Published online Nov 14, 2008. doi: 10.3748/wjg.14.6572
Revised: September 22, 2008
Accepted: September 29, 2008
Published online: November 14, 2008
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is a rare cholestatic liver disease. Such liver disease can get worse by female hormone disorder. Albumin dialysis or Molecular Adsorbent Recirculating System (MARS) has been reported to reverse severe cholestasis-linked pruritus. Here, we report the first use of MARS during a spontaneous pregnancy and its successful outcome in a patient with PFIC3 and intractable pruritus. Albumin dialysis could be considered as a pregnancy-saving procedure in pregnant women with severe cholestasis and refractory pruritus.
- Citation: Lemoine M, Revaux A, Francoz C, Ducarme G, Brechignac S, Jacquemin E, Uzan M, Ganne-Carrié N. Albumin liver dialysis as pregnancy-saving procedure in cholestatic liver disease and intractable pruritus. World J Gastroenterol 2008; 14(42): 6572-6574
- URL: https://www.wjgnet.com/1007-9327/full/v14/i42/6572.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6572
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is a rare autosomal recessive cholestatic liver disease due to mutations in the adenosine triphosphate-binding-cassette, subfamily B, member 4 gene (ABCB4) involved in biliary phospholipid excretion. A few years ago, we reported a case of a young female with PFIC3 revealed by oestro-progestative contraceptive pill-related cholestasis[1]. Here, we report, in the same patient, her first spontaneous pregnancy that resulted in a successful outcome after the use of Molecular Adsorbent Recirculating System (MARS) for intractable pruritus.
A 23-year-old woman, gravida 1, was referred to our liver unit for pruritus and recent diagnosis of pregnancy in November, 2005. This woman was diagnosed with PFIC3 five years ago and it was revealed by contraceptive pill consumption[1]. After stopping the contraceptive pill and the starting of a treatment with ursodeoxycholic acid (UDCA, 20 mg/kg per day), the patient remained asymptomatic with a normal physical examination, normal liver tests, no endoscopic signs of portal hypertension and no abdominal ultrasonographic abnormalities.
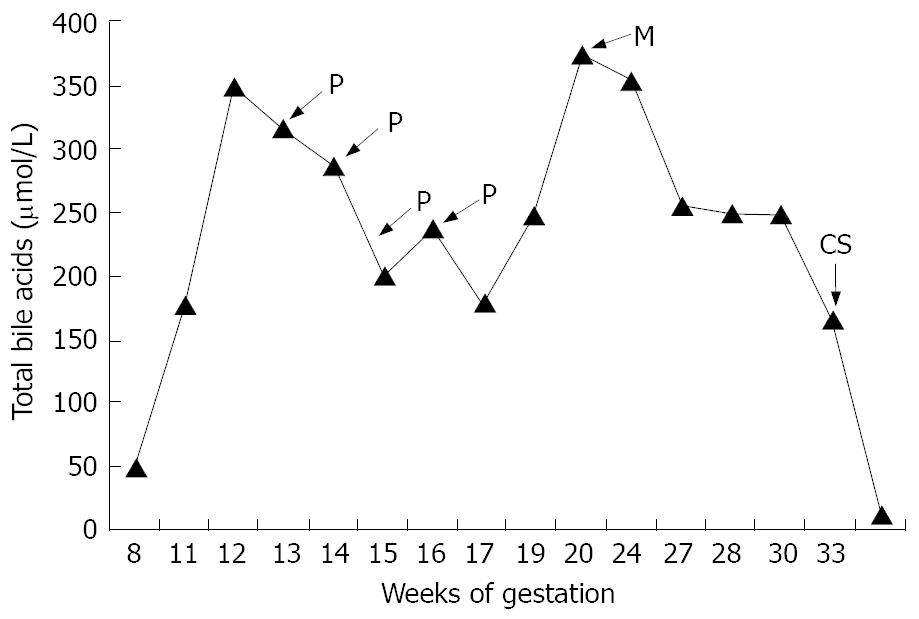
In November, 2005, the patient was at the 7th week of gestation. Because of potential teratogenic risks, UDCA was stopped until the end of the first trimester. As a consequence, itching recurred and moderate liver tests abnormalities were observed. Despite administration of emollients, hydroxyzine, zolpidem tartrate, cetirizine and cholestyramine, pruritus remained very intense leading to sleep disturbance. At the 11th week of gestation, UDCA (20 mg/kg per day) was started again. Within 15 d, no clinical effectiveness was observed: Pruritus became unbearable with insomnia and depressed mood. In addition, total serum bile acids rose to 316 μmol/L. At the 13th week of gestation, a first plasmapheresis was performed, and then continued weekly for 1 mo. The patient reported improvement in pruritus immediately after each procedure and total bile acids slowly decreased, but rose again over 300 μmol/L (Figure 1). Despite the four plasma exchanges (with 2000 mL using 5% albumin as replacement fluid) without any maternal or fetal complications, the pruritus worsened again. Procedure of extracorporeal albumin dialysis using the MARS was decided after giving explanations to the patient regarding the fetal risks. The fetus was careful monitored every week using ultrasonography, Doppler velocimetry and cardiotocography. Two consecutive