- 1Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2The Affiliated Hospital of Ningbo University, Ningbo, China
Objective: Cancer stem cell marker CD44 and its variant isoforms (CD44v) may be correlated with tumor growth, metastasis, and chemo-radiotherapy resistance. However, the prognostic power of CD44 and CD44v in advanced cancer remains controversial. Therefore, the purpose of our study was to generalize the prognostic significance of these cancer stem cell markers in advanced cancer patients.
Methods: Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated from multivariable analysis to assess the associations among CD44, CD44v6, and CD44v9 positivity and overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), and recurrence-free survival (RFS). Trial sequential analysis (TSA) was also conducted.
Results: We included 15 articles that reported on 1,201 patients with advanced cancer (CD44: nine studies with 796 cases, CD44v6: three studies with 143 cases, and CD44v9: three studies with 262 cases). CD44 expression was slightly linked to worse OS (HR = 2.03, P = 0.027), but there was no correlation between CD44 expression and DFS, RFS, or PFS. Stratified analysis showed that CD44 expression was not correlated with OS at ≥5 years or OS in patients receiving adjuvant therapy. CD44v6 expression was not associated with OS. CD44v9 expression was closely associated with poor 5-years CSS in patients treated with chemo/radiotherapy (HR = 3.62, P < 0.001). However, TSA suggested that additional trials were needed to confirm these conclusions.
Conclusions: CD44 or CD44v9 might be novel therapeutic targets for improving the treatment of advanced cancer patients. Additional prospective clinical trials are strongly needed across different cancer types.
Introduction
Cancer remains a pressing worldwide health issue (1), although surgery, chemotherapy, radiotherapy therapy, and targeted molecular therapy have greatly changed clinical outcomes for cancer patients in recent years. Advanced cancer patients (advanced-stage or metastatic disease) are resistant to chemotherapy, radiotherapy and targeted therapies (due to secondary mutations). Thus, effective treatment strategies for advanced cancer patients are still limited and disappointing. The prognosis of patients with advanced cancer remains very poor such as a low 5-years survival rate (2–6). For all cancers combined, the 5-years survival rate is >60%. For different cancer types, it varies; for example, the 5-years survival rate is 14% for advanced colorectal cancer and 29% for advanced ovarian cancer (7). Therefore, novel molecular therapeutic targets to prolong survival in advanced cancer are warranted and could help physicians to stratify cancer patients.
Increasing evidence has suggested that cancer stem cells (CSCs) represent a small subset of cancer cells with the major capabilities of self-renewal and multidifferentiation and may be responsible for tumor relapse, metastasis, progression, a poor prognosis, and resistance to chemotherapy or radiation therapy (8, 9). Several CSC marker molecules, such as CD166, epithelial cell adhesion molecule (EpCAM) and CD44, have been identified and have become useful markers in human cancers (10–12). Among these CSCs, the CD44 family is one of the most commonly reported markers in cancer.
CD44 was originally described as a hyaluronan and lymphocyte-homing receptor (13). Exons 1–5 and 16–20 produce the standard form of CD44 and the remaining exons 6–15 encode variant exons 1–10 (v1–v10). The isoform with none of the 10 variable exons is denoted CD44 standard (CD44s) and the alternative mRNA splicing of variant isoforms of CD44 are named CD44v. CD44 has been reported to be involved in the regulation of cell growth, survival, differentiation, motility, tumor growth, proliferation, and metastasis (14–16). CD44s and CD44v have overlapping and distinct functions. CD44v contains additional binding motifs that can contribute to the interaction of CD44 with molecules in the microenvironment (17). The pattern of CD44 alternative splicing is differentially regulated during epithelial-mesenchymal transition (EMT). EMT is an important step in the metastatic process and the acquisition of stemness in cancer cells. CD44s is more highly expressed by EMT CSCs, and CD44v is more highly expressed by non-EMT CSCs (14, 18). CD44 or CD44v6 expression has been shown to be correlated with poor overall survival (OS) in gastric cancer (19), but CD44 or CD44v6 expression has been found to not be correlated with OS in ovarian cancer (20); these findings suggest that some prognostic information regarding CD44 is still conflicting.
The role of CD44 and its isoforms in advanced cancer patients remains unclear as to which markers may be of value in determining prognosis. Therefore, we present here the first systemic meta-analysis and TSA on the relationship of CD44 and its isoforms expression with clinical outcomes in patients with advanced cancer.
Materials and Methods
Search Strategies
Meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement (21). A systematic literature search was conducted in the PubMed, EMBASE, EBSCO, Web of Science, and Cochrane Library databases through April 2018 without language restrictions. The following key words and search terms were used as follows: “CD44,” “metastatic OR advanced OR metastasized OR recurrent,” “cancer OR tumor OR carcinoma OR neoplasm,” “survival OR outcome OR prognosis.” Moreover, the references listed in the eligible articles were also manually searched to avoid the omission of relevant papers.
