HTLV-1 and Co-infections
- 1Section of Virology, Department of Infectious Disease, Imperial College London, London, United Kingdom
- 2National Centre for Human Retrovirology, Division of Medicine and Integrated Care, St. Mary's Hospital, Imperial College Healthcare NHS Trust, London, United Kingdom
Human T lymphotropic virus type 1 (HTLV-1) is a retrovirus that causes lifelong T-cell infection in humans, impacting the host immune response. This virus causes a range of clinical manifestations, from inflammatory conditions, including neuronal damage (HTLV-1 associated myelopathy, HAM) to life-threatening leukemia (adult T-cell leukemia, ATL). Human T lymphotropic virus type 1 is also associated with increased risk of all-cause mortality, but the mechanisms remain unclear. As a blood-borne and sexually transmitted infection (STI), HTLV-1 shares transmission routes to many other pathogens and although it has worldwide distribution, it affects mainly those in low- and middle-income tropical areas, where the prevalence of other infectious agents is high. These factors contribute to a high incidence of co-infections in people living with HTLV-1 (PLHTLV). This comprehensive review addresses the impact of HTLV-1 on several co-infections and vice-versa. There is evidence of higher rates of HTLV-1 infection in association with other blood borne (HCV, HBV) and sexually transmitted (Syphilis, Chlamydia, HPV, HSV) infections but whether this represents increased susceptibility or opportunity is unclear. Higher frequency of Mycobacterium tuberculosis (MTb) and Mycobacterium leprae (M. leprae) is observed in PLHTLV. Reports of opportunistic infections and high frequency of crusted scabies in patients with HTLV-1 points to immune impairment in those individuals. Human T lymphotropic virus type 1 may influence the persistence of pathogens, exemplified by the higher rates of Schistosoma mansoni and Strongyloides stercoralis (St. stercoralis) treatment failure observed in PLHTLV. This retrovirus is also associated with increased tuberculosis (TB) severity with some evidence pointing to a deleterious impact on leprosy outcome as well. These findings are supported by immune alterations observed in those co-infected individuals. Although the role of HTLV-1 in HCV outcome is debatable, most data indicate that HTLV may negatively impact the clinical course of hepatitis C. Co-infections may also influence the risk of developing HTLV-1 associated disease, but data are still limited. The impact of HTLV-1 on the response to more common infections, might contribute to the increased mortality rate of HTLV-1. Large scale prospective controlled studies on the prevalence and impact of HTLV-1 in co-infections and vice-versa are needed. Human T lymphotropic virus type 1 impact in public health is broad. Measures to increase awareness and to prevent new infections are needed.
Introduction
Human T lymphotropic virus type 1 (HTLV-1) causes lifelong infection mainly in CD4+ cells. The immune imbalance that occurs in HTLV-1 infection is linked to the pathogenesis of HTLV-1 associated diseases. The neurological disease HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), that greatly impacts patient‘s quality of life and can result in wheelchair dependency, and other conditions, such as uveitis and thyroiditis are examples of the many inflammatory manifestations associated with HTLV-1. Adult T-cell leukemia/lymphoma (ATL), the usually fatal neoplasm caused by HTLV-1 is also linked to immunological impairment as well as monoclonal expansion and transformation of an infected T-cell. There are several evidences that patients with ATL have severe immunosuppression which is associated with opportunistic infections and other malignancies (1–4). However, even in the absence of clinical disease, HTLV-1 can cause immune impairment (2, 5). Unprotected sexual intercourse, contact with blood or tissue and breastfeeding may expose individuals not only to HTLV-1 but to a variety of other pathogens, such as human immunodeficiency virus (HIV), Treponema pallidum, hepatitis C virus (HCV), hepatitis B virus (HBV), human papillomavirus (HPV), and herpes simplex virus (HSV). Moreover, the distribution of HTLV-1, with high prevalence observed mainly in low- and middle-income countries (LMIC) (6), overlaps with areas where the prevalence of other infections, such as Strongyloides stercoralis (St. stercoralis), Mycobacterium tuberculosis (MTb), and Mycobacterium leprae (M. leprae), is high. Thus, HTLV-1 infected individuals can be co-infected with a range of pathogens. Indeed, the prevalence of co-infection is high in some settings. The interactions between coexisting pathogens are complex and may affect the natural course of both infections. While an anti-viral immune response involves a dominant Th1 type response, the ideal response to some parasites, e.g., helminths, usually involves a dominant Type-2 response. Therefore, the response to co-infections can be conflicting from an immunological perspective (5). Furthermore, clinical conditions associated with different pathogens, including HTLV-1 itself, may involve an exacerbated response in one arm of the immunological system. Thus, co-infections can be harmful or occasionally even beneficial to the host. Recently, a meta-analysis showed that HTLV-1 infection is associated with increased risk of death and identified 16 clinical conditions associated with, or occurring more frequently with, HTLV-1 infection [seborrheic dermatitis, Sjogren's syndrome, eczema, pulmonary alteration, asthma, fibromyalgia, rheumatoid arthritis, arthritis, tuberculosis (TB), kidney and bladder infections, dermatophytosis, community acquired pneumonia, strongyloides hyperinfection syndrome, liver cancer; lymphoma other than ATL, and cervical cancer] (7). However, the range of reported disease associations is broader still and here a number of questions concerning the impact of HTLV-1 infection on the susceptibility and outcome of other infections and vice-versa are addressed.
Do Other Infections Increase the Likelihood of HTLV-1 Co-infection or Vice-Versa?
Mycobacteria
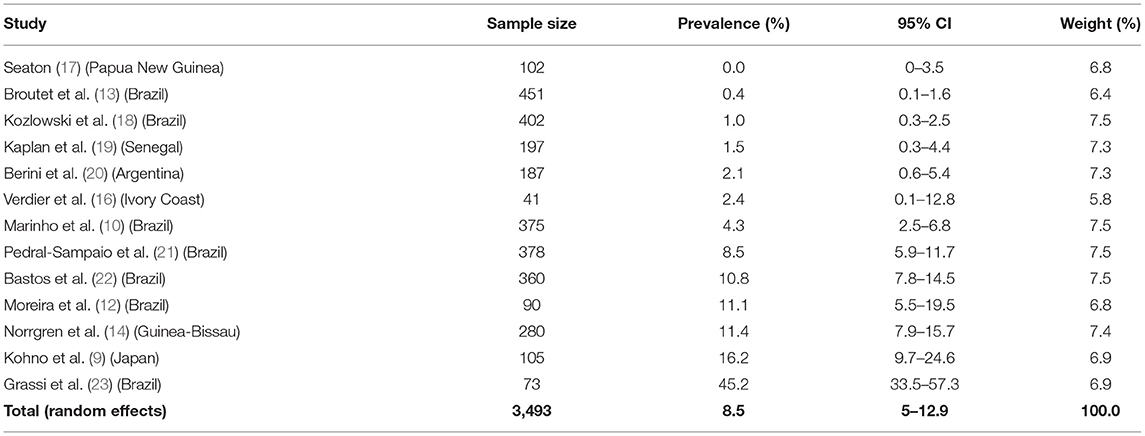
In a Japanese study from the 80's Hanada et al. demonstrated a higher prevalence of HTLV-1 infection among patients with pulmonary TB when compared to healthy individuals [29.5% (64/217) vs. 11.9% (562/4,741), p < 0.01] but confirmatory tests for HTLV-1 infection were not performed (8). Also in Japan, the prevalence of anti-HTLV-1 antibodies was higher in patients with TB than in a control group with bronchial asthma (17/105 vs. 4/58, OR = 2.61, 95% CI 0.83–8.16) (9). In a case-control study of 375 patients with TB and 378 control subjects in Brazil, HTLV-1 prevalence was higher in TB patients (4.3%, 16/375) than in a control group (1.3%, 5/378), resulting in a crude OR 3.31 (95% CI, 1.20–9.13) that remained statistically significant after controlling for numerous variables (age, sex, education, income, ethnicity, sexual history, history of blood transfusion, and intravenous drug use) (10). Similar results were observed in two other cross-sectional studies in Brazil (10.9 and 11.1% HTLV-1 infection in TB patients vs. 4.5 and 1.8% in control patients without TB, respectively) (11, 12). In the same country, the prevalence of HTLV-1 infection in patients with TB was higher than in pregnant women (2/451 vs. 1/814, OR = 3.62, 95% CI 0.33–40.05) (13). In Bissau, although the prevalence of HTLV-1 infection was higher in TB patients when compared to a population-based control group [11.4% (32/280) vs. 3.5% (74/2, 117), OR = 3.6 (95% CI = 2.2–5.6), p < 0.05], the results were not statistically significant when separately evaluating HIV-negative TB patients [3.7% (6/162) vs. 2.6% (51/1,930), OR = 1.18 (95% CI = 0.48–2.89), p = 0.71] (14). The authors hypothesized that the immunosuppression caused by HTLV-1 alone was insufficient to increase the risk of TB, but it adds to the risk of TB among HIV-infected individuals. Later, the same group observed higher mortality in patients with pulmonary TB when coinfected with HIV-2/HTLV-1 compared to HIV-2-positive HTLV-1-negative patients in Guinea-Bissau (15). There was no statistically significant difference between HTLV-1 prevalence in patients with TB comparing to general population from Ivory Coast [1/41 (2.4%) vs. 23/1,291 (1.8%)] (16) and Senegal [3/197(1.5%) vs. 2/181 (1.1%)] (Table 1) (19).
A study conducted in Japan evaluated different diseases among men with HTLV-1 infection and showed that 6.1% (17/278) of HTLV-1 patients had a history of TB compared with 2.9% (74/2,569) of uninfected patients (OR = 3.1 95% CI 1.1–3.3, p < 0.05) (24). When analyzing the incidence of TB amongst HTLV-1 infected individuals from Brazil, by cross-matching of records on the Brazilian national registry and the database of the referral center for HTLV, Grassi et al. reported a relative risk of developing TB in HTLV-1 infected patients of 2.6 (CI 95% 1.6–4.2) when compared to the HTLV-1 uninfected group (23). No differences in clinical presentation or TB outcome were observed. In Peru, the history of active TB was analyzed in relatives of HTLV-1 infected patients. History of active TB was more frequent (11.4%, 45/394) among HTLV-1 infected compared to HTLV-1 uninfected relatives (4.3%, 36/839, p < 0.001). In a multivariate analysis HTLV-1 infection was associated with TB history in Peru (Adjusted OR 2.5, 95% CI 1.6–3.9) (25). This was also observed in the USA (OR = 2.4, 95% CI 0.82–7.02) (26).
