- Department of Radiation Oncology, Georgetown University, Washington, DC, USA
Introduction: Colorectal cancer (CRC) is the third leading cause of cancer-related death in the U.S. Many patients with CRC develop hepatic metastases as the sole site of metastases. Historical treatment options were limited to resection or conventional radiation therapy. Stereotactic body radiation therapy (SBRT) has emerged as a rational treatment approach. This study reviews our experience with SBRT for patients with liver metastases from CRC. Materials and Methods: Fourteen histologically confirmed hepatic CRC metastases in 11 consecutive patients were identified between November, 2004 and June, 2009 at Georgetown University. All patients underwent CT-based treatment planning; a few also had MRI or PET/CT. All patients had fiducial markers placed under CT guidance and were treated using the CyberKnife system. Treatment response and toxicities were examined; survival and local control were evaluated. Results: Most patients were treated to a single hepatic lesion (n = 8), with a few treated to two lesions (n = 3). Median treatment volume was 99.7 cm3, and lesions were treated to a median BED10 of 49.7 Gy (range: 28–100.8 Gy). Median follow-up was 21 months; median survival was 16.1 months, with 2 year actuarial survival of 25.7%. One year local control was 72%. Among patients with post-treatment imaging, eight had stable disease (80%) and two had progressive disease (20%) at first follow-up. The most common grade 1–2 acute toxicities included nausea and alterations in liver function tests; there was one grade 3 toxicity (elevated bilirubin), and no grade 4–5 toxicities. Discussion: SBRT is safe and feasible for the treatment of limited hepatic metastases from CRC. Our results compare favorably with outcomes from previous studies of SBRT. Further studies are needed to better define patient eligibility, study the role of combined modality treatment, optimize treatment parameters, and characterize quality of life after treatment.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer, with 141,210 estimated new diagnoses in 2011 in the U.S., and it is the third leading cause of death, with 49,380 estimated deaths in 2011 (Siegel et al., 2011). Metastatic disease is common, and has been shown to be present in 25% of patients at initial diagnosis, and up to 50% of patients over the course of their disease (Taylor, 1996). Autopsy series have demonstrated that some patients with metastatic CRC die with the liver being the first and solitary site of metastatic disease (Weiss et al., 1986).
Limited metastatic disease to the liver is primarily managed by surgical resection (Scheele et al., 1990). For patients undergoing resection, historical series have shown 5 year survivals of 25–39%, and more modern studies of highly selected patients have demonstrated 5 year survival rates up to 67% (Fong et al., 1995; Simmonds et al., 2006). However, the best surgical outcomes are obtained in patients with low CEA levels, few metastases, small lesions, limited resection volume, and clear post-operative margins (Cady et al., 1998; Fong et al., 1999). Traditionally, surgical resection has only been offered to 10–20% of presenting patients, although the criteria for operability have expanded. Using multimodality approaches and re-centering resectability on hepatic functional reserve and the likelihood of negative margins (Adam, 2003; Pawlik et al., 2008). However, many patients with metastatic disease will either have lesions that are not amenable to surgery due to compromised hepatic reserve or the small likelihood of negative margins; or they may be unfit for surgeries due to medical comorbidities, functional status, or progression of disease outside of the liver. Twenty percent of patients may refuse resection (Alberts et al., 2005; Garden et al., 2006).
Non-conformal RT has had a limited role in treatment of hepatic metastases since radiation-induced liver disease (RILD) has been linked to mean liver dose (Lawrence et al., 1992; Cheng et al., 2002). Modern RT techniques can minimize dose to normal liver with 3D-conformal radiation therapy or intensity-modulated radiation therapy (IMRT; Krishnan et al., 2006). However, even these technical advances can still deposit low-dose radiation in a large volume of liver, continuing to limit dose-escalation for effective tumor control. The majority of liver tumors move with the respiratory cycle. This movement requires the addition of treatment margin, which increases the dose delivered to normal tissue. While it is important to limit dose to normal liver, further studies have demonstrated the important impact of dose-escalation of RT on control of hepatic metastases from CRC (Mohiuddin et al., 1996; Dawson et al., 2000).
Stereotactic body radiation therapy (SBRT) has been successfully employed for the treatment of tumors in various disease sites. Historically, single- or multi-fraction SBRT has been employed for the treatment of brain metastases (Kondziolka, 2005; Aoyama et al., 2006), and more recently it has been successfully used for the treatment of early stage lung cancer (Grills et al., 2010; Timmerman et al., 2010). For patients with limited liver metastasis from CRC who are not surgical candidates or refuse surgery, SBRT is a rational treatment approach, allowing for dose-escalation to the tumor volume while minimizing dose delivered to adjacent normal tissue. SBRT with respiratory tracking for liver tumors has been shown to be feasible (Wurm et al., 2006), while permitting effective dose-escalation and maintaining quality of life (Schefter et al., 2005; Mendez Romero et al., 2008; Goodman et al., 2010). In this study, we aim to review our experience with treating patients with liver metastases from CRC and demonstrate the safety, feasibility, and early outcomes of hypofractionated, image-guided SBRT with implanted fiducial markers.