sessions of MARS were performed at the 20th week of gestation. The procedure was well tolerated without maternal or fetal hemodynamic changes. The pruritus improved without recurrence until the delivery despite high serum total bile acids levels (Figure 1). During pregnancy, several ultrasound scans showed normal fetus growth without morphologic abnormalities and arterial Doppler showed normal uterine arteries and umbilical flow velocity. The patient was hospitalized for preterm labour at 33 wk of gestation and received nifedipine tocolysis, antibiotic prophylaxis and antenatal corticosteroids. Because fetal bowel dilatation observed by ultrasonography and maternal persistent cholestasis with high bile acids, a caesarean section was performed at 34 wk of gestation and the mother gave birth of a healthy, Apgar 10/10 (at 1 and 5 min), boy weighting 1960 g (20th percentile for gestational age), with a height of 42 cm (15th percentile) who was admitted in the neonatology unit. The newborn was discharged on the 15th day, his development was normal and 6 mo later he was still healthy. No intestinal pathology was found and his liver tests were normal. The histopathology analysis of the placenta showed multiple microscopic hypoxic lesions. The postoperative course of the mother was uncomplicated. UDCA was reintroduced just after the delivery and the mother bile acids normalized in few days in postpartum (Figure 1).
Regarding the medical literature, the course of pregnancy in PFIC3 has never been reported. In pregnant patients with chronic liver disease, such as primary biliary cirrhosis, primary sclerosing cholangitis or viral hepatitis, the high systemic and hepatic sex-hormones load levels may induce cholestasis and pruritus[2]. Our patient, who was affected by a cholestatic liver disease clearly aggravated by female hormones rapidly complained of intractable pruritus during the first half of pregnancy. Most likely, the withdrawn of UDCA at the 7th week of gestation triggered the onset of cholestasis with pruritus. Although UDCA has been widely and successfully used in the second and third trimesters of pregnancy in women with intrahepatic cholestasis of pregnancy (ICP)[3,4], we stopped its administration early during the first trimester of pregnancy of our patient for the lack of teratogenic effects of the drug is not well established in early pregnancy.
Refractory pruritus is a serious manifestation of cholestasis and its physiopathology remains unknown. Interventions using extra corporeal removal of pruritogens (bile acids, histamine) have been proposed including hemodialysis, charcoal hemoperfusion, or plasmapheresis[5]. Among these methods, only plasmapheresis provided relief, but with transient efficiency[6]. Plasmapheresis, an extracorporeal exchange procedure used to remove large-molecular-weight substances from the plasma, is generally safe although rare complications have been reported (bleeding, infection, coagulation abnormalities and electrolyte disturbances)[7]. Only one case of plasmapheresis has been reported in a woman with ICP-related pruritus[7]. Although four sessions of plasmapheresis were performed in our patient, the pruritus recurred and the serum bile acids levels remained high, largely above 40 μmol/L, a threshold value that has been shown to be associated with an increased risk preterm delivery, fetal distress and fetal loss[8]. Therefore, considering the failure of plasmapheresis to control the dangerous consequences of the cholestasis of our patient during her pregnancy, we decided to use MARS as a pregnancy-saving procedure.