Higher prevalence of HTLV-1 infection among patients with leprosy has been reported from Japan, Democratic Republic of Congo, Congo, and Ivory Coast (8, 16, 27–29) compared to healthy residents, including blood donors (Japan) and the general population (Democratic Republic of Congo, Congo, and Ivory Coast). Similar results were observed even in patients with leprosy from HTLV-1 non-endemic areas from Japan (30). However, other studies found no significant difference regarding HTLV-1 prevalence in patients with M. leprae from Yemen, Senegal, Ethiopia, and a non-endemic area for HTLV-1 from Brazil (Curitiba). However, the sample size in each setting was small (27, 31, 32). Prevalence studies are summarized in Table 2 (32, 33, 35).
The data indicate that HTLV-1 is more common in patients with MTb than expected (combined OR = 2.6, 95% CI 2.1–3.2) suggesting an increased susceptibility to develop TB if HTLV-1 infected or vice versa, the first option being most biologically plausible (Table 1; Figure 1). While patients with a history of TB were twice as likely to have HTLV-1 infection, those with active TB had three times more chance of being co-infected with HTLV-1. The low heterogeneity observed between this subgroup strengthens this finding. Although limited, current data suggests an association between HTLV-1 and M. leprae (Pooled OR = 4.67, 95% CI 2.23–9.77) (Table 2; Figure 2), which would fit with the association between HTLV-1 and MTb.
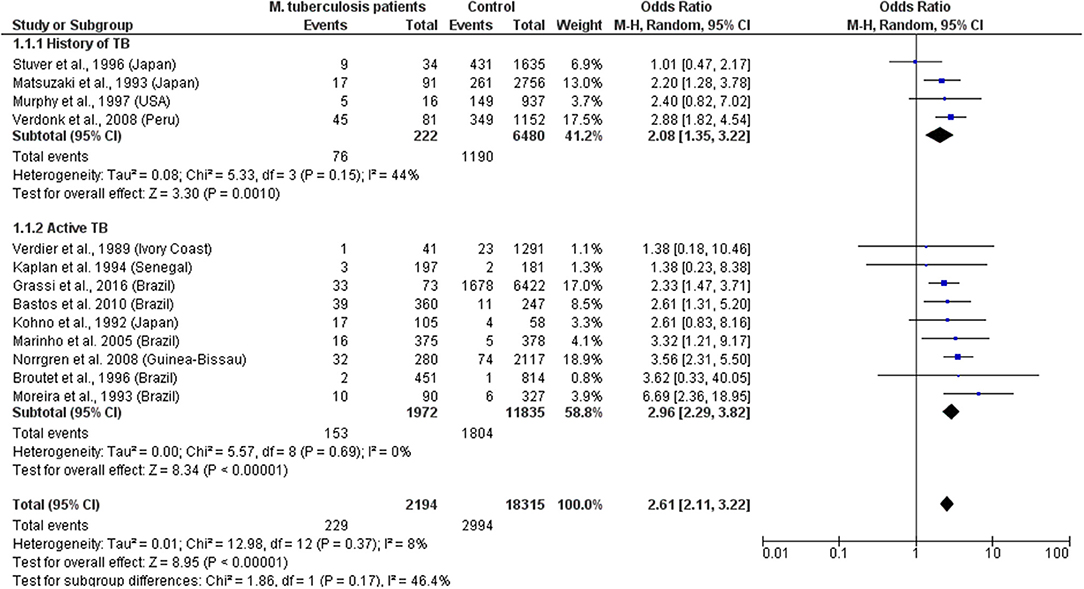
Figure 1. Forest Plot and meta-analysis showing the Odds Ratio of HTLV-1 infection in patients with Mycobacterium tuberculosis. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies in each subgroup (those with History of TB and those with Active TB) and between subgroups is shown.
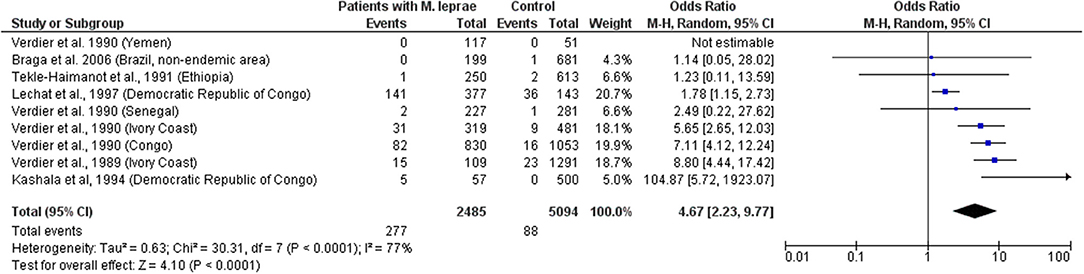
Figure 2. Forest Plot and meta-analysis showing the Odds Ratio of HTLV-1 infection in patients with Mycobaterium leprae. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies is shown.
Sexually Transmitted Infections
Murphy et al. demonstrated current diagnosis of syphilis to be an independent risk factor for HTLV-1 infection in both females [OR = 2.12 (1.12–3.99)] and males [OR = 3.56 (1.24–10.22)] and hypothesized that although the efficiency of HTLV-1 sexual transmission is lower from female-to-male, it can be increased by the presence of a penile ulcer or concurrent syphilis (36). In the US, a history of syphilis (OR = 4.2, p = 0.02, in Baltimore) and positive syphilis serology (OR = 4.4; p = 0.01 in Baltimore and OR = 3.9; p = 0.02 in New Orleans) were associated with confirmed HTLV-1/2 infection (37). This association persisted after adjusting for HIV status and was more robust for men (OR = 8.5; 95% CI: 3.1–23.6) than for women (OR= 3.1; 95% CI: 0.9–10.5) (37). The frequency of T. pallidum antibodies was also higher among HTLV-1 positive blood donors from Guadeloupe, French West Indies, than in seronegative HTLV-1 donors (10.5 vs. 1.7%, p < 0.001; OR= 7.01; 95% CI: 2.33–23.11; Adjusted OR = 2.83; 95% CI: 0.62–12.9) (38). An association between seropositivity for syphilis and HTLV-1 infection was observed in the general population in Gabon (19/106 vs. 83/1,134, OR = 2.77, 95% CI 1.6–4.77) (39) and Brazil (12/51 vs. 33/3,400, OR = 31.4, 95% CI 15.1–65.3) (40). In Japanese women attending antenatal care no association was found between syphilis and HTLV-1 infection (OR = 0.48, 95% CI 0.49) (41). In Haitian pregnant women although the prevalence of positive syphilis serology was higher in HTLV-1 infected women this was not statistically significant [6/44 (14%) vs. 7/89 (8%), p = 0.22] (42), while in Brazil an association was reported in parturient women (OR = 2.48, 95% CI 0.31–20.01) (43). In Peruvian women (sex workers and those attending antenatal care) positive serology for syphilis was also associated with HTLV-1 infection (OR = 1.78; 95% CI: 1.05–3.04, p = 0.03) and the prevalence of HTLV-1 infection was higher in patients seropositive for syphilis than in seronegative patients (30.3 vs. 10.2%, p < 0.0001) (44). Another study of female sex workers in Peru, confirmed that HTLV-1 infection was associated with history of syphilis (OR = 2.3, 95% CI 0.9–5.9) and positive syphilis serology (OR = 1.5, 95% CI 0.6–3.6). The later association did not persist after adjusting for condom use and duration of prostitution (AOR = 0.8, 95% CI 0.3–2.2) (45). In a STD clinic in Rome, Italy, 9/1,457 patients with at-risk sexual behavior were found to be HTLV-1 seropositive and the presence of antibodies to T. pallidum was associated with risk of HTLV-1 infection (OR = 15.62; 95% CI: 3.2–75.6 and Adjusted OR = 9.52; 95% CI: 1.7–52.3) (46). In Zaire, the OR for HTLV-1 seropositivity in syphilis co-infected sex workers was 2.8 (95% CI: 1.7–4.7), [15.2% (28/184) 15.2 vs. 5.9% (58/977)] (47). Two studies found no association between HTLV-1 infection and evidence of syphilis past or present: a study of police officers from Guinea-Bissau where no association was found between HTLV-1/2 infection and history of syphilis evaluated by T. pallidum hemagglutination test (48), nor in 100 HTLV-1 positive Japanese pregnant women compared to 100 uninfected controls [OR = 0.5 (95% CI: 0–5.5), p > 0.05] (41). Meta-analysis shows an association between syphilis and HTLV-1 co-infection, with a pooled OR ranging from 1.6 to 6.99, according to the population being considered (Figure 3). Whether T. pallidum seropositivity is associated with mucosal disruption and increased risk of HTLV-1 infection or is only a surrogate marker of unsafe sexual activity is unclear.
