Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3342
Revised: February 3, 2007
Accepted: February 8, 2007
Published online: June 28, 2007
AIM: To study the capacity of bone marrow mesenchymal stem cells (BM-MSCs) trans-differentiating into islet-like cells and to observe the effect of portal vein transplantation of islet-like cells in the treatment of streptozotocin-induced diabetic rat.
METHODS: BM-MSCs were isolated from SD rats and induced to differentiate into islet-like cells under defined conditions. Differentiation was evaluated with electron microscopy, RT-PCR, immunofluorescence and flow cytometry. Insulin release after glucose challenge was tested with ELISA. Then allogeneic islet-like cells were transplanted into diabetic rats via portal vein. Blood glucose levels were monitored and islet hormones were detected in the liver and pancreas of the recipient by immunohistochemistry.
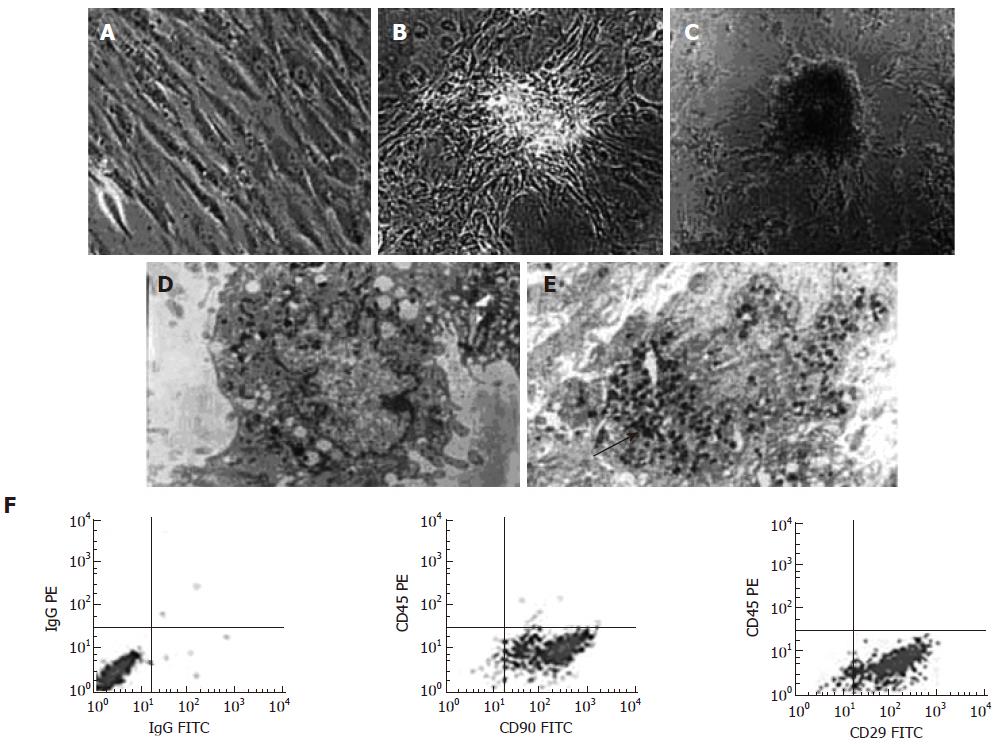
RESULTS: BM-MSCs were spheroid adherent monolayers with high CD90, CD29 and very low CD45 expression. Typical islet-like cells clusters were formed after induction. Electron microscopy revealed that secretory granules were densely packed within the cytoplasm of the differentiated cells. The spheroid cells expressed islet related genes and hormones. The insulin-positive cells accounted for 19.8% and mean fluorescence intensity increased by 2.6 fold after induction. The cells secreted a small amount of insulin that was increased 1.5 fold after glucose challenge. After transplantation, islet-like cells could locate in the liver expressing islet hormones and lower the glucose levels of diabetic rats during d 6 to d 20.
CONCLUSION: Rat BM-MSCs could be transdiffe-rentiated into islet-like cells in vitro. Portal vein transplantation of islet-like cells could alleviate the hyperglycemia of diabetic rats.