Selection Criteria
Eligible publications were included when the given eligibility criteria were satisfied: (1) studies reporting patients with advanced/metastatic cancer or stage III cancer or stage IV cancer; (2) studies investigating the prognostic value of the expression of CD44 and its isoforms using immunohistochemical (IHC) assays; (3) studies reporting multivariable survival analysis with hazard ratio (HR) with 95% confidence interval (CI) for overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), relapse/recurrence-free survival (RFS), metastasis-free survival (MFS) or cancer-specific survival (CSS); (4) In the case of insufficient data, such as only P-value with HR or only the 95% CI, HR and 95% CI were calculated using the previously described method (22, 23), or the corresponding author with an available email was contacted to request the relevant information. Case reports, reviews, comments, letters, cell lines, animal studies, articles unrelated to our topic, studies with no available prognostic data and studies with advanced cancer patients analyzed using univariable survival analysis were excluded. We did not include overlapping sample data in multiple publications from the same research institution.
Data Extraction and Study Assessment
The study assessment was conducted following Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines (24). The REMARK checklist reported 20 items for published tumor prognostic markers, consisting of Introduction (1 item), Materials and Methods (10 items), Results (7 items), and Discussion sections (2 items), with a maximum score of 40 (Table S1). An item had possible scores of 0, 1, and 2. An item was given a score of 2 if it described all aspects of an item, a score of 1 if it reported some aspects and an item was given a score of 0 when an item was lacking reporting of any aspect. Multivariable survival analysis adjusted for potential factors such as traditionally prognostic factors is more valuable compared to a study that reported a univariable survival analysis. Therefore, this meta-analysis only included prognostic information obtained using multivariable analysis. The following data items were extracted from eligible full-text papers: first author's surname, year of publication, number of patients, study source of patients, mean or median age, tumor type, detection method, therapy regime, study design, sample type, cut-off value, median or mean follow-up time, survival rate, adjusted factors, and clinical outcomes. Any inconsistency was discussed until a consensus was reached.
Statistical Analysis
Pooled HR and 95% CI were calculated to estimate the prognostic effect of the expression of CD44 and its isoforms on patients with advanced cancer, including OS, DFS, PFS, CSS, RFS, or MFS using multivariable analysis. A HR >1 showed a worse survival, whereas an observed HR <1 showed a favorable survival. Heterogeneity was tested using Cochran's Q statistic (25), with P < 0.1 indicating substantial heterogeneity. The random-effects model (DerSimonian-Laird) was applied to estimate the HR (26, 27). Subgroup analyses were performed in ≥8 of the included studies and publication bias was measured by using the Egger's and Begg's funnel plots (28, 29).
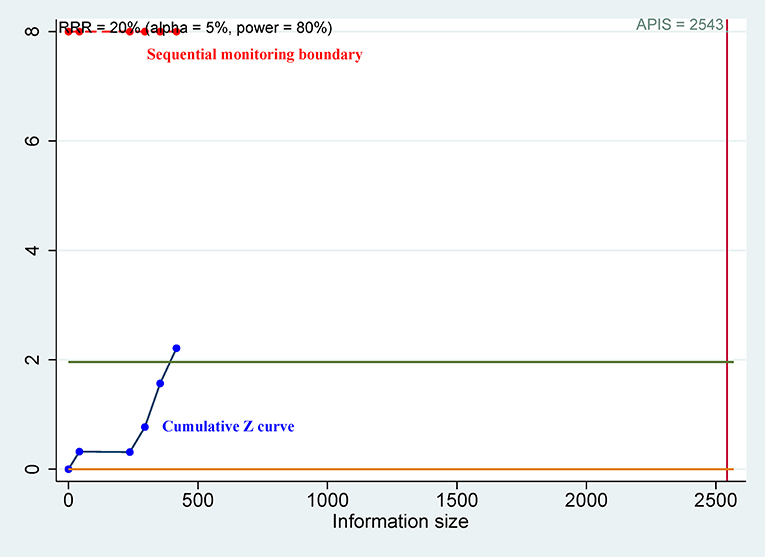
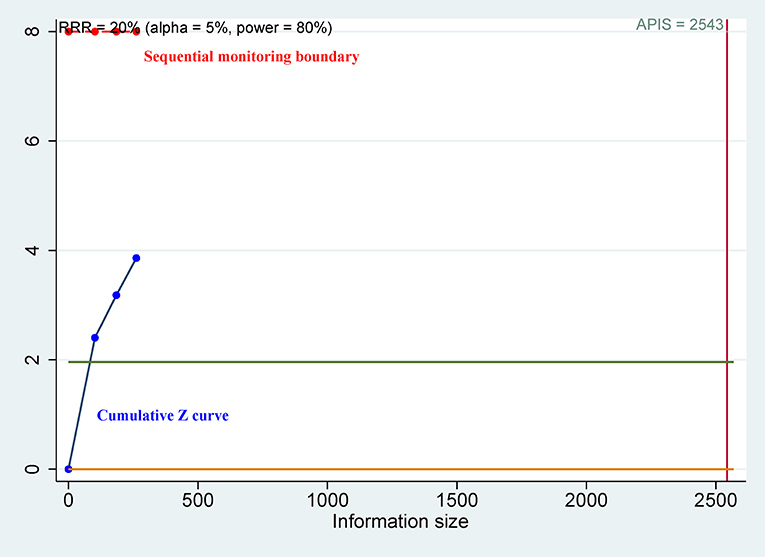
Since the meta-analysis included only a small number of patients and the associated random errors may cause spurious results (30, 31), trial sequential analysis (TSA) was performed to control for random errors and to assess the required sample information (32). The relative risk reduction (RRR) of 20% was applied for the minimum intervention effect. Type I error (α) level of 5%, type II error (β) level of 20% (giving a statistical power of 80%) and the optimal a priori anticipated information size (APIS) method were used. Monitoring boundaries were applied to decide whether a trial could be terminated early. When the cumulative Z-curve passed through the trial sequential monitoring boundary or required information size (RIS) boundary, this suggested the evidence was conclusive and reliable. Otherwise, additional clinical studies are essential. Meta-analyses were performed by using Stata software, version 12.0 (Stata Corp., College Station, TX, USA) and R software, version 3.4.2 (The R Foundation for Statistical Computing; Vienna, Austria).