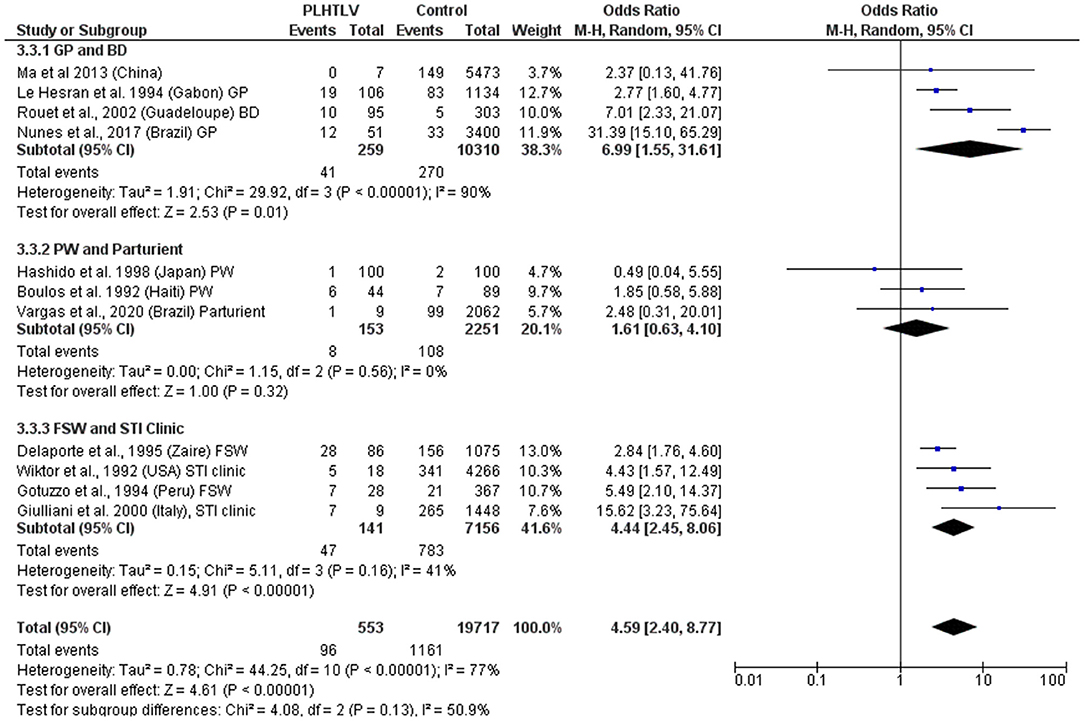
Figure 3. Forest Plot and meta-analysis showing the Odds Ratio of Syphilis infection in people living with HTLV-1. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies in each subgroup (population under investigation) and between subgroups is shown. PLHTLV, people living with HTLV; GP, general population; BD, blood donors; PW, pregnant women; FSW, female sex workers; STI, sexually transmitted infection.
An association has also been observed between HTLV-1 and seropositivity for Chlamydia trachomatis (C. trachomatis) amongst Peruvian female sex workers (with an OR = 3.7; 95% CI 1.4–13.2 after adjusting for condom use) (45). This association was previously reported for sex workers and pregnant women from Ivory Coast (16) and in a case control study in Guadeloupean blood donors where the detection of antibodies to C. trachomatis was higher in cases [63.2% (60/102)] than controls [43.4% (126/306), p < 0.001, OR = 2.23; 95% CI 1.38–3.61; Adjusted OR = 1.95; 95% CI 1.03–3.68] (38). Although the prevalence of antibodies to C. trachomatis was also higher in HTLV-1 seropositive pregnant women from Japan, the difference was not statistically significant (21/100 vs. 12/100; OR = 1.9; 95% CI 0.8–4.2; p > 0.05) (41). When these studies are analyzed altogether it is observed that PLHTLV are 2.4 times more likely to be infected by C. trachomatis than those without HTLV-1 (Figure 4). Zunt et al. analyzed the cervical shedding of HTLV-1 by detecting HTLV-1 proviral DNA in cervical samples collected from Peruvian sex workers. They observed that cervicitis (characterized by increased number of polymorphonuclear cells in cervical mucus) increased the cervical shedding of HTLV-1, but not Chlamydia or Gonorrhea (OR = 1.5; 95% CI 0.6–3.8 and OR=1.1; 95% CI 0.2–5.2, respectively). As the authors highlighted, the limited number of co-infected individuals may have influenced the results (49).
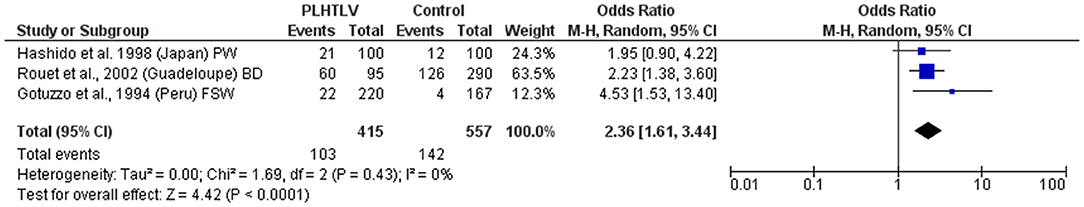
Figure 4. Forest Plot and meta-analysis showing the Odds Ratio Chlamydia trachomatis infection in people living with HTLV-1. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies is shown. PLHTLV, people living with HTLV; BD, blood donors; PW, pregnant women; FSW, female sex workers.
Human T lymphotropic virus type 1 infection was associated with HSV-2 seropositivity in pregnant women from Haiti (OR = 3.7, 95% CI 1.6–11.5) (42) and female sex workers from Peru [Adjusted OR (condom use and duration of prostitution) = 3.7, 95% CI 0.5–28.4] (45). The authors hypothesized that HSV-2 may lead to disruption of the mucous membrane increasing susceptibility to sexual transmission of HTLV-1 (42). Changes in the frequency of immune cells at the mucosa, including HTLV-1 target cells (lymphocytes) or transporters (dendritic cells) as well as the expression of activation markers in lymphocytes are factors that may influence susceptibility to HTLV-1 infection.
Human T lymphotropic virus type 1 and HPV are both sexually transmitted oncogenic viruses that cause persistent infection. The progression to cervical cancer due to HPV is more frequent among immunosuppressed women, such as those co-infected with HIV. Studies concerning HTLV-1/HPV co-infection are limited. In Brazil, the prevalence of HPV infection was higher among HTLV-1 infected (mean age 38 years) than uninfected women (mean age 36 years) attending a gynecology clinic (44 vs. 22.5%, p = 0.03) (50). Human T lymphotropic virus type 1 proviral load (PVL) was similar in HTLV-1 mono-infected and HTLV-1/HPV co-infected women, no cervical cancer and only one case of high grade squamous intraepithelial lesion was observed (50). In HTLV-1 infected women from the Peruvian Amazon the prevalence of HPV infection was also higher than in HTLV-1 negative individuals (43.6 vs. 29.3%). They were twice as likely to have HPV infection of any type (OR = 2.1 95% CI 1.53–2.87) and to have high-risk HPV infection (OR =1.93 95% CI 1.04–3.59) than those not infected by HTLV-1, after adjusting for confounding variables, including age, education, age of sexual partner, number of sexual partners within the last 12 months, and condom use at last sexual intercourse (51). However, no difference was found between the prevalence of epithelial cell abnormalities in the cervix or presence of low-grade squamous intraepithelial lesion (LSIL) or higher grade lesion, according to HTLV-1 infection status (51). Figure 5 shows the forest plot and meta-analysis regarding the association of HPV and HTLV-1 infection.
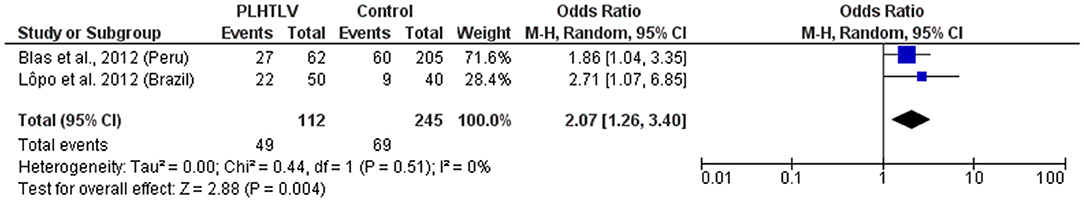
Figure 5. Forest Plot and meta-analysis showing the Odds Ratio HPV infection in people living with HTLV-1. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies is shown.
There is a consistency in the literature to indicate a relationship between HTLV-1 and coinfection with other sexually transmitted infections (STIs) [Pooled OR (95% CI): Syphilis = 4.6 (2.4–8.8); Chlamydia = 2.36 (1.6–3.4); HPV = 2.07 (1.3–3.4)]. However, causation has not been demonstrated. Prospective studies are required and would be helpful to distinguish between HTLV-1 infection acquired sexually and vertically.
Hepatitis
Several studies report a high frequency of HCV and HBV infection in PLHTLV. Unfortunately, most of them do not include a control group. Studies from China, Brazil, and Sweden confirmed a higher prevalence of HTLV-1 infection among patients that are seropositive for HCV (52–54). Pooled OR revealed that individuals with anti-HCV antibodies are 20 times more likely to be infected with HTLV-1 (Figure 6).
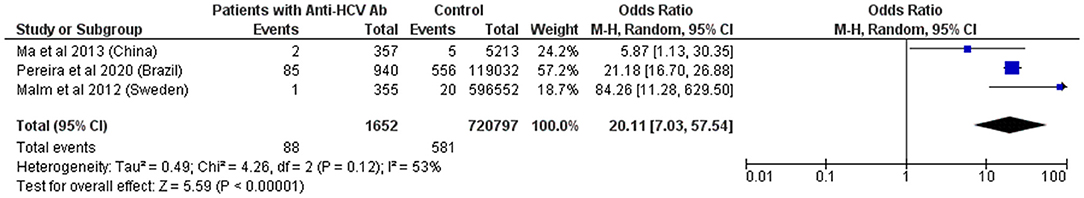
Figure 6. Forest Plot and meta-analysis showing the Odds Ratio HTLV-1 infection in individuals with anti-HCV antibodies. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies is shown.
Respiratory Infections
Human T lymphotropic virus type 1 can cause pulmonary disease that may result in important parenchymal damage and bronchiectasis (55). However, little is known about HTLV-1 and respiratory co-infections. A study conducted in Australia revealed an association between HTLV-1 and lower respiratory tract infection, after adjusting for covariates (adjusted negative binomial regression, coefficient 0.19; 95% CI, 0.04–0.34) (56). It would also be interesting to know the impact of HTLV-1 and SARS-CoV-2 coinfection, but no data are available yet.
Does HTLV-1 Infection Increase the Probability of the Persistence of a Co-existing Pathogen?
Human T lymphotropic virus type 1 infection may also influence the ability of other parasites to persist within the co-infected host. This was described in HTLV-1 and St. stercoralis co-infection. Patients with HTLV-1 have more frequently positive stool test for Ss and treatment failure (persistent parasite excretion despite treatment). This topic is addressed in another study.