- Citation: Wu XH, Liu CP, Xu KF, Mao XD, Zhu J, Jiang JJ, Cui D, Zhang M, Xu Y, Liu C. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol 2007; 13(24): 3342-3349
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3342.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3342
Islet transplantation has recently been shown to be an efficient therapy for type 1 diabetic patients. However, immune rejection, recurrent autoimmune attack against transplanted islets and the lack of donor islets restrict its application in clinical practice[1]. Alternatively, much effort has been made to use the renewable source of stem cells[2-5]. The development of a simple, reliable procedure to obtain autologous stem cells capable of differentiating into functional insulin-producing cells for transplantation would alleviate the major limitations of islet availability and allogeneic rejection. Recent studies have shown that embryonic stem cells, hepatic oval cells and pancreatic stem cells could be differentiated into pancreatic islet-like cells in vitro and in vivo[6-8]. However, these sources are still not enough to provide abundant autologous stem cells. In contrast, bone marrow (BM) has been known to be a safe and abundant source for large quantities of adult stem cells. Some data have revealed that stem cells derived from BM are capable of being reprogrammed to become functional insulin-producing cells and normalize hyperglycemia in streptozotocin-induced diabetic mice and rats by renal subcapsular transplantation[9-11]. The current article reports a potential procedure to generate islet-like cells from BM mesenchymal stem cells (BM-MSCs) by high glucose, nicotinamide and exendin-4. After transplantation via portal vein, allogeneic islet-like cells could locate in the recipient’s liver, expressing islet hormones and alleviate the hyperglycemia of diabetic rats.
Sprague-Dawley (SD) rats of closed colony were purchased from Animal Center, Nanjing Medical University. All the procedure was accordant with animal experiment guidelines of the university. BM was obtained from the femurs and tibias of 10 male SD rats (200-250 g) under aseptic condition, separated by Ficoll density gradients centrifugation and dispersed into a single cell suspension. BM cells (1 × 106 cells/mL) were cultured in 75 cm2 flask with low glucose (5.6 mmol/L) Dulbecco’s modified eagle’s medium (LG-DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Hyclone, USA), HEPES (20 mmol/L), L-glutamine (2 mmol/L), penicillin (100 μ/mL) and streptomycin (100 mg/mL) at 37°C in a humidified atmosphere of 95% air and 5% carbon dioxide. Suspended cells were disposed 24 h later and adherent cells were cultured in 10% FBS LG-DMEM which was changed every 3 d. BM-MSCs gaining 80%-90% confluence were passaged by digestion with 0.25% trypsin and 0.02% EDTA. Following two to three passages, the cells became morphologically homogeneous.
After the third passage, BM-MSCs were released by trypsinization. The cells were incubated with anti-rat phycoerythrin (PE)-labeled CD45 antibody (1:20) and fluorescein isothiocyanate (FITC)-labeled CD90 antibody (1:20) (Caltag, USA) or FITC-labeled CD29 antibody (1:20) (Biolegend, USA) for 20 min, then resuspended in 1% paraformaldehyde/PBS and acquired onto FACSCalibur (BD, USA), the positive rates were assessed by Cellqust software. Isotypematched rat immunoglobulins served as controls for autofluorescence.
At the third passage, BM-MSCs with 80% confluence were induced to differentiate into pancreatic islet cells. Cells were induced with 5% FBS HG-DMEM (25 mmol/L glucose) for 14 d, and added 10 mmol/L nicotinamide (Sigma, USA) for 7 d, and then 10 nmol/L exendin-4 (Sigma) for 7 d.
During differentiation, morphological changes of BM-MSCs were investigated under a converted microscope. BM-MSCs and differentiated cells (D-MSCs) were fixed in 5% glutaraldehyde for 2 h at 4°C, washed in PBS, transferred to 1% osmic acid for 2 h at 4°C, washed in PBS, then dehydrated in acetonic acid and embedded. Ultra thin sections were counterstained using uranyl acetate and lead citrate, then viewed by electron microscope (JEM-1010, Japan).