MARS was developed as an extracorporeal hemofiltration system using an albumin-enriched dialysate to remove albumin-bound substances in patients with liver failure. However, MARS was also reported as a valuable and safe procedure for intractable pruritus either after orthotopic liver transplantation or in patients with primary biliary cirrhosis[9-11]. To date, 25 cases of successful use of the MARS in patients with refractory pruritus have been reported[9-13]. The mechanisms by which MARS ameliorates pruritus remain unclear. In patients with symptomatic cholestasis, removal of bile acids from serum should lead to a decline of their concentration in the skin causing amelioration of pruritus. However, several studies did not observe changes of serum bile acids concentration. A further possible explanation for efficacy of MARS in cholestasis-related pruritus is the clearance of other lipophilic, albumin-bound substances from the patient’s plasma that could induce pruritus, either as peripheral pruritogens or by binding to central serotonin or opioid receptors. To our knowledge, the use of MARS as a pregnancy-saving therapy in cholestasis-induced pruritus during pregnancy was not previously reported. In our patient, MARS led to a spectacularly rapid and protracted improvement in pruritus and allowed significant prolongation of pregnancy.
In conclusion, the successful use of MARS in our patient suggests that the procedure could be considered as a pregnancy-saving procedure in pregnant women with severe cholestasis and intractable pruritus.
Peer reviewer: Tom H Karlsen, MD, Institute of Immunology, Rikshospitalet University Hospital, Oslo N-0027, Norway
S- Editor Li DL L- Editor Negro F E- Editor Yin DH
| 1. | Ganne-Carrié N, Baussan C, Grando V, Gaudelus J, Cresteil D, Jacquemin E. Progressive familial intrahepatic cholestasis type 3 revealed by oral contraceptive pills. J Hepatol. 2003;38:693-694. [Cited in This Article: ] |
| 2. | Janczewska I, Olsson R, Hultcrantz R, Broome U. Pregnancy in patients with primary sclerosing cholangitis. Liver. 1996;16:326-330. [Cited in This Article: ] |
| 3. | Palma J, Reyes H, Ribalta J, Iglesias J, Gonzalez MC, Hernandez I, Alvarez C, Molina C, Danitz AM. Effects of ursodeoxycholic acid in patients with intrahepatic cholestasis of pregnancy. Hepatology. 1992;15:1043-1047. [Cited in This Article: ] |
| 4. | Palma J, Reyes H, Ribalta J, Hernandez I, Sandoval L, Almuna R, Liepins J, Lira F, Sedano M, Silva O. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27:1022-1028. [Cited in This Article: ] |
| 5. | Jones EA, Bergasa NV. The pathogenesis and treatment of pruritus and fatigue in patients with PBC. Eur J Gastroenterol Hepatol. 1999;11:623-631. [Cited in This Article: ] |
| 6. | Cohen LB, Ambinder EP, Wolke AM, Field SP, Schaffner F. Role of plasmapheresis in primary biliary cirrhosis. Gut. 1985;26:291-294. [Cited in This Article: ] |
| 7. | Warren JE, Blaylock RC, Silver RM. Plasmapheresis for the treatment of intrahepatic cholestasis of pregnancy refractory to medical treatment. Am J Obstet Gynecol. 2005;192:2088-2089. [Cited in This Article: ] |
| 8. | Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474. [Cited in This Article: ] |
| 9. | Bellmann R, Graziadei IW, Feistritzer C, Schwaighofer H, Stellaard F, Sturm E, Wiedermann CJ, Joannidis M. Treatment of refractory cholestatic pruritus after liver transplantation with albumin dialysis. Liver Transpl. 2004;10:107-114. [Cited in This Article: ] |
| 10. | Pares A, Cisneros L, Salmeron JM, Caballeria L, Mas A, Torras A, Rodes J. Extracorporeal albumin dialysis: a procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:1105-1110. [Cited in This Article: ] |
| 11. | Macia M, Aviles J, Navarro J, Morales S, Garcia J. Efficacy of molecular adsorbent recirculating system for the treatment of intractable pruritus in cholestasis. Am J Med. 2003;114:62-64. [Cited in This Article: ] |
| 12. | Huster D, Schubert C, Achenbach H, Caca K, Mossner J, Berr F. Successful clinical application of extracorporal albumin dialysis in a patient with benign recurrent intrahepatic cholestasis (BRIC). Z Gastroenterol. 2001;39 Suppl 2:13-14. [Cited in This Article: ] |
| 13. | Mullhaupt B, Kullak-Ublick GA, Ambuhl PM, Stocker R, Renner EL. Successful use of the Molecular Adsorbent Recirculating System (MARS) in a patient with primary biliary cirrhosis (PBC) and treatment refractory pruritus. Hepatol Res. 2003;25:442-446. [Cited in This Article: ] |