Similarly, high frequency of Schistosoma mansoni infection among HTLV-1 infected individuals from Brazil [26/309 (8.4%) vs. 6/331 (1.8%) controls, p = 0.0003] has been reported (57). Two available studies combined give an OR = 4.6 (p < 0.001), but both were carried out in Brazil by the same group, and probably have an overlap of patients (Figure 7). Treatment failure, defined as the persistence of S. mansoni eggs in stool samples 2 months after therapy with praziquantel was more frequent in HTLV-1 co-infected patients than in patients with schistosomiasis only [4/20 (20%) vs. 1/44 (2.3%), p < 0.05]. Re-exposure to parasite was excluded in all patients. One co-infected patient was not able to eradicate the helminthic infection even after eight courses of praziquantel (58). Although limited, evidence points to therapeutic failure and persistence of Schistosoma mansoni in people living with HTLV-1 (PLHTLV) but this is better characterized for Strongyloides infection.
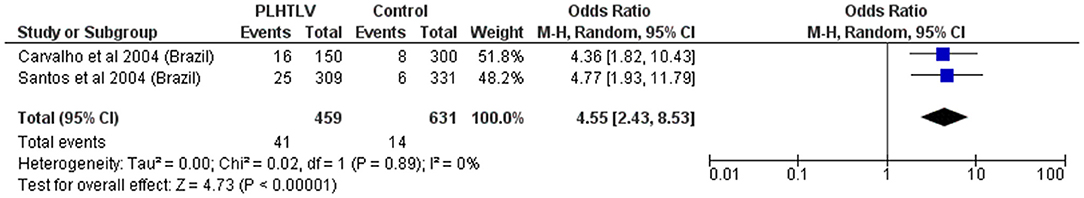
Figure 7. Forest Plot and meta-analysis showing the Odds Ratio Schistosoma mansoni infection in people living with HTLV-1. The boxes and lines indicate the odds ratios (ORs) and their confidence intervals (CIs) for each study. The pooled odds ratio is represented by a black diamond. The size of the blue squares indicates the relative weight of each estimate. Statistical analysis and graph were performed using RevMan 5 Software. Odds Ratio was calculated using Random effects model and Mantel-Haenszel statistical method. Measures of heterogeneity between studies is shown.
Does HTLV-1 Impact the Pathology of Co-infection?
Unlike HTLV-1, Schistosoma sp infection elicits a Th-2 type response. Although it seems to be important for parasite clearing, it can be associated with the disease‘s pathophysiology (5). High degree of infection associated with host's immune reaction to parasite can lead to granuloma formation and consequently liver fibrosis, which is the most important pathological finding and occurs in up to 5% of chronic infected individuals. Thus, HTLV-1/S. mansoni co-infection may result in some beneficial effect to the host. Indeed, the number of eggs/gram of stool was lower in co-infected patients (58) and an inverse association was found between HTLV-1 infection and liver fibrosis in patients with schistosomiasis (evaluated by ultrasound in 22 co-infected patients and 40 mono-infected with S. mansoni) (57, 58). However, Goon and Bangham (5) hypothesized that lower rates of egg excretion observed in the stool of co-infected individuals may be associated with either lower levels of IL-4 (as this cytokine seems to be necessary to translocate eggs from mesenteric venules through intestinal wall followed by secretion in animal models), or with an impaired control of Schistomosoma's reproduction which could result in egg excretion even in individuals with low parasite burden. As discussed before, persistence of S. mansoni eggs in stool samples after therapy is more frequent in HTLV-1 co-infected patients than in patients with schistosomiasis only (58).
Regarding TB severity in HTLV-1 infected individuals, the findings are contradictory. In Peru, HTLV-1 patients co-infected with TB reported more frequently a history of relatives‘ deaths due to TB infection (25). Human T lymphotropic virus type 1 co-infected patients also were more likely to have a highly positive sputum smear (more than 10 acid-fast bacilli in at least 20 fields in microscopy stained by Ziehl-Neelsen) than those mono-infected individuals (59), indicating that HTLV-1 patients may have an impaired ability to control bacilli replication. This finding is reinforced by the decreased skin reaction to tuberculin skin test (TST) observed in co-infected HTLV-1/TB patients and HTLV-1 mono-infected asymptomatic carriers (60–62). Human T lymphotropic virus type 1/HIV-2 co-infected patients hospitalized for TB have a higher CD4 count and higher mortality rate when comparing to HIV-TB infected subjects from Guinea-Bissau (15). Pedral-Sampaio et al. showed an increase in mortality in co-infected individuals: amongst HIV-1 and HTLV-1 negative TB patients the mortality was lower (8%, 25/319) than in patients with HTLV-1 (25%, 8/32) or HIV-1 (33%, 6/18) co-infections. Five out of nine (56%) patients with triple infection (TB-HIV-1-HTLV-1) died (21). On the other hand, in another study from Brazil (Bahia), no differences were observed in clinical presentation of TB according to HTLV-1 status, nor in the number of hospitalizations, sputum and TST response, however the number of patients was small (13 HTLV-1/TB and 24 TB mono-infected), they all had severe active TB (as they were all hospitalized) and most were malnourished, which may have impacted the findings (63).
A study from the Democratic Republic of Congo analyzed 378 leprosy patients for 22 years and reported a significant decrease in the survival of M. leprae/HTLV-1 co-infected patients: Mortality rates 5.5/100 vs. 3.6/100 person-years of observation, resulting in a risk ratio of 1.4 (95% CI 1.04–1.89) for those infected with HTLV-1 (28). In a study of the impact of viral co-infections (including HTLV-1, HIV, HBV, and HCV) on leprosy outcome in Brazil, co-infected patients showed higher rates of neuritis, nerve function impairment and relapse, but those viruses were analyzed altogether (34). A study from Japan and another one conducted at Congo and Ivory Coast, did not observe differences among HTLV-1 prevalence between patients with lepromatous and tuberculoid forms of leprosy (27, 30).
The impact of HTLV-1 on HCV infection is still debatable. Most studies report a deleterious impact on HCV outcome and disease severity on co-infected patients. Higher HCV viral load (64, 65), higher IFN-γ serum concentration (66), higher degree of liver steatosis (66), higher risk of hepatocellular carcinoma (67), reduced HCV clearance (both natural and in response to IFN treatment) (65, 68, 69), and decreased survival rates after HCV-related liver transplantation were already reported in HCV/HTLV-1 co-infected patients comparing to HCV mono-infected individuals (70, 71). A study from Miyazaki, Japan, showed that HTLV-1 co-infection increased the risk of self-reported incident liver disease (RR = 3.5, 95% CI 1.9–6.4) and registered death due to liver cancer (RR = 8.2, 95% CI 1.6–441.4) (72). Human T lymphotropic virus type 1 Tax protein also facilitates HCV replication in vitro (73). On the other hand, HTLV-1/HCV patients were reported to have lower levels of markers of hepatic damage (ALT, AST, GGT, bilirubin) (74–76) and increased platelet counts (74). No difference between HTLV-1 PVL and HCV viral load between co-infected patients and mono-infected individuals were reported by others from Brazil (74).
The association between syphilis and HTLV-1 has been described above. However, there are no data concerning the impact of this coinfection on each disease outcome. There is just one case report of atypical long-lasting secondary syphilis with a localized and atypical eruption in a HTLV-1 co-infected individual (77).
Crusted scabies (also called Norwegian scabies) is an infrequent, albeit severe and highly contagious infection caused by massive infestation of the mite, Sarcoptes scabiei (S. scabiei) and may be considered a marker of HTLV-1 infection (78). In Bahia, Brazil, severe scabies (more than 80% of body surface affected but not fulfilling the crusted scabies criteria) was associated with HTLV-1 infection (OR = 3.0; 95% CI 1.85–4.86, p < 0.01) (79). History of chronic scabies were also associated with HTLV-1 infection in Peruvian women (OR = 13, 95% CI = 1.6–82, p < 0.02) (80). In Peru, in two different studies, 60–70% of patients with crusted scabies were infected with HTLV-1 (81, 82), while in two case series all six patients in French Guiana (83) and all 21 patients from Brazil were co-infected with HTLV-1 (79). Among HTLV-1 seropositive hospitalized patients in Dominica, 7.6% had crusted scabies (84). In the indigenous Australian population scabies was more frequent in HTLV-1 infected patients than seronegative individuals [14.2% (72/507) vs. 8.5% (80/944), p < 0.001] (56). Crusted scabies was already reported in patients with ATL (82, 83, 85–87), with HAM/TSP (81, 82, 88) and in asymptomatic individuals co-infected with other infectious agents (Ss and TB) or other comorbidities (82, 83, 89, 90). During 30 months' follow-up of 30 children with HTLV-1 associated infective dermatitis (ID) in Brazil, 70% had scabies, and one had crusted scabies (91). Therefore, HTLV-1 may be associated with severe cases of scabies, a clinical marker of immunosuppression.
Fungal infections are frequently considered opportunistic infections and may be used as a warning sign of immunological impairment. The prevalence of HTLV-1 infection was higher in Japan among patients with pulmonary cryptococcosis than controls with other pulmonary disorders [32.6% (6/19) vs. 13.8% (49/356), p = 0.033] (9). Dissemination of C. neoformans to the CNS was not observed regardless of HTLV-1 status (9). Cryptococcal lymphadenitis was reported in one HTLV-1 individual in a small case series including two patients with HIV-1. The HTLV-1/cryptococcus infected patient had a smaller number of fungal cocci in the focal area and a higher CD4+ cell count compared to the two HIV/Cryptococcus co-infected individuals (92). Disseminated Cryptococcus infection with CNS involvement was described in a HTLV-1 patient from the Caribbean (93). Pulmonary cryptococcosis in a HTLV-1 positive, HIV-negative patient with disseminated molluscum contagiosum was also described (84). Cryptococcosis has been reported in patients with ATL (94–96).
Histoplasma capsulatum is a ubiquitous fungus that usually causes asymptomatic infection. Patients co-infected with HIV, those with primary immunodeficiencies or under immunosuppressive treatments are at risk of developing progressive disseminated histoplasmosis (PDH), a clinical illness with extra-pulmonary involvement. The mortality of PDH can be as high as 85% in untreated patients, and 25% despite a standard “adequate” therapeutic regimen. Severe gastrointestinal histoplasmosis in Peru (97), spinal cord histoplasmoma (98) brain granulomas and lymphoma associated with several other opportunistic infections (99) have been reported in HTLV-1 co-infected individuals, but no conclusive evidence of an association between HTLV-1 and histoplasmosis outcome was provided (100).