Total RNA from pre-induced BM-MSCs, D-MSCs and normal rat pancreas tissue was isolated using TRIzol reagent (Gibco) and pretreated with DNase to remove genomic DNA contamination. Transcriptional gene expressions related to pancreatic endocrine development and function were determined by RT-PCR kit (Promega, USA). GAPDH was used as an internal control. PCR cycles were as follows: initial denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, annealing temperature (Tab 1) for 30 s, 72°C for 30 s, and final extension at 72°C for 10 min. PCR products were separated by electrophoresis in 1.0% agarose gels and photographed by Kodak digital camera. The name and sequences of the primers, the sizes of PCR products, and annealing temperature for each pair are listed in Table 1. The primers were synthesized by Shanghai BIOASIA Biologic Technology CO. LTD.
| Genes | Forward primer | Reverse primer | Annealingtemperature (°C) | GenBankaccession no. | Size of PCRproduct (bp) |
| InsulinI | CCGTCGTGAAGTGGAG | CAGTTGGTAGAGGGAGCAG | 57 | NM_019129 | 156 |
| InsulinII | ATGGCCCTGTGGATCCGCTT | CTAGTTGCAGTAGTTCTCCA | 53 | NM_019130 | 333 |
| Glucagon | ATCATTCCCAGCTTCCCAGA | CGGTTCCTCTTGGTGTTCAT | 54 | NM_012707 | 152 |
| Somatostatin | CAGGAACTGGCCAAGTAC | AGTTCTTGCAGCCAGCTTTG | 54 | NM_012659 | 187 |
| IAPP | AGTCCTCCCACCAACCAATGT | AGCACAGGCACGTTGTTGTA | 54 | NM_012586 | 220 |
| GLUT-2 | TTACTCTCCATTTCAGTCCTTTGT | TAGAGCAGCTCTTTATTCCAGATTT | 53 | J03145 | 165 |
| GK | ACCAGAAAGGGGAGGCCT | ATTAAAAACTCCCCCACAGTCC | 51 | NM_012565 | 179 |
| GLP-1R | TCCTGTTAAAGCTGCAAGGC | TTGTCCGAGAGGAAGGCTG | 54 | NM_012728 | 232 |
| PDX-1 | GGTGCCAGAGTTCAGTGCTAA | CCAGTCTCGGTTCCATTCG | 53 | NM_022852 | 249 |
| Ngn3 | CTTCACAAGAAGTCTGAGAACACCAG | CTGCGCATAGCGGACCACAGCTTC | 57 | NM_021700 | 233 |
| NeuroD1 | TGTCGTTACTGCCTTTGGAA | CGATCTGAATACAGCTACACGAA | 53 | NM_019218 | 151 |
| PAX-6 | CGACAAGATTTGCCATGGAT | CAACCTTTGGAAAAACCAACA | 54 | NM_013001 | 179 |
| Nkx2.2 | CACGCAGGTCAAGATCTG | TGCCCGCCTGGAAGGTGGCG | 55 | X81408 | 188 |
| GAPDH | CACCCTGTTGCTGTAGCCATATTC | GACATCAAGAAGGTGGTGAAGCAG | 57 | NM_017008 | 196 |
Pre-induced BM-MSCs, D-MSCs and RIN-m5F cells were grown in plastic six-well plates on slide coverslips (22 × 22 mm2). Cells were fixed in methanol for 15 min, washed with PBS, incubated with 0.01% Triton-100 and first antibody for 20 min, washed with PBS, and cultured with secondary antibody for 20 min, washed with PBS. Insulin, C-peptide, glucagons (Gcg), somatostatin (SS) and islet amyloid polypeptide (IAPP) expressions were observed under laser confocal microscope (LSM510, Carl Zeiss, Germany). First antibody: guinea pig anti-insulin (1:50) (Zymed, USA), rabbit anti-Gcg (1:50) (Zymed), rabbit anti-SS (1:50) (Zymed), rabbit anti-IAPP (1:50) (Zymed), goat anti-rat C-peptide (1:50) (Linco Research Inc., USA); Secondary antibody: anti-Guinea pig IgG (1:20) FITC conjugated (KPL,USA), anti-rabbit IgG (1:20) FITC/Cy5 conjugated (KPL), anti-goat IgG (1:20) FITC conjugated (KPL).