Materials and Methods
Patient Selection
This retrospective review was approved by the Institutional Review Board (IRB) of Georgetown University. Fourteen lesions in 11 consecutive patients treated with SBRT for liver metastases from histologically confirmed CRC between November, 2004 and June, 2009 were identified from treatment records at Georgetown University Hospital’s Department of Radiation Oncology. All patients were not considered candidates for surgical resection. Lesions were considered for treatment in any location within the liver, including right, left, and caudate lobes, as well as proximal to the porta hepatis. Patients were included irrespective of prior treatment, including prior treatment with chemotherapy, surgery, or radiation therapy. Patients generally were expected to have a life expectancy greater than 6 months and adequate hepatic function.
SBRT Planning and Treatment
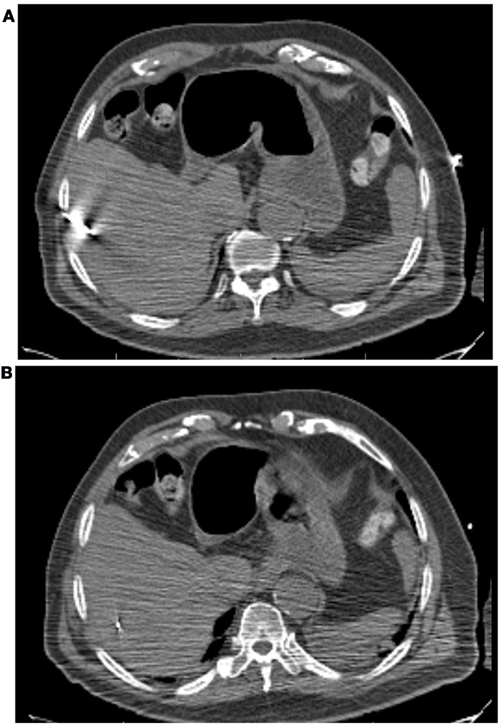
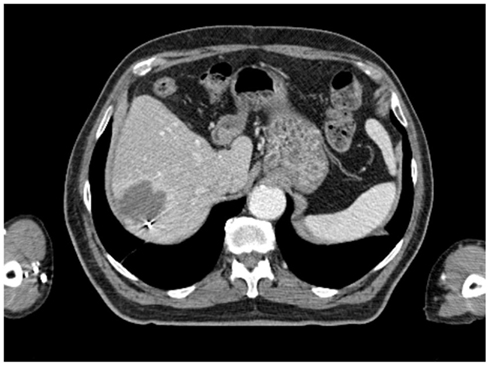
All patients had three to five gold fiducials placed under CT guidance (Best Medical, Springfield, VA, USA). An example of CT-guided placement of fiducial markers is shown in Figure 1. A treatment planning CT scan with IV contrast and slice thickness of 1–3 mm was obtained greater than 5 days after fiducial placement. Patients were simulated in the supine position. A gross tumor volume (GTV) was delineated on the CT scan. To aid in target delineation, two patients also had MRIs and one patient had a PET scan fused with their treatment planning CT scans. Typically, a margin of at least 3–5 mm was added to the GTV to form the clinical target volume (CTV). This expansion is similar to the 5 mm expansion used in an ongoing Accuray trial of CyberKnife liver SBRT (Clinicaltrials.gov, 2011a), and the 5 mm expansion used in the RTOG 0438 Phase I trial for liver SBRT (Radiation Therapy Oncology Group, 2011). No additional margin was added to form the planning target volume (PTV), due to the accuracy of fiducial tracking. Adjacent critical structures were delineated. An example of pre-treatment CT scan is shown in Figure 2.
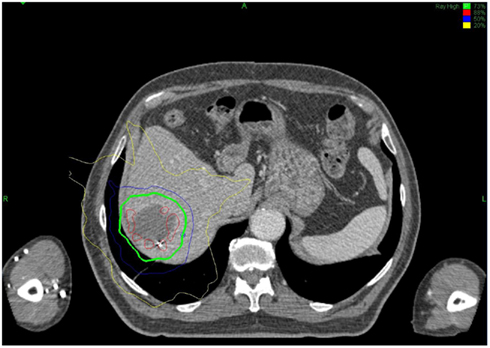
All treatments were performed using the CyberKnife system and were planned using Multiplan treatment planning software. Radiation plans and dose prescriptions were developed using an inverse-planning methodology. Treatments were delivered using 6MV photons from the CyberKnife system and were prescribed to the isodose line that provided adequate coverage of the PTV (>95%). Synchrony Respiratory Tracking System was used to continuously track fiducial position and adjust for respiratory motion during treatment. Patients were treated to a median dose of 28.5 Gy (range: 16–42 Gy), in a median of three fractions (range: 2–5). A biologic equivalent dose (BED) was calculated for each fractionation scheme by the formula: BED10 = (prescription dose) × [1+ (dose per fraction/α/β)]; were α/β is assumed to be 10. A sample treatment plan is shown in Figure 3.