Results
Study Characteristics
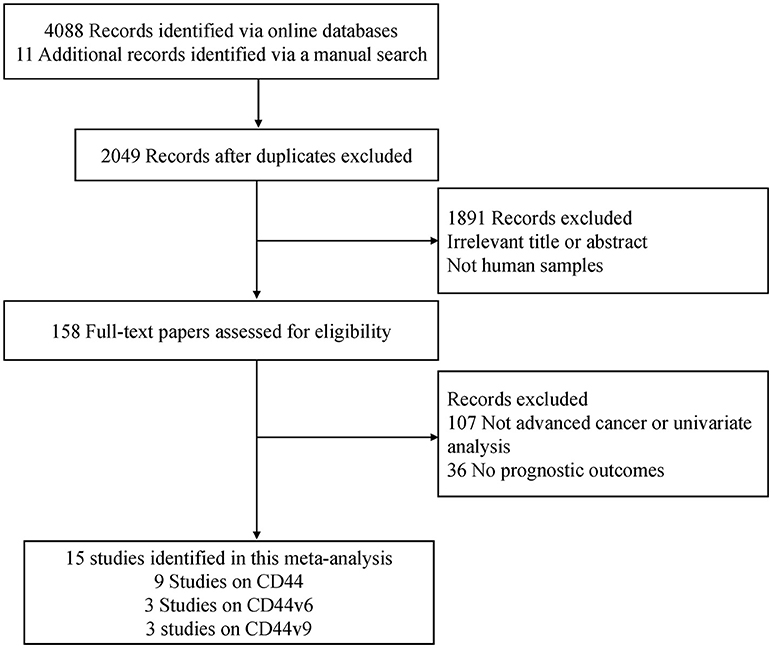
A flow chart of the literature search strategies is shown in Figure 1. After carefully reviewing the titles, abstracts and full text, a total of 1,201 patients with advanced cancer from 15 full text articles published from 1999 to 2018 met the inclusion criteria and were included in the current meta-analysis (33–47). Six studies were conducted in Japan, four studies in the USA, two studies in Germany, one study in Greece, one study in Brazil, and one study in China. One study was a prospective trial and the remaining studies were retrospective in design. The mean REMARK scores were 19, ranging from 14 to 24.
The majority (14 articles) of the eligible 15 articles reported advanced cancer patients treated with surgery and/or adjuvant therapy. Nine studies involving 796 advanced cancer patients evaluated the association between CD44 expression and the prognosis (34–36, 40, 42–46) and only six studies evaluated 5-years survival. Three studies assessed the association of CD44v6 expression and 5-years prognosis (33, 38, 39), including 143 cases treated with surgery and/or adjuvant therapy. Three studies evaluated the correlation between CD44v9 expression and 5-years prognosis (37, 41, 47), including 262 cases treated with surgery and chemo/radiotherapy. The characteristics of the included studies using multivariable survival analysis are presented in Table 1 and Table S2.
Association Between CD44 Expression and the Prognosis
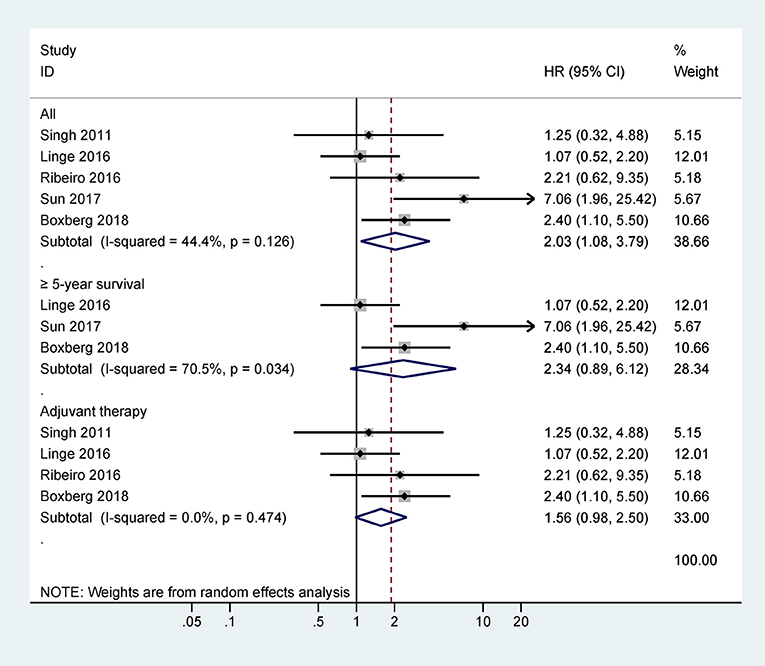
Five studies with 417 cases were included in the final analysis of CD44 expression and OS and the pooled data showed that CD44 expression was associated with worse OS (HR = 2.03, 95% CI = 1.08–3.79, P = 0.027) (Figure 2), with no obvious evidence of heterogeneity (P = 0.126).
