRNA level of interferon-γ, interleukin (IL)-17, IL-4 and FoxP3 in the sequentially collected blood samples (d0, d2 and d7 after surgery) were evaluated.
Peer reviewer: Ji-Ping Wang, MD, PhD, Division of Surgical Oncology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, United States
S- Editor Gou SX L- Editor Webster JR E- Editor Zheng XM
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6341] [Article Influence: 487.8] [Reference Citation Analysis (1)] |
| 2. | Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Ben-Eliyahu S. The price of anticancer intervention. Does surgery promote metastasis? Lancet Oncol. 2002;3:578-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695-2702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 396] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 9. | Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888-898. [PubMed] [Cited in This Article: ] |
| 10. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3445] [Cited by in F6Publishing: 3595] [Article Influence: 224.7] [Reference Citation Analysis (0)] |
| 11. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 845] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 12. | Nagai H, Miyaki D, Matsui T, Kanayama M, Higami K, Momiyama K, Ikehara T, Watanabe M, Sumino Y, Miki K. Th1/Th2 balance: an important indicator of efficacy for intra-arterial chemotherapy. Cancer Chemother Pharmacol. 2008;62:959-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74:549-563. [PubMed] [Cited in This Article: ] |
| 14. | Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 15. | Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Locksley RM. Nine lives: plasticity among T helper cell subsets. J Exp Med. 2009;206:1643-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 18. | Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205-2211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Olson JA, Marcom PK. Benefit or bias? The role of surgery to remove the primary tumor in patients with metastatic breast cancer. Ann Surg. 2008;247:739-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14-18. [PubMed] [Cited in This Article: ] |
| 21. | Hamra JG, Yaksh TL. Equianalgesic doses of subcutaneous but not intrathecal morphine alter phenotypic expression of cell surface markers and mitogen-induced proliferation in rat lymphocytes. Anesthesiology. 1996;85:355-365. [PubMed] [Cited in This Article: ] |
| 22. | Goldmann A, Hoehne C, Fritz GA, Unger J, Ahlers O, Nachtigall I, Boemke W. Combined vs. Isoflurane/Fentanyl anesthesia for major abdominal surgery: Effects on hormones and hemodynamics. Med Sci Monit. 2008;14:CR445-CR452. [PubMed] [Cited in This Article: ] |
| 23. | Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, Shingu K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Xiang L, Marshall GD. Immunomodulatory effects of in vitro stress hormones on FoxP3, Th1/Th2 cytokine and costimulatory molecule mRNA expression in human peripheral blood mononuclear cells. Neuroimmunomodulation. 2011;18:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Takaki A, Tatsukawa M, Iwasaki Y, Koike K, Noguchi Y, Shiraha H, Sakaguchi K, Nakayama E, Yamamoto K. Hepatitis C virus NS4 protein impairs the Th1 polarization of immature dendritic cells. J Viral Hepat. 2010;17:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94:1066-1073. [PubMed] [Cited in This Article: ] |
| 29. | Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, Sugahara S, Kazama T. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499-506. [PubMed] [Cited in This Article: ] |
| 30. | Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660-664. [PubMed] [Cited in This Article: ] |
| 31. | Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res. 2000;10:165-169. [PubMed] [Cited in This Article: ] |