Data Analysis
Treatment response was evaluated by serial CT, PET, and/or MRI scans. Estimates of initial treatment response were determined using the response evaluation criteria in solid tumors (RECIST; Eisenhauer et al., 2009). Local control was defined as no evidence of tumor growth of the treated lesion. Actuarial survival and local control rates were evaluated by the Kaplan–Meier method. Univariate analysis was performed with the log rank test. Toxicities were evaluated according to the common terminology criteria for adverse events (CTCAE), Version 4.0 (National Cancer Institute, 2010). Toxicities occurring less than or equal to 3 months following SBRT were considered acute, while toxicities occurring after 3 months were considered late.
Results
Patient Characteristics
Patient and lesion characteristics are noted in Table 1. Most patients were male (10) and had a median age at the time of SBRT of 69 years old. Three patients were treated to two liver lesions each, and eight patients were each treated to a single hepatic lesion. Most lesions were in the right lobe of the liver (eight), with few in the left lobe (three), porta hepatis (two), or caudate (two).
Treatment Parameters
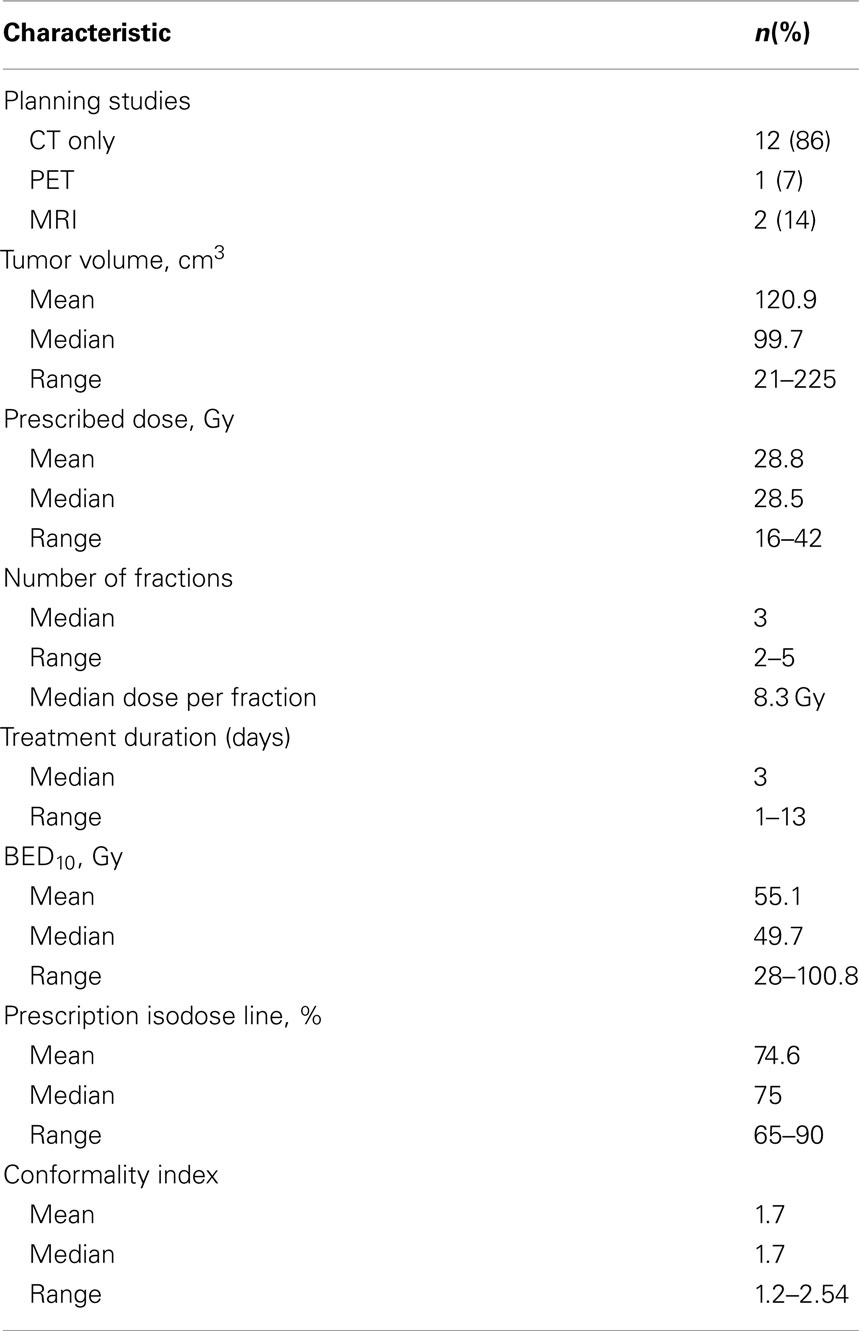
For 12 of the lesions (86%), CT was the only treatment planning study performed. Lesions had a wide range of volumes, with median volume of 99.7 cm3 (range: 21–225 cm3; Table 2). Patients were treated to a median BED10 of 49.7 Gy (range: 28–100.8 Gy). Patients were treated over a median course of 3 days (range: 1–13 days). The median prescription isodose line was 75% (65–90%). Comprehensive treatment variables are listed in Table 2.