Further analysis from three studies with 317 cases indicated that CD44 expression was not associated with OS at ≥ 5 years (HR = 2.34, 95% CI = 0.89–6.12, P = 0.084) (Figure 2). Data from four studies with 358 cases receiving adjuvant therapy showed that no significantly statistical association was observed between CD44 expression and OS (HR = 1.56, 95% CI = 0.98–2.50, P = 0.062) (Figure 2).
Only one study with 63 cases reported that CD44 expression was correlated with poor 5-years CSS (HR = 3.1, 95% CI = 1.2–8.5) (Figure 3). However, there was no statistical significance between CD44 expression and DFS, RFS, MFS, or PFS (Figure 3).
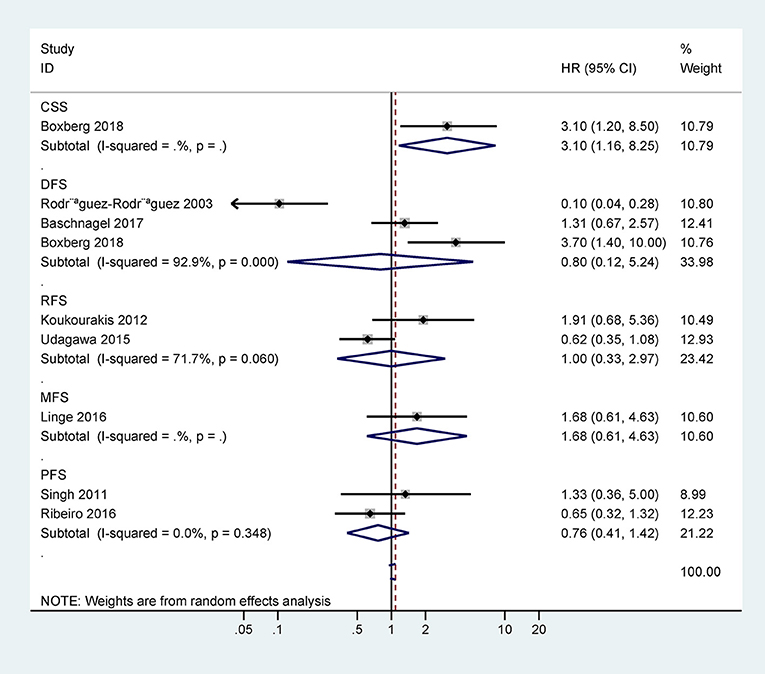
Figure 3. Forest plot for the association between CD44 expression and disease-free survival (DFS), progression-free survival (PFS), relapse/recurrence-free survival (RFS), metastasis-free survival (MFS), or cancer-specific survival (CSS).
Association Between CD44v6 Expression and the Prognosis
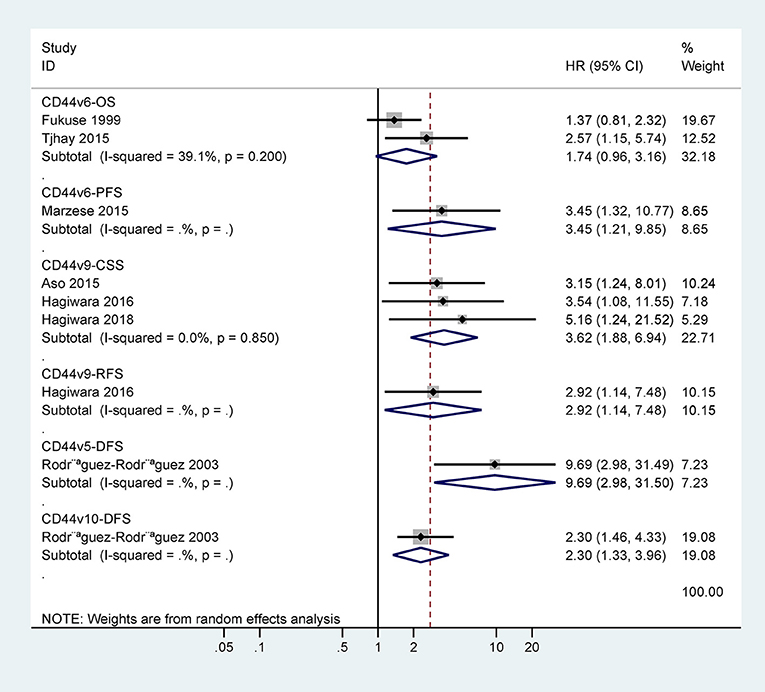
The data from two studies with 93 cases demonstrated no association between CD44v6 expression and 5-years OS (HR = 1.74, 95% CI = 0.96–3.16, P = 0.07) (Figure 4). One study involving 50 cases reported a significant association between CD44v6 expression and poor 5-years PFS (HR = 3.45, 95% CI = 1.32–10.77) (Figure 4).