Pre-induced BM-MSCs and D-MSCs (n = 10) were fixed in methanol for 15 min, washed with PBS, incubated with 0.01% Triton-100 and Guinea pig anti-Insulin (1:50) for 20 min, washed with PBS, and cultured with anti-Guinea pig IgG FITC conjugated (1:20) for 20 min, washed with PBS, then resuspended in 1% paraformaldehyde/PBS solution and acquired onto FACSCalibur. The insulin expression and mean immunofluorescence intensity were assessed by Cellqust software. Isotypematched rat immunoglobulins served as controls for autofluorescence.
Pre-induced BM-MSCs and D-MSCs (106/mL, n = 5) were switched to serum-free LG-DMEM containing 0.5% BSA for 12 h, washed twice with PBS, then stimulated by HG-DMEM for 2 h. The culture medium was collected and frozen at -70°C. Serum-free LG-DMEM containing 0.5% BSA was used as a control for secreted insulin measurement. Insulin release was detected by rat insulin enzyme-linked immunosorbent assay (ELISA kit, Linco) according to the manufacturer’s instructions.
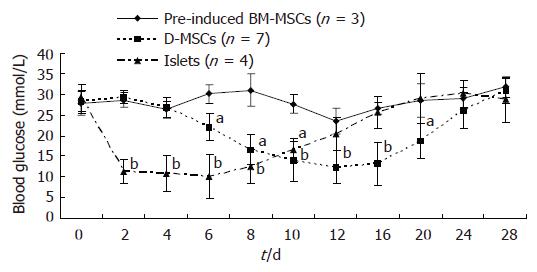
Hyperglycemia was induced in 16 male SD rats of closed colony (Body weight 180-200 g) through intraperitoneal injection of 60 mg/kg of streptozotocin (STZ). Blood glucose levels were determined using Roche ACCU-CHEK glucose meter. Stable hyperglycemia (blood glucose levels ranging between 16.7-33.3 mmol/L) developed in 14 rats one week later. Under general anesthesia, the rats received a transplant of 5 × 106 D-MSCs (n = 7) or pre-induced BM-MSCs (n = 3) or 600 freshly isolated islets (n = 4) via portal vein. Glucose levels were monitored by tapped tail-vein blood under non-fasting condition every two days after transplantation for 28 d.
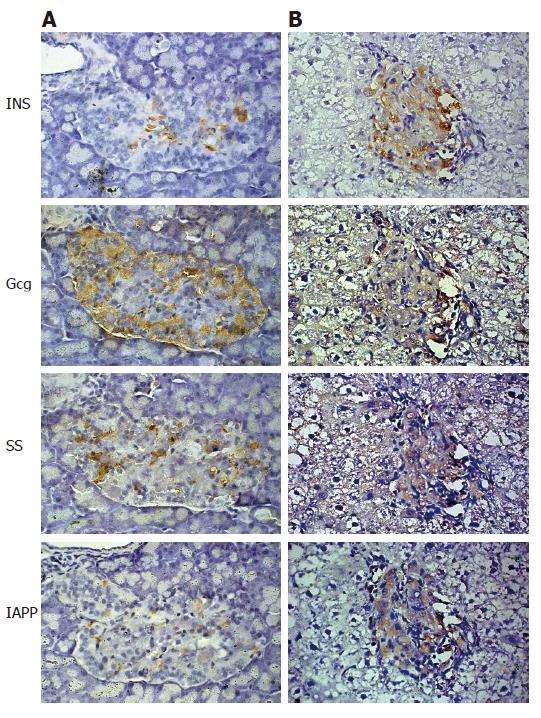
Liver and pancreas of the recipient at d 12 after D-MSCs transplantation were removed and fixed in 4% formaldehyde and embedded in paraffin. To detect D-MSCs grafts in the liver, all liver lobes were sampled. Sections were stained with anti-insulin (1:50) (DAKO, Denmark), anti-Gcg (1:50) (Zymed), anti-SS (1:50) (Zymed) and anti-IAPP (1:50) (Zymed), respectively. Immunohistochemical analysis was performed using an EnVisionTM + System-HRP (DAB) (DAKO) following the manufacture’s instruction.
Data are presented as mean ± SD. Results were analyzed using one-way ANOVA. Statistical significance was set at P < 0.05.