Clinical Outcomes
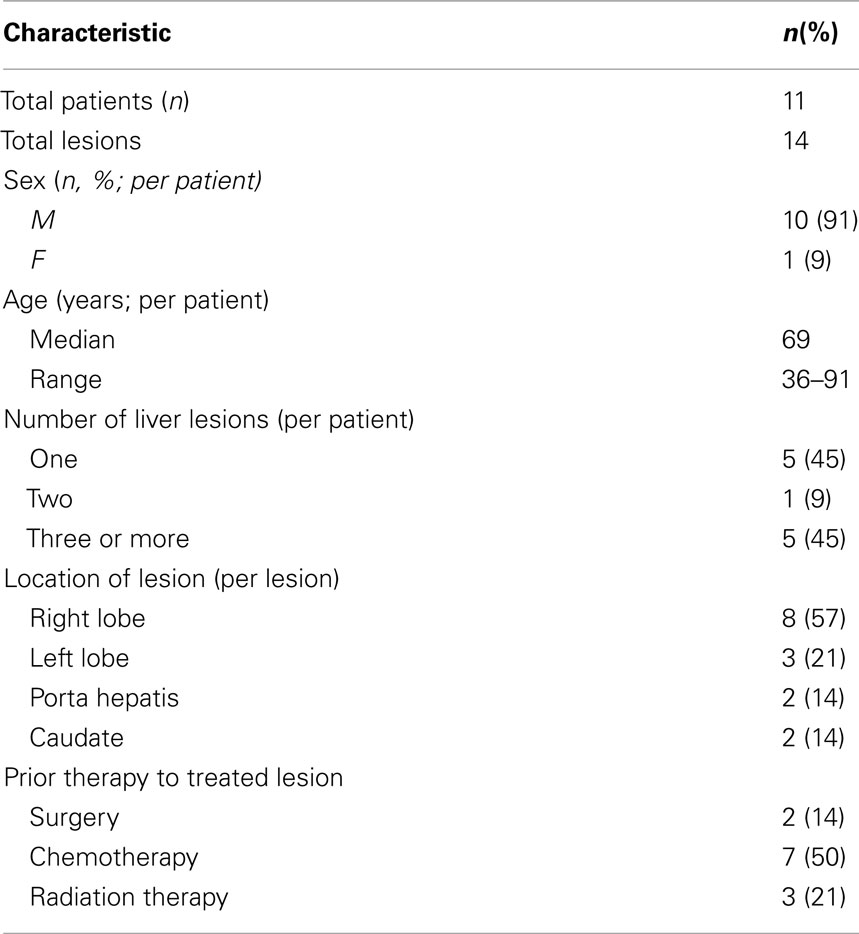
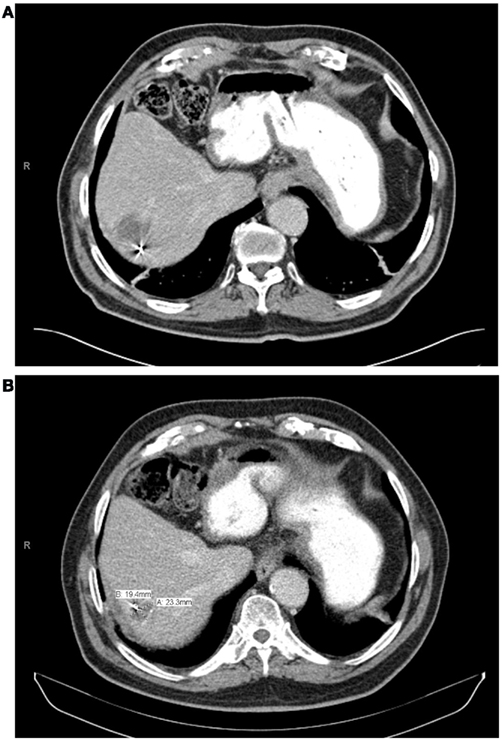
With a median follow-up of 21 months, the median survival was 16.1 months and the 2 year actuarial overall survival was 25.7%. Of the lesions with evaluable post-treatment radiographic imaging, eight had stable disease (80%), and two had progressive disease (20%) at first imaging follow-up. An example of treatment response as determined by CT scan is demonstrated in Figure 4. None of these patients had a complete or partial response. One year Kaplan–Meier estimate of local control was 72%, and only three lesions demonstrated radiographic evidence of local failure at last follow-up. On univariate analysis, the only factor associated with local failure was initial RECIST response to SBRT (p = 0.0047). None of the remaining factors, including BED10, conformality index, prescription isodose line, and location within the liver were statistically significantly associated with local failure.