Association Between CD44v9 Expression and the Prognosis
Only one study with 83 cases reported that CD44v9 expression was linked to poor 5-years RFS (HR = 2.92, 95% CI = 1.14–7.48) (Figure 4). CD44v9 expression was significantly linked to worse 5-years CSS (HR = 3.62, 95% CI = 1.88–6.94, P < 0.001), including three studies with 262 cases receiving surgery and chemo/radiotherapy (Figure 4).
TSA
For OS of CD44, the cumulative Z curve did not reach the sequential monitoring boundary (Figure 5), a finding which indicated that more studies are needed to achieve the required information size. For CSS of CD44v9, the cumulative Z curve did not cross the trial sequential monitoring boundary (Figure 6), which demonstrated that additional studies were needed for stable conclusions.
Discussion
Although advances in surgical techniques and treatment methods have been used for advanced cancer patients, the 5-years survival rate for these patients remains very disappointing. Drug resistance and frequent recurrence are the major obstacles to the treatment of advanced cancers (48, 49). According to the CSC theory, CSCs contribute to cancer progression, metastasis and chemotherapy/radiotherapy resistance (50, 51).
To date, CD44s remains the most commonly reported isoform in cancer, but the other CD44 variants (CD44v) are also correlated with neoplasia and metastasis in some cancers (52) CD44 is a frequently observed CSC marker in solid tumors, and CD44 was revealed to be a major Wnt target, involved in the Ras-Raf-Mek-Erk-Cyclin D1 pathway, phosphoinositide 3-kinase (PI3K)-Akt signaling, and the Wnt pathway, as well as stimulating EMT, which promotes tumor invasion, progression and metastasis (53–55). Some studies suggest that expression of total CD44 may be correlated with distant metastasis, tumor recurrence and a worse prognosis (19, 56, 57). CD44 or CD44v6 may also play a key role in drug resistance (39, 58, 59).
CD44v6 is one of the most common variants of CD44, and some meta-analyses have shown that the expression of CD44v6 is related to a poor prognosis in gastric and hepatocellular carcinomas (60, 61) but is not related to prognosis in ovarian cancer (20). CD44v9 may be correlated with lymph node/liver metastasis and tumor stage, and contribute to EMT-mediated invasion and migration (62, 63). Recent studies suggest that CD44v9 is associated with increased resistance to chemotherapy or radiation therapy and a poor prognosis in gastric cancer (64–66).
Despite numerous studies having investigated whether CD44, CD44v6, and CD44v9 may be potential prognostic indicators in many cancers, some findings are controversial. The impact of the expression of CD44, CD44v6, and CD44v9 on the prognosis of patients with advanced cancer has not been fully understood. Therefore, we conducted the first systematic meta-analysis to reveal the association of CD44 and its isoform CD44v6 and CD44v9 with the prognosis of advanced cancer patients by using multivariable survival data.
No statistical association was found between CD44 expression and DFS, RFS, MFS, or PFS. The expression of CD44 was not significantly associated with OS using multivariable analysis (35, 42, 43), but other studies showed a significant association between CD44 expression and shorter OS (45, 46). We integrated all eligible studies with a relatively large population, and found that CD44 expression was slightly linked to unfavorable OS of advanced cancer (HR = 2.03, P = 0.027). However, TSA suggested that this result was not reliable, and additional studies are needed.
To further investigate the impact of 5-years survival and adjuvant therapy, stratified analysis showed that CD44 expression was not correlated with OS at ≥5 years or in patients treated with adjuvant therapy. No relationship was reported between CD44v6 expression and OS in metastatic non-small cell lung carcinoma (33), but CD44v6 expression was correlated with worse OS in advanced epithelial ovarian cancer (39). Pooled results demonstrated no correlation between CD44v6 expression and OS in advanced cancer.
CD44v9 expression was closely associated with unfavorable CSS (37, 41, 47), and pooled data from three studies also showed a correlation in advanced cancer patients treated with surgery and chemo/radiotherapy (5-years CSS: HR = 3.62, P < 0.001). The above analyses suggested that CD44v9 may be a major variant of the CD44 family and play an important role in the prognosis of patients with advanced cancer. These results suggest that CD44v9 may provide more useful prognostic value for advanced cancer patient classification and survival benefit and could become a targeted selective treatment approach. However, we used TSA to check the reliability of these results, and TSA suggested that the available study population was insufficient to provide conclusive evidence. Additional studies are needed to further validate these conclusions in the future.
Although the analysis of the eligible studies revealed deficiencies in some items of the REMARK guidelines, in the present meta-analysis, the included studies based on multivariate survival analysis showed higher methodologic quality than studies based on univariate survival analysis. Several limitations should be acknowledged in this meta-analysis. First, the number of the included studies and sample sizes were relatively small, although all eligible studies were well-performed with data from multivariable survival analysis. Our conclusions should be interpreted with caution based on the TSA. Second, most studies were conducted in Japan and the USA and other study sources were lacking. Only one study was a prospective phase II trial and the other remaining studies were of retrospective design. Additional prospective clinical trials are necessary. The cut-off values of expression from the immunohistochemical studies may differ, and in the future, CD44s or CD44v expression should be defined as positive or negative based on a standard. Third, although CD44 expression was linked to worse CSS, CD44v6 expression was associated with worse PFS, CD44v9 was correlated with unfavorable RFS, and CD44v5 and CD44v10 were linked to poor DFS (34). These results were only reported by an individual study each. Finally, studies of different cancer types are strongly needed to further confirm the results regarding CD44s and CD44v1–v10 in advanced tumors.