Paracoccidioides brasiliensis is a dimorphic fungus restricted to Latin America. It is the agent of paracoccidioidomicosis, a systemic fungal disease. Four cases of HTLV-1/P. brasiliensis co-infection seen during a 2 years-period in a Peruvian hospital were reported. Two had severe chronic clinical presentation and two had aberrant clinical picture and co-infections (one co-infected with H. capsulatum and the other had sepsis caused by Listeria monocytogenes) (101).
The strongest evidence points to increased pathology due to mycobacterium in the presence of HTLV-1. There is a suggestion that HTLV-1 infection may favorably modulate the impact on S mansoni but this needs to be substantiated. Whilst it is plausible that HTLV-1 infection may impact the course of the many mycotic infections that co-exist in HTLV-1 endemic areas the need for better studies to determine the epidemiological and biological associations between HTLV-1 and infections such as histoplasmosis, paracoccidioidomycosis, and cryptococcosis is clear (100).
Does Co-infection Impact Pathology of HTLV-1?
Data concerning the impact of co-infections in HTLV-1 outcome is scarce. It is known that Schistosoma antigens downregulate in vitro pro-inflammatory cytokine response (IFN-γ and CXCL9) by peripheral blood lymphocytes from HTLV-1 infected individuals (102, 103). These antigens also upregulate IL-10 production (103). Interestingly, high prevalence of helminthic infection (Schistosomo mansoni and St. stercoralis) was observed among asymptomatic carriers when compared to individuals with HAM [23% (71/310) vs. 3% (1/32), p < 0.05] (104). Human T lymphotropic virus type 1 PVL was lower in co-infected patients [2.2 copies/100 cells vs. 3.7 copies/100 cells, p < 0.05]. They also showed lower levels of IFNg, CD8+IFN+ (57, 104), CD4+IFNg+, and an increase in T cells expressing IL-5 and IL-10 compared to HTLV-1 monoinfected patients (104). These observations, together with the immunological findings gave rise to the hypothesis that HTLV-1/helminthic co-infection in comparison to HTLV-1 single infection may decrease Th1 response, which may influence the clinical outcome of HTLV-1 infection (104). As the majority of the data were obtained by evaluating patients with HTLV-1/St. stercoralis and HTLV-1/Schistosoma sp. together, more studies are needed to clarify the impact of Schistosoma sp. on HTLV-1 clinical outcomes.
Regarding TB and HTLV-1 infection outcome, a history of TB was associated with an increased risk of HAM development among HTLV-1 infected individuals in Brazil (OR 3.8; 95% CI 1.9–9.1, p = 0.0001) in a retrospective association (105). Once again, changes in immunological response may be responsible for the observed alteration. Among patients with HAM, IFN-γ/IL-10, and TNF-α/IL-10 ratios were higher in those individuals with active TB than those HAM patients without co-infection. These differences were not observed in HTLV asymptomatic carriers nor in those with probable HAM (105). However, no differences in HTLV-1 PVL and serum CCXCL9 and CXCL10 levels according to TB infection status (not infected, latent infection, and active infection) were observed among patients with HAM. These findings led to the hypothesis that the higher susceptibility to develop TB in HTLV-1 infected individuals may be due to an impaired innate immune response (such as lower levels of TNF-α). However, more severe clinical presentation observed in co-infection may be due to an enhancement of Th1 response. Thus, the co-infection TB/HTLV-1 could increase not only TB severity, but also HAM incidence. More studies regarding HTLV-1 associated diseases in patients co-infected with TB are necessary.
A recent study amongst the Indigenous population of Central Australia, demonstrated that although HBV co-infection did not impact HTLV-1 PVL, HBV was associated with increased expansion of HTLV-1 T-cell infected clones. Moreover, the degree of clonal expansion positively correlated with the titer of HBsAg. This finding indicates that HBV/HTLV co-infection may have a role in the development of ATL (106) and this study should be replicated for other infections. Although clinical data to support this hypothesis are still limited, chronic HBV co-infection was found in 47% (8/17) and chronic HCV in 35% (6/17) of patients with ATL in a case series from Taiwan (compared to 15–20 and 2% prevalence found in general population) (107). Hepatitis C virus infection was identified as a predictor of mortality in PLHTLV in Brazil [HR (95% CI) = 5.2 (2.4–11.1), p < 0.0001] (108). However, there are evidences that HCV co-infection does not impact HTLV-1 PVL, T-cell proliferation rate (109) nor the frequency of HAM (74).
During the follow-up (mean 6.75 years) of 36 HTLV-1 infected patients with ID from Bahia, 47.2% developed HAM and one individual developed ATL (110). In a more recent study, the same group showed that during follow-up of 37 patients with ID 54% developed HAM before the age of 19 years old (111). HTLV-1 associated myelopathy was observed in 30% of children with ID in Brazil (112), and a history of ID was more frequent in patients with HAM than in asymptomatic carriers (3/73 vs. 0/120, p = 0.02) (113). Moreover, in 74 ATL cases in Bahia, 44% had history of severe eczema in childhood, suggestive of ID (114, 115). Interestingly, 30% of patients with ID had abnormal lymphocyte in peripheral blood and 16.6% had flower cells (116). A number of factors may contribute to these associations between ID and HAM and ATL. First, infection in early life is strongly associated with ATL, but the young age of ATL onset must still be explained. Second, ID is associated with high HTLV-1 PVL, a risk for both HAM and ATL. Third, ID which is characterized by both lymphocytic infiltration in the skin and by persistence of, and pathology due to, bacteria, may represent both the inflammatory and the immunosuppressive consequences of HTLV-1 infection.
Data regarding the impact of co-infections on HTLV-1 outcome is extremely limited and more studies are needed.
Conclusion
There is evidence of higher rates of HTLV-1 infection in association with other blood borne and STIs but whether this represents increased susceptibility or opportunity is unclear. The data suggest that HTLV-1 increases susceptibility to a range of infection related disease resulting in either higher risk of disease if exposed, longer persistence or greater severity. This indicates that PLHTLV may have a degree of immunosuppression regardless of symptomatic or asymptomatic HTLV-1 clinical status. Co-infections may also impact the risk of developing HTLV-1 associated disease, but data are still extremely limited.
Human T lymphotropic virus type 1 infection is a neglected infection that is responsible not only for diverse and severe inflammatory diseases, such as HAM, uveitis, pulmonary diseases, Sjogren syndrome, and arthritis, and the malignant disease ATL, but also a degree of immunosuppression that can increase susceptibility to and/or severity of co-infections, many of which are also neglected tropical diseases. More subtle impact of HTLV-1 on the response to more common infections, might contribute to the increased mortality rate of HTLV-1, but this will only be determined by large scale prospective controlled studies. Identification of HTLV-1 status should therefore be included in population-based studies. Human T lymphotropic virus type 1 should be considered a global health concern as its impact in public health is evident. Measures to improve the global awareness about HTLV-1 and to prevent its transmission are necessary.
Author Contributions
GT designed the study, supervised the project, and revised the manuscript. CR performed data compilation, analysis, and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
GT was supported by NIHR Imperial College Trust.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kawano N, Nagahiro Y, Yoshida S, Tahara Y, Himeji D, Kuriyama T, et al. Clinical features and treatment outcomes of opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia-lymphoma (ATL) at a single institution from 2006 to 2016. J Clin Exp Hematop. (2019) 59:156–67. doi: 10.3960/jslrt.18032
2. Tanaka T, Sekioka T, Usui M, Imashuku S. Opportunistic infections in patients with HTLV-1 infection. Case Rep Hematol. (2015) 2015:943867. doi: 10.1155/2015/943867
3. Bunn PA, Schechter GP, Jaffe E, Blayney D, Young RC, Matthews MJ, et al. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. (1983) 309:257–64. doi: 10.1056/NEJM198308043090501
4. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. (1991) 79:428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x
5. Goon PKC, Bangham CRM. Interference with immune function by HTLV-1. Clin Exp Immunol. (2004) 137:234–6. doi: 10.1111/j.1365-2249.2004.02524.x
6. Gessain A, Cassar O, Grossi P, Taylor G. Geographical Distribution of Areas with a High Prevalence of HTLV-1 Infection. European Centre for Disease Prevention and Control (2015). p. 8–17. doi: 10.2900/047633
7. Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis. (2019) 20:133–43. doi: 10.1016/S1473-3099(19)30402-5
8. Hanada S, Uematsu T, Iwahashi M, Nomura K, Utsunomiya A, Kodama M, et al. The prevalence of human T-cell leukemia virus type I infection in patients with hematologic and nonhematologic diseases in an adult T-cell leukemia-endemic area of Japan. Cancer. (1989) 64:1290–5. doi: 10.1002/1097-0142(19890915)64:6<1290::aid-cncr2820640620>3.0.co;2-z
9. Kohno S, Koga H, Kaku M, Yasuoka A, Maesaki S, Tanaka K, et al. Prevalence of HTLV-I antibody in pulmonary cryptococcosis. Tohoku J Exp Med. (1992) 167:13–8. doi: 10.1620/tjem.167.13
10. Marinho J, Galvao-Castro B, Rodrigues LC, Barreto ML. Increased risk of tuberculosis with human T-lymphotropic virus-1 infection a case-control study. J Acquir Immune Defic Syndr. (2005) 40:625–8. doi: 10.1097/01.qai.0000174252.73516.7a
11. de Lourdes Bastos M, Osterbauer B, Mesquita DL, Carrera CA, Albuquerque MJ, Silva L, et al. Prevalence of human T-cell lymphotropic virus type 1 infection in hospitalized patients with tuberculosis. Int J Tuberc Lung Dis. (2009) 13:1519–23.
12. Moreira ED, Ribeiro TT, Swanson P, Sampaio Filho C, Melo A, Brites C, et al. Seroepidemiology of human T-cell lymphotropic virus type I/II in northeastern Brazil. J Acquir Immune Defic Syndr. (1993) 6:959–63.