Pre-induced BM-MSCs were typical of spindle and fibrocyte-like adherent monolayers (Figure 1A) with high CD90 positive rate (96.3% ± 1.3%), CD29 positive rate (93.9% ± 0.8%) and very low CD45 expression (0.3% ± 0.4%) (Figure 1F). When being switched into 5% FBS HG-DMEM, BM-MSCs began to form three dimensional, islet-like clusters (Figure 1B). After induced by nicotinamide and exendin-4, clusters were increased and some half suspended in the culture medium (d = 80-200 μm) (Figure 1C). An electron micrograph of D-MSCs revealed structures typical of a secretory cell, including rough endoplasmic reticulum, Golgi complex, a few large vacuoles and secretory vesicles containing dense granules (Figure 1E). However, few could be found within the cytoplasm of pre-induced BM-MSCs (Figure 1D).
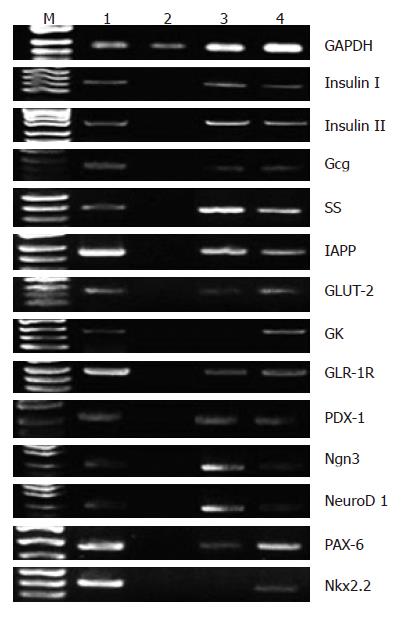
Transcriptional gene expressions related to pancreatic endocrine development and function were not detected in pre-induced BM-MSCs. However, when BM-MSCs were induced with 5% FBS HG-DMEM for 14 d, insulin (Iand II), Gcg, SS, IAPP, glucagon-like peptide (GLP)-1 receptor (GLP-1R), pancreatic duodenal homeobox-1 (PDX-1), Ngn3, NeuroD1, PAX-6 and GLUT-2 messages were positively expressed. After adding nicotinamide and exendin-4 for another 14 d, D-MSCs showed the expression of the aforementioned genes and GK, Nkx2.2 mRNAs (Figure 2).