Toxicity
Toxicities were evaluated according to the CTCAE, Version 4.0. The most common acute toxicities included nausea (three) and alterations in liver function tests (two). There was only one grade 3 toxicity (elevated bilirubin), and no grade 4 or 5 toxicities.
Discussion
The results from our retrospective case series demonstrate the safety and feasibility of hypofractionated SBRT for the treatment of liver metastases from CRC. During treatment, tumors were continuously tracked by implanted fiducial markers, allowing a reduction in tumor margins. With a median follow-up of 21 months, the 1 year local control rate was 72% and 2 year overall survival rate was 26%. The treatment was well tolerated, with only one patient developing a grade 3 elevation of bilirubin. Importantly, treatment was generally delivered in less than 2 weeks, allowing for rapid resumption of systemic therapy.
Previous Experience with Liver SBRT
Several previous series have been published evaluating the use of SBRT for the treatment of liver metastases from multiple primary sites (Katz et al., 2007; Lee et al., 2009; Rule et al., 2011). In a phase I dose-escalation study, local control was 57% at 20 months, with median OS of 14.5 months, and 1 year progression-fee survival 24% (Katz et al., 2007). A review of several case series demonstrated excellent local control rates (78–100%), limited grade 1–2 acute toxicity (0–29%), and minimal grade 3–4 toxicity (0–5%; Katz et al., 2007). A recent dose-escalation study that treated a large median volume of normal liver (39%) demonstrated safety and efficacy of SBRT doses up to 60 Gy delivered in up to six fractions over 2 weeks, noting that the maximum safely tolerated doses may be even higher (Lee et al., 2009).
Specifically for CRC metastases to the liver, one group treated patients in the same regimen as a current prospective trial, with 15 Gy in three fractions; 19% of those patients were without disease progression after 2 years, and OS at 2 years was 38% (Hoyer et al., 2006). In contrast to our study, significant toxicity was observed, including hepatic failure leading to death, colonic perforation, and duodenal ulcerations (Hoyer et al., 2006). A pooled analysis demonstrated that total dose, dose per fraction, and BED correlated with local control; inactive extrahepatic disease was associated with overall survival; and local control was strongly correlated with OS, emphasizing the significance of controlling intra-hepatic disease (Chang et al., 2011).
As demonstrated by previous studies of SBRT for liver metastases in multiple different histologies and specifically for colorectal metastases, the optimal dose, and fractionation scheme has not yet been determined. A dose–response effect has been observed in heterogeneous groups of patients, with improved local control with higher fractionated SBRT dose (60 Gy as compared with 30 Gy), or higher single-fraction SBRT dose (22–26 vs. 14–20 Gy; Herfarth et al., 2004; Rule et al., 2011). The safety and efficacy of higher–dose fractionated SBRT (60 Gy in three fractions) has been demonstrated, with local control rates of 92% at 2 years and low rates of grade 3 or higher toxicity (Rusthoven et al., 2009). We did not observe a dose–response relationship.
Ongoing Studies
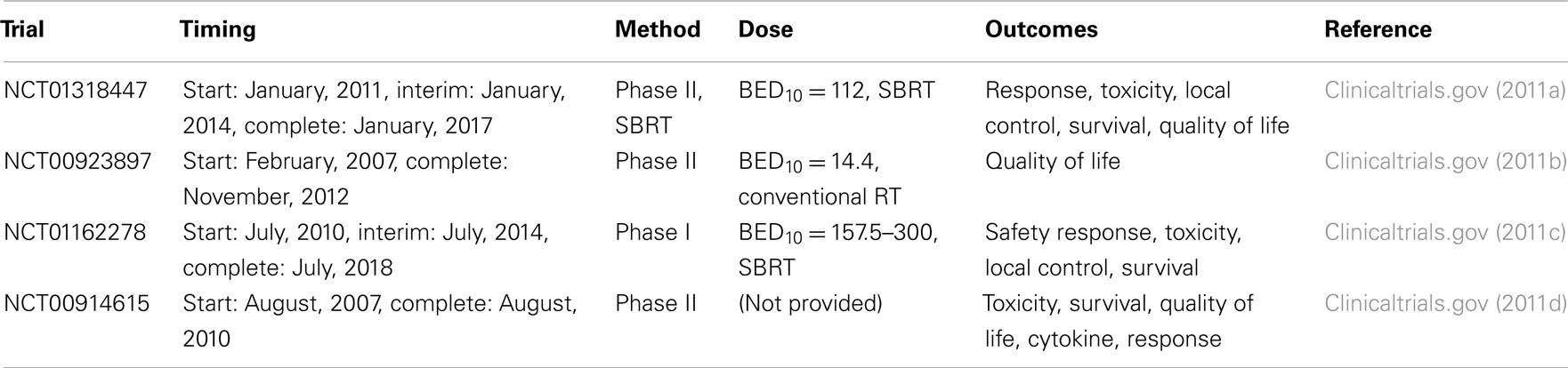
A summary of ongoing studies regarding the use of SBRT and conventional radiotherapy in the treatment of liver metastases is provided in Table 3. These trials utilize higher doses of RT compared with our study and will provide important data regarding clinical outcomes and quality of life after SBRT. The outcomes from all of these trials will help to inform future therapeutic decision-making regarding patient selection, prognosis, and quality of life after treated with SBRT for liver metastases.