13. Broutet N, de Queiroz Sousa A, Basilio FP, Sa HL, Simon F, Dabis F. Prevalence of HIV-1, HIV-2 and HTLV antibody, in Fortaleza, Ceara, Brazil, 1993–1994. Int J STD AIDS. (1996) 7:365–9. doi: 10.1258/0956462961918103
14. Norrgren HR, Bamba S, Larsen O, Silva Z Da, Aaby P, Koivula T, et al. Increased prevalence of HTLV-1 in patients with pulmonary tuberculosis coinfected with HIV, but not in HIV-negative patients with tuberculosis. J Acquir Immune Defic Syndr. (2008) 48:607–10. doi: 10.1097/QAI.0b013e31817efb83
15. Norrgren H, Bamba S, Da Silva ZJ, Koivula T, Andersson S. Higher mortality in HIV-2/HTLV-1 co-infected patients with pulmonary tuberculosis in Guinea-Bissau, West Africa, compared to HIV-2-positive HTLV-1-negative patients. Int J Infect Dis. (2010) 14:142–7. doi: 10.1016/j.ijid.2009.11.040
16. Verdier M, Denis F, Sangaré A, Barin F, Gershy-Damet G, Rey JL, et al. Prevalence of antibody to human T cell leukemia virus type 1 (HTLV-1) in populations of Ivory Coast, West Africa. J Infect Dis. (1989) 160:363–70. doi: 10.1093/infdis/160.3.363
17. Seaton RA. Tuberculosis and human T-cell lymphotropic virus type 1 infection. Clin Infect Dis. (1997) 24:1026. doi: 10.1093/clinids/24.5.1026
18. Kozlowski AG, Carneiro MADS, Matos MAD De, Teles SA, Araújo Filho JA, Otsuki K, et al. Prevalence and genetic characterisation of HTLV-1 and 2 dual infections in patients with pulmonary tuberculosis in Central-West Brazil. Mem Inst Oswaldo Cruz. (2014) 109:118–21. doi: 10.1590/0074-0276130230
19. Kaplan JE, Camara T, Hanne A, Green D, Khabbaz R, LeGuenno B. Low prevalence of human T-lymphotropic virus type I among patients with tuberculosis in Senegal. J Acquir Immune Defic Syndr. (1994) 7:418–20.
20. Berini CA, Pando MA, Bautista CT, Eirin ME, Martinez-Peralta L, Weissenbacher M, et al. HTLV-1/2 among high-risk groups in Argentina: molecular diagnosis and prevalence of different sexual transmitted infections. J Med Virol. (2007) 79:1914–20. doi: 10.1002/jmv.21036
21. Pedral-Sampaio DB, Martins Netto E, Pedrosa C, Brites C, Duarte M, Harrington W Jr. Co-infection of tuberculosis and HIV/HTLV retroviruses: frequency and prognosis among patients admitted in a brazilian hospital. Braz J Infect Dis. (1997) 1:31–5.
22. Bastos ML, Osterbauer B, Mesquita DL, Carrera CA, Albuquerque MJ, Silva L, et al. Prevalence of human T-cell lymphotropic virus type 1 infection in hospitalized patients with tuberculosis. Int J Tuberc Lung Dis. (2009) 13:1519–23.
23. Grassi MFR, dos Santos NP, Lírio M, Kritski AL, Chagas Almeida M da C, Santana LP, et al. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis. (2016) 16:1–7. doi: 10.1186/s12879-016-1428-z
24. Matsuzaki T, Otose H, Hashimoto K, Shibata Y, Arimura K, Osame M. Diseases among men living in human T-lymphotropic virus type I endemic areas in Japan. Intern Med. (1993) 32:623–8. doi: 10.2169/internalmedicine.32.623
25. Verdonck K, Gonzalez E, Schrooten W, Vanham G, Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol Infect. (2008) 136:1076–83. doi: 10.1017/S0950268807009521
26. Murphy EL, Glynn SA, Fridey J, Sacher RA, Smith JW, Wright DJ, et al. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. Retrovirus Epidemiology Donor Study (REDS) Study Group. J Infect Dis. (1997) 176:1468–75. doi: 10.1086/514143
27. Verdier M, Denis F, Sangare A, Léonard G, Sassou-Guesseau E, Gaye A, et al. Antibodies to human T lymphotropic virus type 1 in patients with leprosy in tropical areas. J Infect Dis. (1990) 161:1309–10. doi: 10.1093/infdis/161.6.1309
28. Lechat MF, Shrager DI, Declercq E, Bertrand F, Blattner WA, Blumberg BS. Decreased survival of HTLV-I carriers in leprosy patients from the Democratic Republic of the Congo: a historical prospective study. J Acquir Immune Defic Syndr Hum Retrovirol. (1997) 15:387–90. doi: 10.1097/00042560-199708150-00010
29. Kashala O, Marlink R, Ilunga M, Diese M, Gormus B, Xu K, et al. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J Infect Dis. (1994) 169:296–304. doi: 10.1093/infdis/169.2.296
30. Muneishi H, Taguchi H, Sawada T, Ikezoe T, Matsui S, Tanaka S, et al. Prevalence of HTLV-I in leprosy patients in two sanatoriums in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. (1998) 17:380–3. doi: 10.1097/00042560-199804010-00014
31. de Moraes Braga AC, Reason IJM, Maluf ECP, Vieira ER. Leprosy and confinement due to leprosy show high association with hepatitis C in Southern Brazil. Acta Trop. (2006) 97:88–93. doi: 10.1016/j.actatropica.2005.09.003
32. Tekle-Haimanot R, Frommel D, Tadesse T, Verdier M, Abebe M, Denis F. A survey of HTLV-1 and HIVs in Ethiopian leprosy patients. AIDS. (1991) 5:108–10.
33. Glaser JB, Levis WR, Gruber T, Cabrera A, Poiesz BJ. Prevalence of human T cell lymphotropic virus (HTLV) types I and II and human immunodeficiency virus type 1 infections among persons with Hansen's disease in New York City. J Infect Dis. (1994) 170:1007–9. doi: 10.1093/infdis/170.4.1007
34. Machado PRL, Machado LM, Shibuya M, Rego J, Johnson WD, Glesby MJ. Viral co-infection and leprosy outcomes: a cohort study. PLoS Negl Trop Dis. (2015) 9:1–11. doi: 10.1371/journal.pntd.0003865
35. Milanga M, Kashala LO, Mbayo I, Yajima M, Yamada N, Mbowa KR, et al. Brief survey of leprosy situation in Congo: sero-epidemiologic profile in correlation with some emerging viral infections. Nihon Hansenbyo Gakkai Zasshi. (1999) 68:109–16. doi: 10.5025/hansen.68.109
36. Murphy EL, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann Intern Med. (1989) 111:555. doi: 10.7326/0003-4819-111-7-555
37. Wiktor SZ, Cannon RO, Atkinson WL, Lutz B, Hook EW, Blattner WA, et al. Infection with human T lymphotropic virus types I and II in sexually transmitted disease clinics in Baltimore and New Orleans. J Infect Dis. (1992) 165:920–4. doi: 10.1093/infdis/165.5.920
38. Rouet F, Herrmann-Storck C, Courouble G, Deloumeaux J, Madani D, Strobel M. A case-control study of risk factors associated with human T-cell lymphotrophic virus type-I seropositivity in blood donors from Guadeloupe, French West Indies. Vox Sang. (2002) 82:61–6. doi: 10.1046/j.0042-9007.2001.00143.x
39. Hesran Jy Le Delaporte E, Gaudebout C, Trebuck A, Schrijvers D, Josse R, Peeters M, et al. Demographic factors associated with HTLV-1 infection in a gabonese community. Int J Epidemiol. (1994) 23:812–7. doi: 10.1093/ije/23.4.812
40. Nunes D, Boa-Sorte N, Grassi MFR, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS ONE. (2017) 12:e0171303. doi: 10.1371/journal.pone.0171303
41. Hashido M, Lee FK, Nahmias AJ, Inouye S, Miyata K, Nagata Y, et al. Herpes simplex virus types 1 and 2, chlamydia, syphilis, and toxoplasma in pregnant Japanese women with HTLV-I. J Acquir Immune Defic Syndr Hum Retrovirol. (1998) 17:95–7.