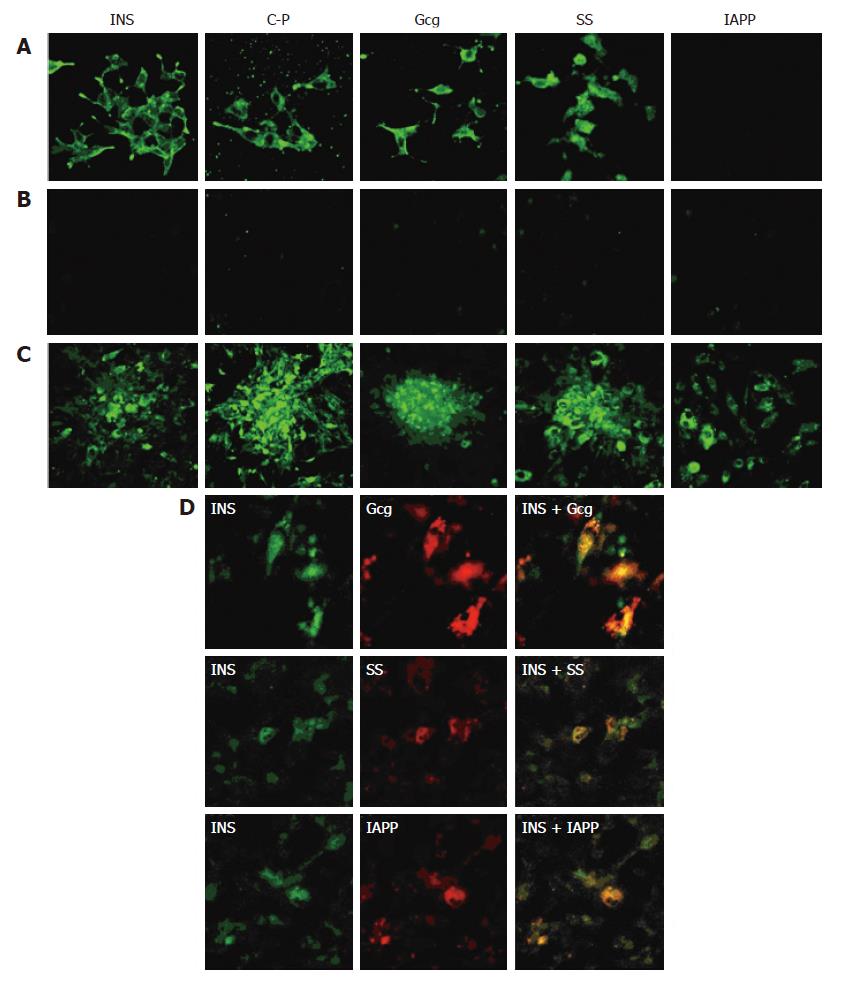
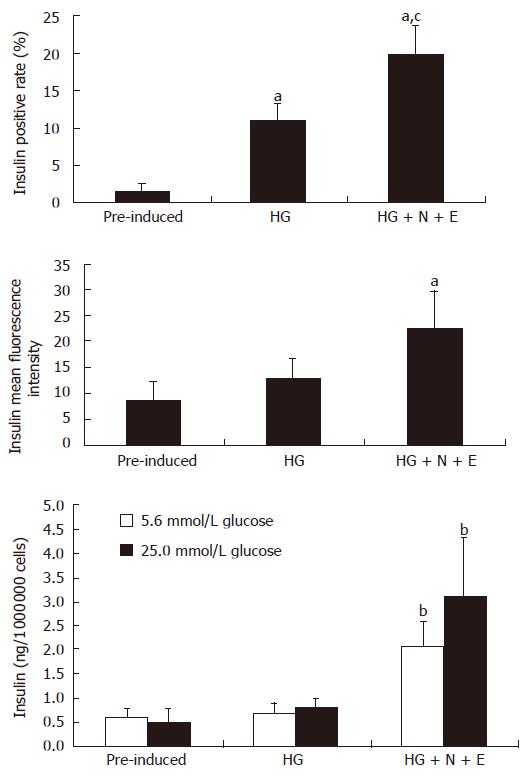
To investigate the expressions of pancreatic islet hormones, immunofluorescence analyses were performed for insulin (INS), C-peptide, Gcg, SS and IAPP in D-MSCs. RIN-m5F cells were shown to strongly express INS, C-peptide, Gcg, SS, but negative for IAPP (Figure 3A). Pre-induced BM-MSCs were negative for the above islet hormones (Figure 3B); However, D-MSCs did express these cytoplasmic proteins (Figure 3C); Some D-MSCs co-expressed INS/Gcg, INS/SS, INS/IAPP (Figure 3D). Flow cytometry showed that the insulin positive rate of cells induced by 5% FBS HG-DMEM for 14 d was about 11.2%. When added with nicotinamide and exendin-4, the insulin positive rate was up to around 19.8% and mean fluorescence intensity was increased by 2.6 folds, which was significantly higher than that of pre-induced BM-MSCs (Figure 4, P < 0.05). To determine whether D-MSCs could secret insulin and response to a glucose challenge, insulin release from pre-induced BM-MSCs and D-MSCs was measured by ELISA. Pre-induced BM-MSCs and cells induced with 5% FBS HG-DMEM for 14 d showed little insulin secretion and glucose response. However, D-MSCs could secret a small amount of insulin (roughly 2.0 ng/106 cells) into medium and increase by 1.5 fold in the presence of glucose challenge (Figure 4).
