- 1Division of Hematologic Malignancies & Cellular Therapeutics, University of Kansas Medical Center, Kansas City, KS, United States
- 2Moffitt Cancer Center, University of South Florida, Tampa, FL, United States
- 3Division of Hematology/Oncology, Roswell Park Cancer Institute Buffalo, NY, United States
- 4Division of Hematology/Oncology, University of Wisconsin School of Medicine & Public Health, Madison, WL, United States
Background: We conducted a systematic review and meta-analysis to evaluate outcomes following chimeric antigen receptor T cell (CAR-T) therapy in relapsed/refractory acute myeloid leukemia (RR-AML).
Methods: We performed a literature search on PubMed, Cochrane Library, and Clinicaltrials.gov. After screening 677 manuscripts, 13 studies were included. Data was extracted following PRISMA guidelines. Pooled analysis was done using the meta-package by Schwarzer et al. Proportions with 95% confidence intervals (CI) were computed.
Results: We analyzed 57 patients from 10 clinical trials and 3 case reports. The pooled complete and overall response rates were 49.5% (95% CI 0.18-0.81, I2 =65%) and 65.2% (95% CI 0.36-0.91, I2 =57%). The pooled incidence of cytokine release syndrome, immune-effector cell associated neurotoxicity syndrome, and graft-versus-host disease was estimated as 54.4% (95% CI 0.17-0.90, I2 =77%), 3.9% (95% CI 0.00-0.19, I2 =22%), and 1.6% (95%CI 0.00-0.21, I2 =33%), respectively.
Conclusion: CAR-T therapy has demonstrated modest efficacy in RR-AML. Major challenges include heterogeneous disease biology, lack of a unique targetable antigen, and immune exhaustion.
Introduction
Acute myeloid leukemia (AML) accounts for only 1% of all new cancers in the United States and remains one of the most aggressive hematological malignancies in adults with a 5-year survival rate of 30.5% (1). The prognosis of AML is poor, with a cure rate of a mere 5-15% in patients above age 60 years, and 35-40% in patients younger than 60 years (2). At the time of diagnosis, in most cases the disease initially responds to high-dose induction chemotherapy (3); nevertheless, 10-40% of patients are primarily refractory to induction chemotherapy (2). Allogenic hematopoietic stem cell transplant (HSCT) is deemed as the only definitive treatment for AML at this time for intermediate and high-risk patients, as it is expected to result in long-lasting complete remission (CR) (4). However, 50% of patients that undergo HSCT and 80% of the patients that are not eligible or while waiting for HSCT will eventually relapse and die of the disease (5). The treatment options for the relapsed disease are limited and median overall survival is in months after the disease relapse (6).
Chimeric antigen receptor (CAR)-T cell therapy has shown promising results for the treatment of chemotherapy-refractory B cell malignancies, including acute lymphoblastic leukemia (ALL) and B cell lymphoma as well as for multiple myeloma (7–10). The use of CAR-T cell therapy is now being investigated for the treatment of AML (11). The road to successful introduction and incorporation of CAR-T cell therapy to the treatment of AML has some key roadblocks that include, but are not limited to, antigenic heterogeneity that is prevalent in AML; the AML tumor microenvironment; and on-target off-tumor toxicities (12). In this paper, we bring forth the current advances made in CAR-T cell therapy for AML treatment as found in published data in the form of case reports, case series, and clinical trials.