42. Boulos R, Ruff AJ, Nahmias A, Holt E, Harrison L, Magder L, et al. Herpes simplex virus type 2 infection, syphilis, and hepatitis B virus infection in haitian women with human immunodeficiency virus type 1 and human T lymphotropic virus type I infections. J Infect Dis. (1992) 166:418–20. doi: 10.1093/infdis/166.2.418
43. Vargas L, Bastos F, Guimarães A, Amaral S, Fausto T, Arriaga M, et al. Seroprevalence and factors associated with human immunodeficiency virus, human T lymphotropic virus and Hepatitis B/C infections in parturient women of Salvador – Bahia, Brazil. Brazilian J Infect Dis. (2020) 24:279–87. doi: 10.1016/j.bjid.2020.05.001
44. Wignall FS, Hyams KC, Phillips IA, Escamilla J, Tejada A, Li O, et al. Sexual transmission of human T-lymphotropic virus type I in Peruvian prostitutes. J Med Virol. (1992) 38:44–8. doi: 10.1002/jmv.1890380110
45. Gotuzzo E, Sanchez J, Escamilla J, Carrillo C, Phillips IA, Moreyra L, et al. Human T cell lymphotropic virus type I infection among female sex workers in Peru. J Infect Dis. (1994) 169:754–9. doi: 10.1093/infdis/169.4.754
46. Giuliani M, Rezza G, Lepri AC, Di Carlo A, Maini A, Crescimbeni E, et al. Risk factors for HTLV-I and II in individuals attending a clinic for sexually transmitted diseases. Sex Transm Dis. (2000) 27:87–92. doi: 10.1097/00007435-200002000-00006
47. Delaporte E, Buvé A, Nzila N, Goeman J, Dazza MC, Henzel D, et al. HTLV-I infection among prostitutes and pregnant women in Kinshasa, Zaïre: how important is high-risk sexual behavior? J Acquir Immune Defic Syndr Hum Retrovirol. (1995) 8:511–5. doi: 10.1097/00042560-199504120-00012
48. Norrgren H, Andersson S, Naucler A, Dias F, Johansson I, Biberfeld G. HIV-1, HIV-2, HTLV-I/II and Treponema pallidum infections. J Acquir Immune Defic Syndr Hum Retrovirology. (1995) 9:422. doi: 10.1097/00042560-199508000-00014
49. Zunt JR, Dezzutti CS, Montano SM, Thomas KK, Alarcón JOV, Quijano E, et al. Cervical shedding of human T cell lymphotropic virus type I is associated with cervicitis. J Infect Dis. (2002) 186:1669–72. doi: 10.1086/345364
50. Lôpo SS, Oliveira PM, Santana IU, Pena GB, Torrales MB, Mascarenhas RE, et al. Evidence of a higher prevalence of HPV infection in HTLV-1-infected women: a cross-sectional study. Rev Soc Bras Med Trop. (2012) 45:305–8. doi: 10.1590/S0037-86822012000300005
51. Blas MM, Alva IE, Garcia PJ, Carcamo C, Montano SM, Muñante R, et al. Association between human papillomavirus and human T-lymphotropic virus in indigenous women from the Peruvian Amazon. PLoS ONE. (2012) 7:e44240. doi: 10.1371/journal.pone.0044240
52. Malm K, Ekermo B, Hillgren K, Britton S, Fredlund H, Andersson S. Prevalence of human T-lymphotropic virus type 1 and 2 infection in Sweden. Scand J Infect Dis. (2012) 44:852–9. doi: 10.3109/00365548.2012.689847
53. Ma Y, Zheng S, Wang N, Duan Y, Sun X, Jin J, et al. Epidemiological analysis of HTLV-1 and HTLV-2 infection among different population in central China. PLoS ONE. (2013) 8:e66795. doi: 10.1371/journal.pone.0066795
54. Pereira FM, Almeida M da CC de, Santos FLN, Carreiro RP, Galvão-Castro B, Grassi MFR. Distribution of human T-lymphotropic virus (HTLV) and hepatitis C co-infection in Bahia, Brazil. PLoS ONE. (2020) 15:e0223087. doi: 10.1371/journal.pone.0223087
55. Einsiedel L, Chiong F, Jersmann H, Taylor GP. Human T-cell leukaemia virus type 1 associated pulmonary disease: clinical and pathological features of an under-recognised complication of HTLV-1 infection. Retrovirology. (2021) 18:1. doi: 10.1186/s12977-020-00543-z
56. Einsiedel L, Spelman T, Goeman E, Cassar O, Arundell M, Gessain A. Clinical associations of human T-lymphotropic virus type 1 infection in an indigenous Australian population. PLoS Negl Trop Dis. (2014) 8:e2643. doi: 10.1371/journal.pntd.0002643
57. Santos SB, Porto AF, Muniz AL, De Jesus AR, Carvalho EM. Clinical and immunological consequences of human T cell leukemia virus type-I and Schistosoma mansoni co-infection. Mem Inst Oswaldo Cruz. (2004) 99:121–6. doi: 10.1590/s0074-02762004000900022
58. Porto AF, Santos SB, Alcântara L, Guerreiro JB, Passos J, Gonzalez T, et al. HTLV-1 modifies the clinical and immunological response to schistosomiasis. Clin Exp Immunol. (2004) 137:424–9. doi: 10.1111/j.1365-2249.2004.02508.x
59. Verdonck K, González E, Henostroza G, Nabeta P, Llanos F, Cornejo H, et al. HTLV-1 infection is frequent among out-patients with pulmonary tuberculosis in northern Lima, Peru. Int J Tuberc Lung Dis. (2007) 11:1066–72.
60. Tachibana N, Okayama A, Ishizaki J, Yokota T, Shishime E, Murai K, et al. Suppression of tuberculin skin reaction in healthy HTLV-I carriers from Japan. Int J Cancer. (1988) 42:829–31. doi: 10.1002/ijc.2910420605
61. Welles SL, Tachibana N, Okayama A, Shioiri S, Ishihara S, Murai K, et al. Decreased reactivity to PPD among htlv-i carriers in relation to virus and hematologic status. Int J Cancer. (1994) 56:337–40. doi: 10.1002/ijc.2910560307
62. Murai K, Tachibana N, Shioiri S, Shishime E, Okayama A, Ishizaki J, et al. Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J Acquir Immune Defic Syndr. (1990) 3:1006–9.
63. Bastos MDL, Santos SB, Souza A, Finkmoore B, Bispo O, Barreto T, et al. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect Dis. (2012) 12:13–5. doi: 10.1186/1471-2334-12-199
64. Alves FA, Campos KR, Lemos MF, Moreira RC. Caterino-de-Araujo A. Hepatitis C viral load in HCV-monoinfected and HCV/HIV-1-, HCV/HTLV-1/-2-, and HCV/HIV/HTLV-1/-2-co-infected patients from São Paulo, Brazil. Brazilian J Infect Dis. (2018) 22:123–8. doi: 10.1016/j.bjid.2018.03.002
65. Campos KR, Alves FA, Lemos MF, Moreira RC, Marcusso RMN, Caterino-de-Araujo A. The reasons to include the serology of human T-lymphotropic virus types 1 and 2 (HTLV-1 and HTLV-2) in the clinical follow-up of patients with viral hepatitis B and C in Brazil. PLoS Negl Trop Dis. (2020) 14:e0008245. doi: 10.1371/journal.pntd.0008245
66. Silva MC, Silva CAC, Machado GU, Atta A, Freire SM, Carvalho E, et al. HCV/HTLV coinfection: does HTLV-1 interfere in the natural history of HCV-related diseases? J Med Virol. (2016) 88:1967–72. doi: 10.1002/jmv.24538
67. Kamihira S, Momita S, Ikeda S, Yamada Y, Sohda H, Atogami S, et al. Cohort study of hepatotropic virus and human T lymphotropic virus type-I infections in an area endemic for adult T cell leukemia. Jpn J Med. (1991) 30:492–7. doi: 10.2169/internalmedicine1962.30.492
68. Kishihara Y, Furusyo N, Kashiwagi K, Mitsutake A, Kashiwagi S, Hayashi J. Human T lymphotropic virus type 1 infection influences hepatitis C virus clearance. J Infect Dis. (2001) 184:1114–9. doi: 10.1086/323890
69. Castro E, Roger E. Hepatitis C virus/human T lymphotropic virus 1/2 co-infection: Regional burden and virological outcomes in people who inject drugs. World J Virol. (2016) 5:68–72. doi: 10.5501/wjv.v5.i2.68
70. Ichikawa T, Taura N, Miyaaki H, Matsuzaki T, Ohtani M, Eguchi S, et al. Human T-cell leukemia virus type 1 infection worsens prognosis of hepatitis C virus-related living donor liver transplantation. Transpl Int. (2012) 25:433–8. doi: 10.1111/j.1432-2277.2012.01434.x
71. Tokunaga M, Uto H, Oda K, Tokunaga M, Mawatari S, Kumagai K, et al. Influence of human T-lymphotropic virus type 1 coinfection on the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J Gastroenterol. (2014) 49:1567–77. doi: 10.1007/s00535-013-0928-5
72. Boschi-Pinto C, Stuver S, Okayama A, Trichopoulos D, Orav EJ, Tsubouchi H, et al. Follow-up study of morbidity and mortality associated with hepatitis C Virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. (2000) 181:35–41. doi: 10.1086/315177
73. Zhang J, Yamada O, Kawagishi K, Yoshida H, Araki H, Yamaoka S, et al. Up-regulation of hepatitis C virus replication by human T cell leukemia virus type I-encoded Tax protein. Virology. (2007) 369:198–205. doi: 10.1016/j.virol.2007.07.032
74. Espíndola OM, Vizzoni AG, Lampe E, Andrada-Serpa MJ, Araújo AQC, Leite ACC. Hepatitis C virus and human T-cell lymphotropic virus type 1 co-infection: impact on liver disease, virological markers, and neurological outcomes. Int J Infect Dis. (2017) 57:116–22. doi: 10.1016/j.ijid.2017.01.037
75. Milagres FAP, Duarte MIS, Viso AT, Segurado AC. Hepatitis C virus and human T-lymphotropic virus coinfection: epidemiological, clinical, laboratory and histopathological features. Rev Soc Bras Med Trop. (2009) 42:363–8. doi: 10.1590/S0037-86822009000400001
76. Cardoso DF, Souza FV de, Fonseca LAM, Duarte AJ da S, Casseb J. Influence of human T-cell lymphotropic virus type 1 (HTLV-1) Infection on laboratory parameters of patients with chronic hepatitis C virus. Rev Inst Med Trop São Paulo. (2009) 51:325–9. doi: 10.1590/s0036-46652009000600003
77. Carnaúba D, Bittencourt A, Brites C. Atypical presentation of syphilis in an HTLV-I infected patient. Braz J Infect Dis. (2003) 7:273–7. doi: 10.1590/S1413-86702003000400008
78. Chosidow O. Scabies and pediculosis. Lancet. (2000) 355:819–26. doi: 10.1016/S0140-6736(99)09458-1
79. Brites C, Weyll M, Pedroso C, Badaró R. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. AIDS. (2002) 16:1292–3. doi: 10.1097/00002030-200206140-00015
80. Sanchez-Palacios C, Gotuzzo E, Vandamme A-MM, Maldonado Y. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int J Infect Dis. (2003) 7:132–7. doi: 10.1016/S1201-9712(03)90009-9
81. Gotuzzo E, Arango C, de Queiroz-Campos A, Istúriz RE. Human T-cell lymphotropic virus-I in Latin America. Infect Dis Clin North Am. (2000) 14:211–39. doi: 10.1016/S0891-5520(05)70225-7
82. Blas M, Bravo F, Castillo W, Castillo WJ, Ballona R, Navarro P, et al. Norwegian scabies in Peru: the impact of human T cell lymphotropic virus type I infection. Am J Trop Med Hyg. (2005) 72:855–7.