To determine whether D-MSCs possessed the capacity to correct hyperglycemia in diabetic rats, D-MSCs (5 × 106/rat) were transplanted via portal vein into STZ-induced diabetic rats. Pre-induced BM-MSCs (5 × 106/rat) and freshly isolated islets (600/rat) were transplanted as controls. As demonstrated in Figure 5, glucose levels in D-MSCs implanted rats began to decrease at d 6 after transplantation, kept below 15 mmol/L during d 12 to d 16, and then elevated again after d 20. In contrast, glucose levels in islets implanted rats decreased at d 2, kept below 15 mmol/L during d 4 to d 8, and then elevated again after d 10. Glucose levels in the pre-induced BM-MSCs implanted rats remained elevated (P < 0.05). Immunohistochemical analysis revealed that D-MSCs grafts were located in the recipient’s liver in close proximity to the portal vein and expressed insulin, Gcg, SS and IAPP (Figure 6B). Very few native islets could be found in the pancreas of STZ-induced diabetic recipient rats. In the remaining pancreatic islets, only few insulin-expressing cells located in the center, rich Gcg-expressing cells setting at the periphery, a few SS-expressing cells and IAPP-expressing cells scattering in the islets (Figure 6A).
In the present study, we generated pancreatic islet-like cells from BM-MSCs under an in vitro differentiation procedure promoted by nicotinamide and exendin-4, and confirmed the presence of insulin production by RT-PCR, immunofluorescence, electron microscopy, glucose stimulating insulin secretion test. After transplantation via portal vein, allogeneic islet-like cells could locate in the recipient’s liver expressing islet hormones and alleviate the hyperglycemia of diabetic rats.
The mammalian pancreas arises initially as dorsal and ventral buds that emanate from the embryonic foregut endodermal layer and differentiates into the endocrine cells forming the pancreatic islets of Langerhans under a cascade of gene activation events controlled by transcription factors including PDX-1, Ngn3, NeuroD1, PAX-6, PAX-4, Nkx2.2, Nkx6.1 and so on[12,13]. Inducing stem cells to differentiate into islet-like cells resembles this reprogrammed process. This strategy has been successfully applied in inducing embryonic stem cells, hepatic oval cells and pancreatic stem cells into pancreatic islet-like cells in vitro under defined condition[6-8]. However, these sources are still not suitable for clinical application. BM harbors large quantities of adult stem cells that could be easily obtained. Among them, BM-MSCs are multipotent and can differentiate into lineages of mesenchymal tissues, endodermal and epidermal cells, such as tendon, muscle, adipocytes, chondrocytes, osteocytes, vascular endothelial cells, neurocytes, lung cells and hepatocytes[14-19]. Moreover, BM-MSCs are of great multiplication potency. Cell-doubling time is 48-72 h, and cells could be expanded in culture for more than 60 doublings[20]. Autologous transplantation of functional cells differentiated from BM-MSCs would not cause any rejection. Several in vitro studies have shown that bone marrow-derived stem cells are capable of being reprogrammed to become functional insulin-producing cells[9-11,21-23]. Their inducing processes are to initiate PDX-1 gene expression directly in BM-MSCs or via nestin-positive cells, using factors such as nicotinamide, glucose, β-mercaptoethanol, dimethyl sulphoxide, trichostatin A and so on.
We attempted to induce BM-MSCs into islet-like cells by high glucose, nicotinamide and exendin-4 (GLP-1 agonist) which were considered as potent inducers for pancreatic islet differentiation. Glucose is a growth factor for β-cells. It promotes β-cell replication in vitro and in vivo at a 20-30 mmol/L concentration, induces adult hepatic stem cells into pancreatic endocrine hormone-producing cells at a 23 mmol/L concentration and increases insulin content in cell lines derived from embryonic stem cells at a 5-mmol/L concentration[7,24,25]. Nicotinamide is a poly (ADP-ribose) synthetase inhibitor and could induce liver stem cells or pancreatic progenitor cells into insulin producing cells[7,8]. Whereas exendin-4 could also stimulate both β-cell replication and neogenesis from ductal progenitor cells, and inhibit apoptosis of β-cell[26,27].