Methods
Data source and search strategy
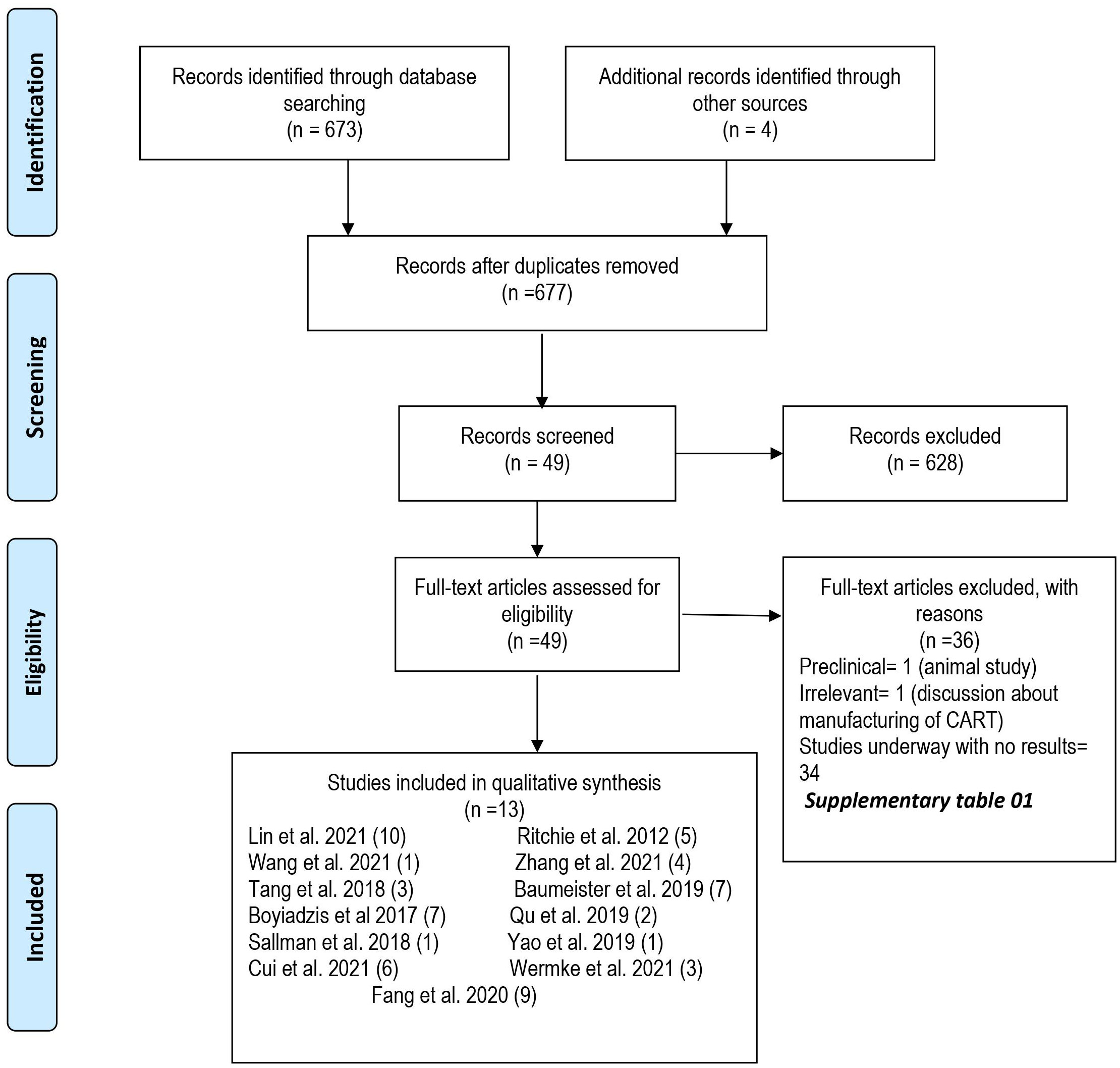
A detailed literature search was performed for the systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A population, intervention, comparison, and outcome table were developed, and three electronic databases (PubMed, Cochrane Register of Controlled Trials, and Clinical trials.gov) were searched using MeSH terms and keywords for “Leukemia, Myeloid, Acute” AND “Receptors, Chimeric Antigen” OR “adoptive immunotherapy” from the date of inception to December 6, 2022. No filters or publication time limits were applied for the search. We also searched conference abstracts of annual meetings e.g., American Society of Hematology, American Society of Clinical Oncology, and American Society of Transplantation and Cellular Therapy. A total of 677 records were identified using the database search. All search results were imported to the Endnote X9.0 reference manager, and duplicates were removed.
Selection criteria
A total of 677 articles were screened independently by two authors. In primary screening, we excluded nonrelevant and review articles. Full texts of the remaining 49 articles were then assessed for eligibility based on predetermined criteria that were set after discussion and consensus between all authors and approved by the principal investigator (M.U.M.). Inclusion criteria were (1) original studies (clinical trials and case-control, retrospective, and prospective cohort studies); (2) studies reporting data for any age; (3) studies reporting only R/R AML patients; and (4) studies reporting CAR-T therapy as the intervention. A total of 13 studies (10 clinical trials and 3 case reports) were included, and 36 studies were excluded in secondary screening based on inclusion criteria (Figure 1). Supplementary Table S1 lists excluded studies along with the reasons for exclusion.