83. del Giudice P, Marie D Sainte, Gérard Y, Couppié P, Pradinaud R. Is crusted (Norwegian) scabies a marker of adult T cell leukemia/lymphoma in human T lymphotropic virus type I-seropositive patients? J Infect Dis. (1997) 176:1090–2. doi: 10.1086/516518
84. Adedayo A, Bascom C, Grell G, Bellot P, Adebiyi R, Chandra P. Disseminated molluscum contagiosum and pulmonary cryptococcosis coexisting in an HTLV-1 seropositive patient. J Eur Acad Dermatology Venereol. (2003) 17:723–4. doi: 10.1046/j.1468-3083.2003.00889.x
85. Takeshita T, Takeshita H. Crusted (Norwegian) scabies in a patient with smoldering adult T-cell leukemia. J Dermatol. (2000) 27:677–9. doi: 10.1111/j.1346-8138.2000.tb02253.x
86. Daisley H, Charles W, Suite M. Crusted (Norwegian) scabies as a pre-diagnostic indicator for HTLV-1 infection. Trans R Soc Trop Med Hyg. (1993) 87:295. doi: 10.1016/0035-9203(93)90134-C
87. Bimbi C, Brzezinski P, Sokolowska-Wojdylo M. Crusted (Norwegian) scabies as a strong marker of adult T-cell leukemia/lymphoma in HTLV-1 infection. Clin Case Rep. (2019) 7:474–6. doi: 10.1002/ccr3.1983
88. Bergman JN, Dodd WAH, Trotter MJ, Oger JJF, Dutz JP. Crusted scabies in association with human T-cell lymphotropic virus 1. J Cutan Med Surg. (1999) 3:148–52. doi: 10.1177/120347549900300310
89. Nobre V, Guedes AC, Martins ML, Barbosa-Stancioli EF, Serufo JC, Proietti FA, et al. Dermatological findings in 3 generations of a family with a high prevalence of human T cell lymphotropic virus type 1 infection in Brazil. Clin Infect Dis. (2006) 43:1257–63. doi: 10.1086/508177
90. Einsiedel LJ, Pepperill C, Wilson K. Crusted scabies: a clinical marker of human T-lymphotropic virus type 1 infection in central Australia. Med J Aust. (2014) 200:633–4. doi: 10.5694/mja14.00458
91. Bittencourt AL, Oliveira M de FP de. Cutaneous manifestations associated with HTLV-1 infection. Int J Dermatol. (2010) 49:1099–110. doi: 10.1111/j.1365-4632.2010.04568.x
92. Kawamoto K, Miyoshi H, Suzuki T, Muto R, Yamada K, Yanagida E, et al. Clinicopathological features of cryptococcal lymphadenitis and a review of literature. J Clin Exp Hematop. (2017) 57:26–30. doi: 10.3960/jslrt.17011
93. Chalkias S, Doweiko JP, Eliopoulos GM. An Enabling Act. Open Forum Infect Dis. (2014) 1:ofu008. doi: 10.1093/ofid/ofu008
94. Tashiro T, Yamasaki T, Nagai H, Kikuchi H, Nasu M. Immunological studies on opportunistic infection and the development of adult T-cell leukemia. Intern Med. (1992) 31:1132–6. doi: 10.2169/internalmedicine.31.1132
95. Desai A, Fe A, Desai A, Ilowite J, Cunha BA, Mathew JP. A case of pneumonia caused by Pneumocystis jirovecii and Cryptococcus neoformans in a patient with HTLV-1 associated adult T-cell leukemia/lymphoma: Occam's Razor blunted. Conn Med. (2016) 80:81–3.
96. Rhew DC, Gaultier CR, Daar ES, Zakowski PC, Said J. Infections in patients with chronic adult T-cell leukemia/lymphoma: case report and review. Clin Infect Dis. (1995) 21:1014–6. doi: 10.1093/clinids/21.4.1014
97. Canelo-Aybar C, Cuadra-Urteaga J, Atencia F, Romani F. Human T lymphotropic virus-1 associated gastrointestinal histoplasmosis in Peru. J Infect Dev Ctries. (2011) 5:484–8. doi: 10.3855/jidc.1030
98. Rivierez M, Heyman D, Brebion A, Landau-Ossondo M, Desbois N, Vally P. [Spinal cord histoplasmoma. A case report]. Neurochirurgie. (2002) 48:44–8.
99. Cappell MS, Chow J. HTLV-I-associated lymphoma involving the entire alimentary tract and presenting with an acquired immune deficiency. Am J Med. (1987) 82:649–54. doi: 10.1016/0002-9343(87)90117-3
100. Leon M, Alave J, Bustamante B, Gotuzzo E, Seas C. A probable association between HTLV-1 and endemic mycosis in Latin America. J Infect Dev Ctries. (2012) 6:301. doi: 10.3855/jidc.2305
101. León M, Alave J, Bustamante B, Cok J, Gotuzzo E, Seas C. Human T lymphotropic virus 1 and paracoccidioidomycosis: a probable association in Latin America. Clin Infect Dis. (2010) 51:250–1. doi: 10.1086/653679
102. Lima LM, Cardoso LS, Santos SB, Oliveira RR, Oliveira SC, Góes AM, et al. Schistosoma antigens downregulate CXCL9 production by PBMC of HTLV-1-infected individuals. Acta Trop. (2017) 167:157–62. doi: 10.1016/j.actatropica.2016.12.030
103. Lima LM, Santos SB, Oliveira RR, Cardoso LS, Oliveira SC, Góes AM, et al. Schistosoma antigens downmodulate the in vitro inflammatory response in individuals infected with human T cell lymphotropic virus type 1. Neuroimmunomodulation. (2013) 20:233–8. doi: 10.1159/000348700
104. Porto AF, Santos SB, Muniz AL, Basilio V, Rodrigues W Jr, Neva FA, et al. Helminthic infection down-regulates type 1 immune responses in human T cell lymphotropic virus type 1 (HTLV-1) carriers and is more prevalent in HTLV-1 carriers than in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. J Infect Dis. (2005) 191:612–8. doi: 10.1086/427560
105. Souza A, Carvalho N, Neves Y, Braga Santos S, Bastos M de L, Arruda S, et al. Association of tuberculosis status with neurologic disease and immune response in HTLV-1 infection. AIDS Res Hum Retroviruses. (2017) 33:1126–33. doi: 10.1089/aid.2015.0340
106. Turpin J, Yurick D, Khoury G, Pham H, Locarnini S, Melamed A, et al. Impact of hepatitis B virus coinfection on human T-lymphotropic virus type 1 clonality in an indigenous population of Central Australia. J Infect Dis. (2019) 219:562–7. doi: 10.1093/infdis/jiy546
107. Lee C-W, Chang M-C, Chang Y-F, Hsieh R-K, Lin J, Chen K-S. Adult T-cell leukemia/lymphoma in Taiwan: an analysis of 17 patients and review of the literature. Asia Pac J Clin Oncol. (2010) 6:161–4. doi: 10.1111/j.1743-7563.2010.01320.x
108. Marcusso RMN, Van Weyenbergh J, de Moura JVL, Dahy FE, de Moura Brasil Matos A, Haziot MEJ, et al. Dichotomy in Fatal Outcomes in a Large Cohort of People Living with HTLV-1 in São Paulo, Brazil. Pathogens. (2019) 9:25. doi: 10.3390/pathogens9010025
109. Assone T, Kanashiro TM, Baldassin MPMM, Paiva A, Haziot ME, Smid J, et al. In vitro basal T-cell proliferation among asymptomatic human T cell leukemia virus type 1 patients co-infected with hepatitis C and/or human immunodeficiency virus type 1. Braz J Infect Dis. (2018) 22:106–12. doi: 10.1016/j.bjid.2018.02.002
110. Marcusso RMN, Van Weyenbergh J, de Moura JVL, Dahy FE, de Moura Brasil Matos A, Haziot MEJ, et al. Infective dermatitis associated with human T-cell lymphotropic virus type 1: evaluation of 42 cases observed in Bahia, Brazil. Clin Infect Dis. (2012) 54:1714–9. doi: 10.1093/cid/cis273
111. Varandas C, Silva JLS, Primo JRL, Oliveira M de FS, Moreno-Carvalho O, Farre L, et al. Early juvenile human T-cell lymphotropic virus type-1 – associated myelopathy/tropical spastic paraparesis: study of 25 patients. Clin Infect Dis. (2018) 67:1427–33. doi: 10.1093/cid/ciy289/4975518
112. Primo JR, Brites C, Oliveira Mde F, Moreno-Carvalho O, Machado M, Bittencourt AL. Infective dermatitis and human T cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis in childhood and adolescence. Clin Infect Dis. (2005) 41:535–41. doi: 10.1086/432058
113. Okajima R, Oliveira ACP, Smid J, Casseb J, Sanches JA. High prevalence of skin disorders among HTLV-1 infected individuals independent of clinical status. PLoS Negl Trop Dis. (2013) 7:e2546. doi: 10.1371/journal.pntd.0002546
114. Lee R, Schwartz RA. Human T-lymphotrophic virus type 1–associated infective dermatitis: a comprehensive review. J Am Acad Dermatol. (2011) 64:152–60. doi: 10.1016/j.jaad.2009.10.021
115. Bittencourt AL. Adult T-cell leukemia/lymphoma (ATL) in Bahia, Brazil. Brazilian J Infect Dis. (2005) 9:437–8. doi: 10.1590/S1413-86702005000500020
Keywords: HTLV-1, co-infection, Mycobacterium tuberculosis, Mycobacterium leprae, HCV, HBV, sexually transmitted infections, Schistosoma mansoni
Citation: Rosadas C and Taylor GP (2022) HTLV-1 and Co-infections. Front. Med. 9:812016. doi: 10.3389/fmed.2022.812016
Received: 09 November 2021; Accepted: 12 January 2022;
Published: 03 February 2022.
Edited by:
Juarez Antonio Simões Quaresma, Universidade do Estado do Pará, BrazilReviewed by:
Luiz C. Rodrigues Junior, Federal University of Health Sciences of Porto Alegre, BrazilLinda B. Adams, The National Hansen's Disease Programs, United States
Copyright © 2022 Rosadas and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graham P. Taylor, g.p.taylor@imperial.ac.uk
 Carolina Rosadas
Carolina Rosadas Graham P. Taylor
Graham P. Taylor