The Future of SBRT for Hepatic Colorectal Metastases
Despite the current ongoing trials, there will still be a significant need for robust data regarding the utility and efficacy of SBRT for the treatment of liver metastases. Future directions for research would optimally evaluate which patients are eligible for liver SBRT, including whether SBRT might be a reasonable alternatively to limited surgery, or only used when surgery is not an option. Additionally, dosimetric parameters need optimization to determine the most effective total and fractional doses to achieve maximal, long-term local control.
Although SBRT can be an important part of treatment for these patients, they also are likely to utilize other therapeutic techniques for local or distant control, which might include radiofrequency ablation (RFA), transarterial chemoembolization (TACE), surgery, or systemic chemotherapy. Further research is needed to understand the interaction of these multiple therapeutic techniques and to determine the best sequencing of these modalities for primary or salvage therapy. In addition to examining optimal therapy in this highly specific patient population, lessons learned in the treatment of hepatic metastases from CRC with SBRT could be extrapolated and further studied to determine optimum treatment strategies for other primary malignancies which might similarly have long-term survival even in the setting of hepatic metastases, such as breast cancer.
Our study focused on the technique of CyberKnife SBRT and on local control, but more research is needed regarding the overall impact of SBRT and control of hepatic disease on patient-reported outcomes (PRO) in the palliative setting, and in overall disease-free and overall survival in both patients treated with curative and palliative intent. Finally, as SBRT techniques improve, comparative effectiveness research will also be needed to compare among local–regional therapies, including surgery, RFA, TACE, and SBRT, to determine the best therapeutic approaches for individual patients.
Limitations
Limitations include the retrospective nature of this series and heterogeneous treatment schedules: there was a wide range of doses prescribed, and a variety of fractionation schema. Although this demonstrates that several different types of technical approaches may be reasonable for the treatment of liver metastases from CRC, our study does not refine the prescription technique to optimize local control. Patients also were not compared with those undergoing alternate loco-regional treatment modalities, including TACE or RFA. Finally, patient follow-up needs to be longer in order to examine both local control and impact of local control on local–regional control and its interplay with distant disease control and, ultimately, on survival.
Conclusion
This study demonstrates that SBRT for hepatic metastases from CRC is safe and feasible in a small patient cohort, including those who would not have been candidates for hepatectomy with negative margins. Although the majority of patients died within our follow-up, many had effectively controlled local disease after treatment. Our methodology is similar to those presented in recently published trials, although our median BED10 is lower than some trials demonstrated to be most effective for dose-escalation. Given the size of our sample, local control and overall survival among our patients compare favorably with published series. Toxicity in the presented series also compares favorably with historical data. Although some trials report small numbers of grade 3–5 events, our study demonstrates only one grade 3 liver enzyme abnormality. This study hypothesis-generating, serving as a basis for designing a prospective clinical study to better characterize patient eligibility, optimize combinations of therapeutic modalities, and define treatment parameters to maximize local control, survival, and quality of life following SBRT.
Conflict of Interest Statement
Dr. Brian Collins and Dr. Sean Collins are clinical consultants for accuray. Clinical consult includes speaking. The remaining authors have no commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was not supported by outside funding.
References
Adam, R. (2003). Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann. Oncol. 14, ii13–ii16.
Alberts, S. R., Horvath, W. L., Sternfeld, W. C., Goldberg, R. M., Mahoney, M. R., Dakhil, S. R., Levitt, R., Rowland, K., Nair, S., Sargent, D. J., and Donohue, J. H. (2005). Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 23, 9243–9249.
Aoyama, H., Shirato, H., Tago, M., Nakagawa, K., Toyoda, T., Katano, K., Kenjyo, M., Oya, N., Hirota, S., Shioura, H., Kunieda, F., Inomata, T., Hayakawa, K., Kato, N., and Kobashi, G. (2006). Stereotactic radiosrugery plus whole-brain radiation therapy vs. stereotactice radiosurgery alone for treatment of brain metastases: a randomized controlled trial. J. Am. Med. Assoc. 295, 2486–2491.