Data extraction
Three authors independently extracted data from the 13 selected studies. Datasheets were double-checked for any discrepancies. Data were collected on baseline characteristics (i.e., number of patients, sex, age, diagnosis, prior and subsequent HSCT, and conditioning therapy before CAR-T) and the following outcomes were extracted: CR, partial response (PR), overall response rate (ORR), overall survival (OS), progression-free survival (PFS), stable disease (SD), progressive disease (PD), cytokine release syndrome (CRS), immune-effector cell associated neurotoxicity syndrome (ICANS), and graft-versus-host disease (GVHD). The response was defined as per the clinical trials or the case reports and was not uniform. The instances where response assessment was not mentioned were excluded from the analysis.
Quality evaluation
The methodological quality of the included studies was evaluated using the National Institute of Health NIH quality assessment tool.
Data analysis
The inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with a 95% confidence Interval (CI) were extracted to compute pooled analysis using the ‘meta’ package by Schwarzer et al. in the R programming language (version 4.16-2).
Results
We identified 57 patients in 13 studies (3 case reports and 10 clinical trials) who received CAR-T therapy for RR-AML (Table 1). The median age of patients was 41 (7-80) years (13–25). Twelve studies mentioned sex ratio and 64% (n=32/50) were male (13, 15–25). Twenty-nine percent (12/41) of patients had a history of allogeneic HSCT before CAR-T therapy (13, 15–24), while subsequent HSCT was performed in 19% (11/57) of patients (13–25). Lin et al. did not specify the source of CAR-T cell therapy (autologous vs allogeneic) (13), while it was autologous in 33/47 (70%) and allogeneic in 14/47 (30%) of the patients (14–25). Eight studies were conducted in China (13, 16–18, 22–25), three in the US (14, 19, 20) and one study in Germany (21) and Australia (15) each. Five studies used fludarabine and cyclophosphamide (FluCy) as conditioning regimen (15, 18, 21, 23, 25) while Que et al. used decitabine with FluCy for conditioning (24). Four studies did not use any conditioning (13, 14, 17, 20), and two studies did not report regarding the conditioning regimen (16, 19). The target antigens were CD123 (21, 22), NKG2D (14, 20), CLL-1 (13, 18), CD33 (16, 17), CD33-CLL1 (25), CD19 (24), CD33, CD34, CD45, CD117 (19), CD38 (23), and Ley Ag (15).
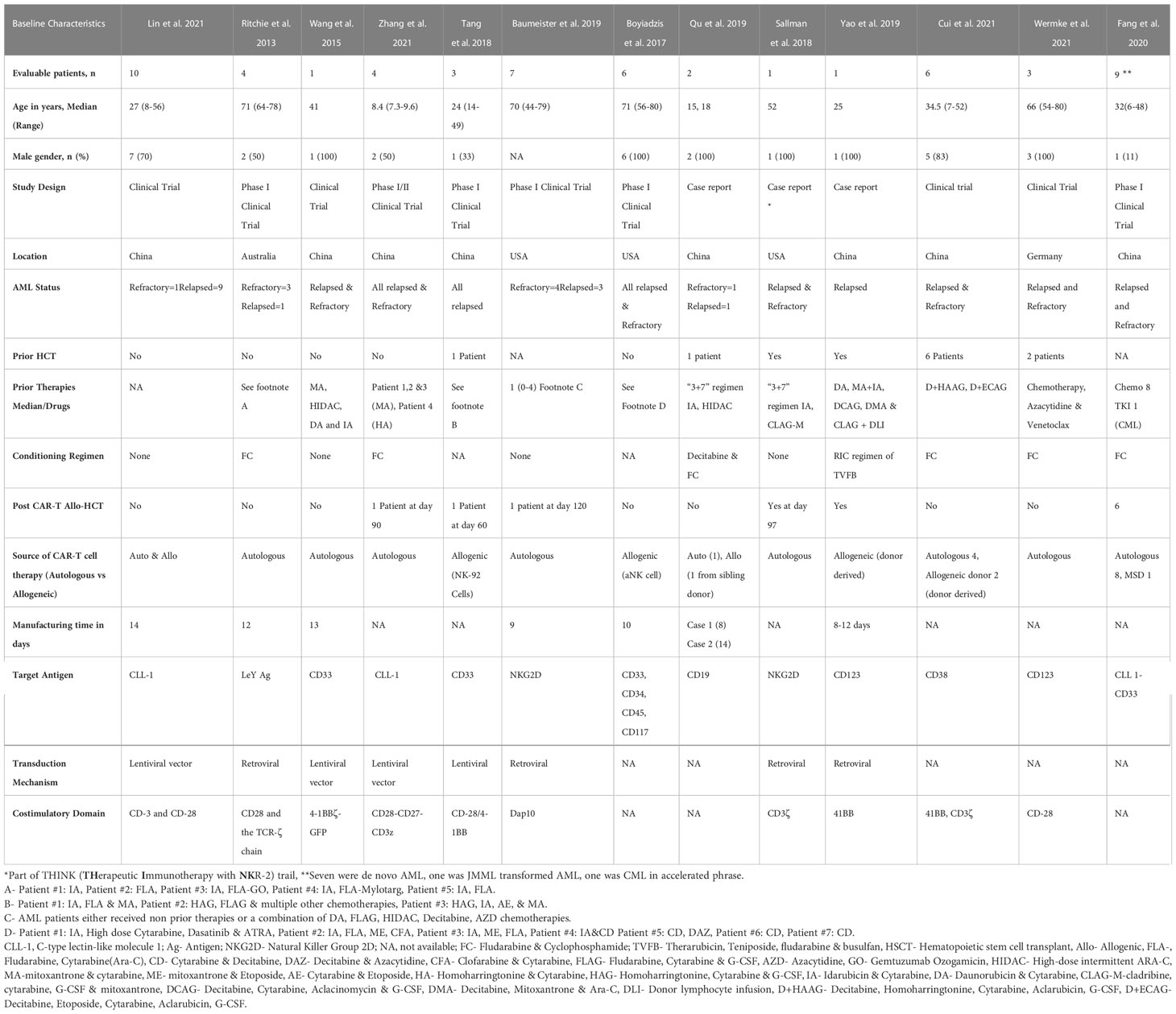