Our data showed that CD45-negative and CD90/CD29 positive BM-MSCs could be manipulated toward a pathway of pancreatic endocrine cell lineages differentiation under low serum HG-DMEM via a still unclear mechanism. These premature cells could express insulin (Iand II), Gcg, SS, IAPP, GLP-1R, PDX-1, Ngn3, NeuroD1, PAX-6, GLUT-2 genes, and a relatively low level of insulin, Gcg, SS and IAPP proteins. However, they showed low insulin secretion and weak glucose response. At this stage, GLP-1 receptor gene was also expressed. Nicotinamide and exendin-4, D-MSCs displayed Nkx2.2, GK and aforementioned genes and proteins, the insulin protein levels were markedly increased. Moreover, D-MSCs could secrete a small amount of insulin and show glucose response to some extent. Although a combination of nicotinamide and exendin-4 effectively promotes further differentiation of BM-MSCs in our experimental system, insulin positive rate of D-MSCs was only around 19.8%, the insulin production and glucose response were still quite lower when compared with pancreatic islets.
In order to test the function of D-MSCs in vivo, we transplanted the differentiated cells in STZ-induced diabetic rats via portal vein. At first, we established the allogeneic islets transplantation system via portal vein. We found that islet grafts could reduce the hyperglycemia of diabetic rats for 6-8 d after transplantation and then lost their function gradually due to allograft rejection. However, blood glucose levels of D-MSCs implanted rats began to decrease at d 6 after transplantation and kept below 15 mmol/L during d 12 to d 16, suggesting that immature D-MSCs needed further differentiation in vivo to display their functions. Immunohistochemical analysis revealed that D-MSCs grafts could survive in the liver of the recipient and express insulin, Gcg, SS and IAPP. Some data have suggested that transplanted stem cells derived from bone marrow could initiate endogenous pancreatic β cell regeneration and then reduce hyperglycemia in mice with STZ-induced pancreatic damage[28]. We also detected the islet hormones expression of pancreas in the recipient. Very few native islets could be found in the pancreas with only few insulin-expressing cells located in the center, just like the STZ-induced pancreatic damage, indicating that D-MSCs did not promote endogenous β cells regeneration in our experiment. After d 20, glucose levels elevated again. D-MSCs grafts lost their functions and displayed lymphocyte infiltration and apoptosis (Data not shown), which might be due to allograft rejection.
Taken together, present studies demonstrate that rat BM-MSCs may be trans-differentiated into islet-like cells in vitro. Portal vein transplantation of islet-like cells may alleviate the hyperglycemia of diabetic rats. These insulin-producing cells may be a potential source for antilogous transplantation without immune rejection. However, because of the transdifferentiation and dedifferentiation potency of BM-MSCs, there are many questions that remain unresolved, such as how to manipulate the differentiation process (e.g. exogenous factors, and timing of factor addition), how to push these cells to become mature β cells, will the autoantibodies responding to β-cell antigens recognize and destroy the newly generated insulin-producing cells obtained from BM-MSCs. Further research is required to address these important questions.
We wish to thank Professor Han Xiao from Nanjing Medical University for kindly providing the RIN-m5F cell line. We also wish to thank Ms Wang Lin and Mr Hu Fan for technical assistance.
S- Editor Wang J L- Editor Alpini GD E- Editor Ma WH
| 1. | Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203-205. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Hardikar AA. Generating new pancreas from old. Trends Endocrinol Metab. 2004;15:198-203. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Peck AB, Ramiya V. In vitro-generation of surrogate islets from adult stem cells. Transpl Immunol. 2004;12:259-272. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Hampton T. Stem cells probed as diabetes treatment. JAMA. 2006;296:2785-2786. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci USA. 2002;99:8078-8083. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278-282. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721-1732. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607-617. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Chakrabarti SK, Mirmira RG. Transcription factors direct the development and function of pancreatic beta cells. Trends Endocrinol Metab. 2003;14:78-84. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Sumi S, Gu Y, Hiura A, Inoue K. Stem cells and regenerative medicine for diabetes mellitus. Pancreas. 2004;29:e85-e89. [PubMed] [Cited in This Article: ] |
| 14. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Hervé P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108 Suppl 1:II253-II258. [PubMed] [Cited in This Article: ] |
| 19. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615-2625. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003;35:2140-2141. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [PubMed] [Cited in This Article: ] |
| 23. | Thatava T, Ma B, Rohde M, Mayer H. Chromatin-remodeling factors allow differentiation of bone marrow cells into insulin-producing cells. Stem Cells. 2006;24:2858-2867. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205-219. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763-770. [PubMed] [DOI] [Cited in This Article: ] |