Cady, B., Jenkins, R. L., Steele, G. D., Lewis, W. D., Stone, M. D., Mcdermott, W. V., Jessup, J. M., Bothe, A., Lalor, P., Lovett, E. J., Lavin, P., and Linehan, D. C. (1998). Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann. Surg. 227, 566–571.
Chang, D. T., Swaminath, A., Kozak, M., Weintraub, J., Koong, A. C., Kim, J., Dinniwell, R., Brierly, J., Kavanagh, B. D., Dawson, L. A., and Schefter, T. E. (2011). Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer [Epub ahead of print].
Cheng, J. C., Wu, J. K., Huang, C. M., Liu, H. S., Huang, D. Y., Cheng, S. H., Tsai, S. Y., Jian, J. J., Lin, Y. M., Cheng, T. I., Horng, C. F., and Huang, A. T. (2002). Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int. J. Radiat. Oncol. Biol. Phys. 54, 156–162.
Clinicaltrials.gov. (2011a). CyberKnife® for Hepatic Metastases from Colorectal Cancer: NCT01318447. Available at: http://clinicaltrials.gov/ct2/show/study/NCT01318447?show_desc=Y#desc [accessed August 12, 2011].
Clinicaltrials.gov. (2011b). Palliative Radiotherapy (RT) for Liver Metastases (Mets) and Hepatocellular Carcinoma (HCC): NCT00923897. Toronto. Available at: http://clinicaltrials.gov/ct2/show/NCT00923897?term=hepatic+metastases&rank=1 [accessed August 12, 2011].
Clinicaltrials.gov. (2011c). Stereotactic Body Radiation Therapy (SBRT) for Patients with Hepatic Metastases: NCT01162278. Dallas. Available at: http://clinicaltrials.gov/ct2/show/NCT01162278?term=hepatic+metastases&rank=2 [accessed August 12, 2011].
Clinicaltrials.gov. (2011d). Stereotactic Body Radiation Therapy (SBRT) in Liver Metastasis: NCT00914615. Toronto. Available at: http://clinicaltrials.gov/ct2/show/NCT00914615?term=hepatic+metastases&rank=20 [accessed August 12, 2011].
Dawson, L. A., Mcginn, C. J., Normolle, D., Ten Haken, R. K., Walker, S., Ensminger, W. D., and Lawrence, T. S. (2000). Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 18, 2210–2218.
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., Dancey, J., Arbuck, S., Gwyther, S., Mooney, M., Rubinstein, L., Shankar, L., Dodd, L., Kaplan, R., Lacombe, D., and Verweij, J. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247.
Fong, Y., Blumgart, L. H., and Cohen, A. M. (1995). Surgical treatment of colorectal metastases to the liver. CA Cancer J. Clin. 45, 50–62.
Fong, Y., Fortner, J., Sun, R. L., Brennan, M. F., and Bulmgart, L. H. (1999). Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 230, 309–318.
Garden, O. J., Rees, M., Poston, G. J., Mirza, D., Saunders, M., Ledermann, J., Primrose, J. N., and Parks, R. W. (2006). Guidelines for resection of colorectal cancer liver metastases. Gut 55, iii1–iii8.
Goodman, K. A., Wiegner, E. A., Maturen, K. E., Zhang, Z., Mo, Q., Yang, G., Gibbs, I. C., Fischer, G. A., and Koong, A. C. (2010). Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int. J. Radiat. Oncol. Biol. Phys. 78, 486–493.
Grills, I., Mangona, V., Welshe, R., Chmielewski, G., McInerney, E., and Martin, S. (2010). Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J. Clin. Oncol. 28, 928–935.
Herfarth, K. K., Debus, J., and Wannenmacher, M. (2004). Stereotactic radiation therapy of liver metastases: update of the initial phase-I/II trial. Front. Radiat. Ther. Oncol. 38, 100–105.
Hoyer, M., Roed, H., Traberg Hansen, A., Ohlhuis, L., Petersen, J., Nellemann, H., Kiil Berthelsen, A., Grau, C., Aage Engelholm, S., and Von Der Maase, H. (2006). Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 45, 823–830.
Katz, A. W., Carey-Sampson, M., Muhs, A. G., Milano, M. T., Schell, M. C., and Okunieff, P. (2007). Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int. J. Radiat. Oncol. Biol. Phys. 67, 793–798.
Kondziolka, D., Niranjan, A., Flickinger, J. C., and Lunsford, L. D. (2005). Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am. J. Clin. Oncol. 28, 173–179.
Krishnan, S., Lin, E. H., Gunn, G. B., Chandra, A., Beddar, A. S., Briere, T. M., Das, P., Delclos, M. E., Janjan, N. A., and Crane, C. H. (2006). Conformal radiotherapy of the dominant liver metastasis: a viable strategy for treatment of unresectable chemotherapy refractory colorectal cancer liver metastases. Am. J. Clin. Oncol. 29, 562–567.