In conclusion, the present study demonstrated that CD44 expression was linked to unfavorable OS, but there was no association between CD44 expression and OS at ≥5-years or OS in patients receiving adjuvant therapy. No association was found between CD44v6 expression and 5-years OS. CD44v9 expression was closely associated with worse 5-years CSS for patients with advanced cancer treated with surgery and chemo/radiotherapy, which suggested that CD44v9 may be a promising prognostic marker. TSA showed that these results cannot be considered conclusive. There is a need for predefined training and validation sets based on the REMARK guidelines, and additional prospective clinical trials in advanced cancer are necessary to further determine whether CD44s and CD44v (v1–v10) may help stratify different cancer patients who could benefit from chemo/radiotherapy.
Ethics Statement
The present study was not primary research involving human samples in the public databases.
Author Contributions
SH, TH, and FH contributed to the conception and design of this research. SH, XWu, XWa, WL, SL, WY, QS, and HL contributed to the drafting of the article and final approval of the submitted version. SH, TH, XWu, XWa, WL, SL, WY, QS, HL, and FH contributed to data analyses and the interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the Natural Science Foundation of China (81473624). The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00039/full#supplementary-material
Table S1. REMARK guidelines.
Table S2. Detailed characteristics of the eligible studies in the meta-analysis.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharm Des. (2010) 16:3–10. doi: 10.2174/138161210789941847
3. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
4. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. (2016) 14:73. doi: 10.1186/s12916-016-0623-5
5. Martinelli E, Morgillo F, Troiani T, Ciardiello F. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: the role of MEK. Cancer Treat Rev. (2017) 53:61–9. doi: 10.1016/j.ctrv.2016.12.001
6. Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. (2018) 2:CD011123. doi: 10.1002/14651858.CD011123.pub2
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
8. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature (2001) 414:105–11. doi: 10.1038/35102167
9. Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell (2017) 20:233–46 e237. doi: 10.1016/j.stem.2016.11.003
10. Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. (2015) 4:1033–43. doi: 10.5966/sctm.2015-0048
11. Han S, Yang W, Zong S, Li H, Liu S, Li W, et al. Clinicopathological, prognostic and predictive value of CD166 expression in colorectal cancer: a meta-analysis. Oncotarget (2017a) 8:64373–84. doi: 10.18632/oncotarget.17442
12. Han S, Zong S, Shi Q, Li H, Liu S, Yang W, et al. Is Ep-CAM expression a diagnostic and prognostic biomarker for colorectal cancer? A systematic meta-analysis. EBioMed. (2017b) 20:61–9. doi: 10.1016/j.ebiom.2017.05.025
13. Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. (1997) 71:241–319. doi: 10.1016/S0065-230X(08)60101-3
14. Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. (2011) 121:1064–74. doi: 10.1172/JCI44540
15. Negi LM, Talegaonkar S, Jaggi M, Ahmad FJ, Iqbal Z, Khar RK. Role of CD44 in tumour progression and strategies for targeting. J Drug Target. (2012) 20:561–73. doi: 10.3109/1061186X.2012.702767
16. Sacks JD, Barbolina MV. Expression and function of CD44 in epithelial ovarian carcinoma. Biomolecules (2015) 5:3051–66. doi: 10.3390/biom5043051
17. Zhao S, Chen C, Chang K, Karnad A, Jagirdar J, Kumar AP, et al. CD44 Expression level and isoform contributes to pancreatic cancer cell plasticity, invasiveness, and response to therapy. Clin Cancer Res. (2016b) 22:5592–604. doi: 10.1158/1078-0432.CCR-15-3115
18. Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene (2013) 32:5191–8. doi: 10.1038/onc.2012.638
19. Fang M, Wu J, Lai X, Ai H, Tao Y, Zhu B, et al. CD44 and CD44v6 are correlated with gastric cancer progression and poor patient prognosis: evidence from 42 studies. Cell Physiol Biochem. (2016) 40:567–78. doi: 10.1159/000452570
20. Zhao L, Gu C, Huang K, Zhang Z, Ye M, Fan W, et al. The prognostic value and clinicopathological significance of CD44 expression in ovarian cancer: a meta-analysis. Arch Gynecol Obstet. (2016a) 294:1019–29. doi: 10.1007/s00404-016-4137-3
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
22. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
23. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ (2011) 343:d2090. doi: 10.1136/bmj.d2090
24. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. (2005) 97:1180–4. doi: 10.1093/jnci/dji237
25. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics (2005) 21:3672–3. doi: 10.1093/bioinformatics/bti536
26. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials (2007) 28:105–14. doi: 10.1016/j.cct.2006.04.004
27. Evangelou E, Ioannidis JP. Meta-analysis methods for genome-wide association studies and beyond. Nat Rev Genet. (2013) 14:379–89. doi: 10.1038/nrg3472
28. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
30. Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. (2009) 38:276–86. doi: 10.1093/ije/dyn179
31. Miladinovic B, Mhaskar R, Hozo I, Kumar A, Mahony H, Djulbegovic B. Optimal information size in trial sequential analysis of time-to-event outcomes reveals potentially inconclusive results because of the risk of random error. J Clin Epidemiol. (2013) 66:654–9. doi: 10.1016/j.jclinepi.2012.11.007
32. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
33. Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Expression of proliferating cell nuclear antigen and CD44 variant isoforms in the primary and metastatic sites of nonsmall cell lung carcinoma with intrapulmonary metastases. Cancer (1999) 86:1174–81.