Lawrence, T. S., Ten Haken, R. K., Kessler, M. L., Robertson, J. M., Lyman, J. T., Lavigne, M. L., Brown, M. B., Duross, D. J., Andrews, J. C., and Ensminger, W. D. (1992). The use of 3-D dose volume analysis to predict radiation hepatitis. Int. J. Radiat. Oncol. Biol. Phys. 23, 781–788.
Lee, M. T., Kim, J. J., Dinniwell, R., Brierley, J., Lockwood, G., Wong, R., Cummings, B., Ringash, J., Tse, R. V., Knox, J. J., and Dawson, L. A. (2009). Phase I study of individualized stereotactic body radiotherapy of liver metastases. J. Clin. Oncol. 27, 1585–1591.
Mendez Romero, A., Wunderink, W., Van Os, R. M., Nowak, P. J., Heijmen, B. J., Nuyttens, J. J., Brandwijk, R. P., Verhoef, C., Ijzermans, J. N., and Levendag, P. C. (2008). Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 70, 1447–1452.
Mohiuddin, M., Chen, E., and Ahmad, N. (1996). Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J. Clin. Oncol. 14, 722–728.
National Cancer Institute. (2010). Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5 × 7.pdf [accessed August 16, 2011].
Pawlik, T. M., Schulick, R. D., and Choti, M. A. (2008). Expanding criteria for resectability of colorectal liver metastases. Oncologist 13, 51–64.
Radiation Therapy Oncology Group. (2011). RTOG 0438. Available at: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study = 0438 [accessed December 1, 2011].
Rule, W., Timmerman, R., Tong, L., Abdulrahman, R., Meyer, J., Boike, T., Schwarz, R. E., Weatherall, P., and Chinsoo Cho, L. (2011). Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann. Surg. Oncol. 18, 1081–1087.
Rusthoven, K. E., Kavanagh, B. D., Cardenes, H., Stieber, V. W., Burri, S. H., Fieigenberg, S. J., Chidel, M. A., Pugh, T. J., Franklin, W., Kane, M., Gaspar, L. E., and Schefter, T. E. (2009). Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 27, 1572–1578.
Scheele, J., Stangl, R., and Altendorf-Hofmann, A. (1990). Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br. J. Surg. 77, 1241–1246.
Schefter, T. E., Kavanagh, B. D., Timmerman, R. D., Cardenes, H. R., Baron, A., and Gaspar, L. E. (2005). A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int. J. Radiat. Oncol. Biol. Phys. 62, 1371–1378.
Siegel, R., Ward, E., Brawley, O., and Jemal, A. (2011). Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 61, 212–236.
Simmonds, P. C., Primrose, J. N., Colguitt, J. L., Garden, O. J., Poston, G. J., and Rees, M. (2006). Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br. J. Cancer 94, 982–999.
Taylor, I. (1996). Liver metastases from colorectal cancer: lessons from past and present clinical studies. Br. J. Surg. 83, 456–460.
Timmerman, R., Paulus, R., Galvin, J., Michalski, J., Straube, W., Bradley, J., Fakiris, A., Bezjak, A., Videtic, G., Johnstone, D., Fowler, J., Gore, E., and Choy, H. (2010). Stereotactic body radiation therapy for inoperable early stage lung cancer. J. Am. Med. Assoc. 303, 1070–1076.
Weiss, L., Grundmann, E., Torhorst, J., Hartveit, F., Moberg, I., Eder, M., Fenoglio-Preiser, C. M., Napier, J., Horne, C. H., and Lopez, M. J. (1986). Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necroscopies. J. Pathol. 150, 195–203.
Wurm, R. E., Gum, F., Erbel, S., Schlenger, L., Scheffler, D., Agaolglu, D., Schild, R., Gebauer, B., Rogalla, P., Plotkin, M., Ocran, K., and Budach, V. (2006). Image guided respiratory gate hypofractionated stereotactic body radiation therapy (H-SBRT) for liver and lung tumors: initial experience. Acta Oncol. 45, 881–889.
Keywords: colorectal cancer, liver, hepatic metastases, stereotactic body radiation therapy, CyberKnife
Citation: Kress M-AS, Collins BT, Collins SP, Dritschilo A, Gagnon G and Unger K (2012) Stereotactic body radiation therapy for liver metastases from colorectal cancer: analysis of safety, feasibility, and early outcomes. Front. Oncol. 2:8. doi: 10.3389/fonc.2012.00008
Received: 28 September 2011;
Accepted: 16 January 2012;
Published online: 02 February 2012.
Edited by:
Silvia C. Formenti, New York Langone Cancer Institute, USAReviewed by:
Peter B. Schiff, NYU School of Medicine, USAJoshua Silverman, New York University Medical Center, USA
Copyright: © 2012 Kress, Collins, Collins, Dritschilo, Gagnon and Unger. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Marie-Adele S. Kress, Department of Radiation Oncology, Georgetown University Hospital, Lower Level Bles Building, 3800 Reservoir Road Northwest, Washington, DC 20007, USA. e-mail: marie-adele.s.kress@gunet.georgetown.edu