34. Rodriguez-Rodriguez L, Sancho-Torres I, Mesonero C, Gibbon DG, Shih WJ, Zotalis G. The CD44 receptor is a molecular predictor of survival in ovarian cancer. Med Oncol. (2003) 20:255–63. doi: 10.1385/MO:20:3:255
35. Singh M, Darcy KM, Brady WE, Clubwala R, Weber Z, Rittenbach JV, et al. Cadherins, catenins and cell cycle regulators: impact on survival in a Gynecologic Oncology Group phase II endometrial cancer trial. Gynecol Oncol. (2011) 123:320–8. doi: 10.1016/j.ygyno.2011.07.005
36. Koukourakis MI, Giatromanolaki A, Tsakmaki V, Danielidis V, Sivridis E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br J Cancer (2012) 106:846–53. doi: 10.1038/bjc.2012.33
37. Aso T, Matsuo M, Kiyohara H, Taguchi K, Rikimaru F, Shimokawa M, et al. Induction of CD44 variant 9-expressing cancer stem cells might attenuate the efficacy of chemoradioselection and Worsens the prognosis of patients with advanced head and neck cancer. PLoS ONE (2015) 10:e0116596. doi: 10.1371/journal.pone.0116596
38. Marzese DM, Liu M, Huynh JL, Hirose H, Donovan NC, Huynh KT, et al. Brain metastasis is predetermined in early stages of cutaneous melanoma by CD44v6 expression through epigenetic regulation of the spliceosome. Pigment Cell Melanoma Res. (2015) 28:82–93. doi: 10.1111/pcmr.12307
39. Tjhay F, Motohara T, Tayama S, Narantuya D, Fujimoto K, Guo J, et al. CD44 variant 6 is correlated with peritoneal dissemination and poor prognosis in patients with advanced epithelial ovarian cancer. Cancer Sci. (2015) 106:1421–8. doi: 10.1111/cas.12765
40. Udagawa H, Ishii G, Morise M, Umemura S, Matsumoto S, Yoh K, et al. Comparison of the expression levels of molecular markers among the peripheral area and central area of primary tumor and metastatic lymph node tumor in patients with squamous cell carcinoma of the lung. J Cancer Res Clin Oncol. (2015) 141:1417–25. doi: 10.1007/s00432-015-1912-7
41. Hagiwara M, Kikuchi E, Kosaka T, Mikami S, Saya H, Oya M. Variant isoforms of CD44 expression in upper tract urothelial cancer as a predictive marker for recurrence and mortality. Urol Oncol. (2016) 34:337 e319–26. doi: 10.1016/j.urolonc.2016.03.015
42. Linge A, Lock S, Gudziol V, Nowak A, Lohaus F, von Neubeck C, et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin Cancer Res. (2016) 22:2639–49. doi: 10.1158/1078-0432.CCR-15-1990
43. Ribeiro KB, da Silva Zanetti J, Ribeiro-Silva A, Rapatoni L, de Oliveira HF, da Cunha Tirapelli DP, et al. KRAS mutation associated with CD44/CD166 immunoexpression as predictors of worse outcome in metastatic colon cancer. Cancer Biomark. (2016) 16:513–21. doi: 10.3233/CBM-160592
44. Baschnagel AM, Tonlaar N, Eskandari M, Kumar T, Williams L, Hanna A, et al. Combined CD44, c-MET, and EGFR expression in p16-positive and p16-negative head and neck squamous cell carcinomas. J Oral Pathol Med. (2017) 46:208–13. doi: 10.1111/jop.12478
45. Sun H, Liu T, Zhu D, Dong X, Liu F, Liang X, et al. HnRNPM and CD44s expression affects tumor aggressiveness and predicts poor prognosis in breast cancer with axillary lymph node metastases. Genes Chromosomes Cancer (2017) 56:598–607. doi: 10.1002/gcc.22463
46. Boxberg M, Gotz C, Haidari S, Dorfner C, Jesinghaus M, Drecoll E, et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphologic parameters and clinicopathological factors. Histopathology (2018) 73:559–72. doi: 10.1111/his.13496
47. Hagiwara M, Kikuchi E, Tanaka N, Kosaka T, Mikami S, Saya H, et al. Variant isoforms of CD44 involves acquisition of chemoresistance to cisplatin and has potential as a novel indicator for identifying a cisplatin-resistant population in urothelial cancer. BMC Cancer (2018) 18:113. doi: 10.1186/s12885-018-3988-3
48. Mentha G, Terraz S, Andres A, Toso C, Rubbia-Brandt L, Majno P. Operative management of colorectal liver metastases. Semin Liver Dis. (2013) 33:262–72. doi: 10.1055/s-0033-1351785
49. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. (2017) 15:109. doi: 10.1186/s12916-017-0872-y
50. Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. (2011) 2011:941876. doi: 10.1155/2011/941876
51. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer (2012) 12:133–43. doi: 10.1038/nrc3184
52. Rajarajan A, Stokes A, Bloor BK, Ceder R, Desai H, Grafstrom RC, et al. CD44 expression in oro-pharyngeal carcinoma tissues and cell lines. PLoS ONE (2012) 7:e28776. doi: 10.1371/journal.pone.0028776
53. Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer (2011) 11:254–67. doi: 10.1038/nrc3023
54. Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther. (2015) 8:3783–92. doi: 10.2147/OTT.S95470
55. Hasebe T, Fujimoto K, Kajita M, Ishizuya-Oka A. Essential roles of thyroid hormone-regulated hyaluronan/CD44 signaling in adult stem cell development during Xenopus laevis intestinal remodeling. Stem Cells (2017) 35:2175–83. doi: 10.1002/stem.2671
56. Chen Y, Fu Z, Xu S, Xu Y, Xu P. The prognostic value of CD44 expression in gastric cancer: a meta-analysis. Biomed Pharmacother. (2014) 68:693–7. doi: 10.1016/j.biopha.2014.08.001
57. Li X, Ma X, Chen L, Gu L, Zhang Y, Zhang F, et al. Prognostic value of CD44 expression in renal cell carcinoma: a systematic review and meta-analysis. Sci Rep. (2015) 5:13157. doi: 10.1038/srep13157
58. Palyi-Krekk Z, Barok M, Isola J, Tammi M, Szollosi J, Nagy P. Hyaluronan-induced masking of ErbB2 and CD44-enhanced trastuzumab internalisation in trastuzumab resistant breast cancer. Eur J Cancer (2007) 43:2423–33. doi: 10.1016/j.ejca.2007.08.018
59. Zhang X, He F, Xiang K, Zhang J, Xu M, Long P, et al. CD44-Targeted facile enzymatic activatable chitosan nanoparticles for efficient antitumor therapy and reversal of multidrug resistance. Biomacromolecules (2018) 19:883–95. doi: 10.1021/acs.biomac.7b01676
60. Fu Y, Geng Y, Yang N, Zhu N, Wang CZ, Su XC, et al. CD44v6 expression is associated with a poor prognosis in Chinese hepatocellular carcinoma patients: a meta-analysis. Clin Res Hepatol Gastroenterol. (2015) 39:736–9. doi: 10.1016/j.clinre.2015.03.001
61. Lu L, Huang F, Zhao Z, Li C, Liu T, Li W, et al. CD44v6: a metastasis-associated biomarker in patients with gastric cancer?: a comprehensive meta-analysis with heterogeneity analysis. Medicine (2016) 95:e5603. doi: 10.1097/MD.0000000000005603
62. Li Z, Chen K, Jiang P, Zhang X, Li X, Li Z. CD44v/CD44s expression patterns are associated with the survival of pancreatic carcinoma patients. Diagn Pathol. (2014) 9:79. doi: 10.1186/1746-1596-9-79
63. Kobayashi K, Matsumoto H, Matsuyama H, Fujii N, Inoue R, Yamamoto Y, et al. Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol Rep. (2016) 36:2852–60. doi: 10.3892/or.2016.5061
64. Kodama H, Murata S, Ishida M, Yamamoto H, Yamaguchi T, Kaida S, et al. Prognostic impact of CD44-positive cancer stem-like cells at the invasive front of gastric cancer. Br J Cancer (2017) 116:186–94. doi: 10.1038/bjc.2016.401
65. Yamakawa Y, Kusuhara M, Terashima M, Kinugasa Y, Sugino T, Abe M, et al. CD44 variant 9 expression as a predictor for gastric cancer recurrence: immunohistochemical and metabolomic analysis of surgically resected tissues. Biomed Res. (2017) 38:41–52. doi: 10.2220/biomedres.38.41
Keywords: advanced cancer, CD44v9, prognosis, CD44, therapy
Citation: Han S, Huang T, Li W, Wang X, Wu X, Liu S, Yang W, Shi Q, Li H and Hou F (2019) Prognostic Value of CD44 and Its Isoforms in Advanced Cancer: A Systematic Meta-Analysis With Trial Sequential Analysis. Front. Oncol. 9:39. doi: 10.3389/fonc.2019.00039
Received: 25 July 2018; Accepted: 15 January 2019;
Published: 06 February 2019.
Edited by:
Barbara Zavan, University of Padova, ItalyReviewed by:
Francine Baumann, University of New Caledonia, FranceJavid P. Mohammed, North Carolina State University, United States
Copyright © 2019 Han, Huang, Li, Wang, Wu, Liu, Yang, Shi, Li and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susu Han, anyasue@163.com
Tao Huang, huangtao334@163.com
Fenggang Hou, fghou555@126.com
†These authors share co-first authorship
 Susu Han1*†
Susu Han1*† Tao Huang
Tao Huang Xing Wu
Xing Wu