Table 1 Baseline Characteristics of chimeric antigen receptor T-Cell (CAR-T) therapy in acute myeloid leukemia (n=57).
At a median duration of 5.7 (1-23) months (15–25), OS was reported from 1.8 months (22) to 23 months (18). Mean OS could not be calculated due to heterogeneity of the included studies (Table 2). Twenty-two patients had complete remission after CAR-T therapy, with an estimated pooled incidence of 49.5% (95% CI 0.18-0.81, I2 =65%) (14–25) (Figure 2A). The pooled incidence of ORR was estimated as 65.2% (95% CI 0.36-0.91, I2 =57%) (14–25) (Figure 2B). One out of the four included patients in Ritchie et al. remained without cytogenetic disease for 23 months, while the other three patients relapsed on day 49, day 28, and at 5 months, respectively (15). Wang et al. reported OS of 3.1 months (17) while in Zhang et al. patients’ number 1, 2, and 3 died at 23, 5, and 12 months respectively, and patient number 4 was alive at 9 months. One out of the three included patients in Tang et al. relapsed at 4 months and no treatment was pursued, the second patient relapsed 6 months post CAR-T and 4 months post-HSCT and died of grade IV GVHD after salvage chemotherapy and donor lymphocyte infusions, and the third patient did not respond to CAR-T (16). At 21 days post-CAR-T, no patient achieved CR, and only one patient had a reduction in blast percentage in Boyiadzis et al. (19) Both included patients by Qu et al. achieved CR and one of them maintained CR for over 10 months, while the other refused further treatment after CR and relapsed at 3 months (24). The patient reported by Sallman et al. maintained CR for 6 months post-HSCT and 9 months following initial CAR-T cell therapy (20), while the patient in Yao et al. received CAR-T as part of conditioning therapy and achieved CR with incomplete count recovery, developed grade IV GVHD on day 32 and died on day 56 (22). Cui et al. reported the 6-month OS and leukemia-free survival (LFS) rates were both 50%, and the median OS and LFS were 7.9 and 6.4 months, respectively (23). Two patients in Wermke et al. achieved complete remission with incomplete hematologic recovery, one of which remained in remission at 100 days, while the other relapsed at one month after the CAR-T cell therapy (21). Fang et al. reported that seven out of nine patients were minimal residual disease negative (MRD-) at four weeks follow-up post CAR-T cell therapy. Six out of these seven MRD- patients moved to subsequent HSCT with successful engraftment and persistent full chimerism in five patients. One patient died of sepsis on day +6 before engraftment (25).
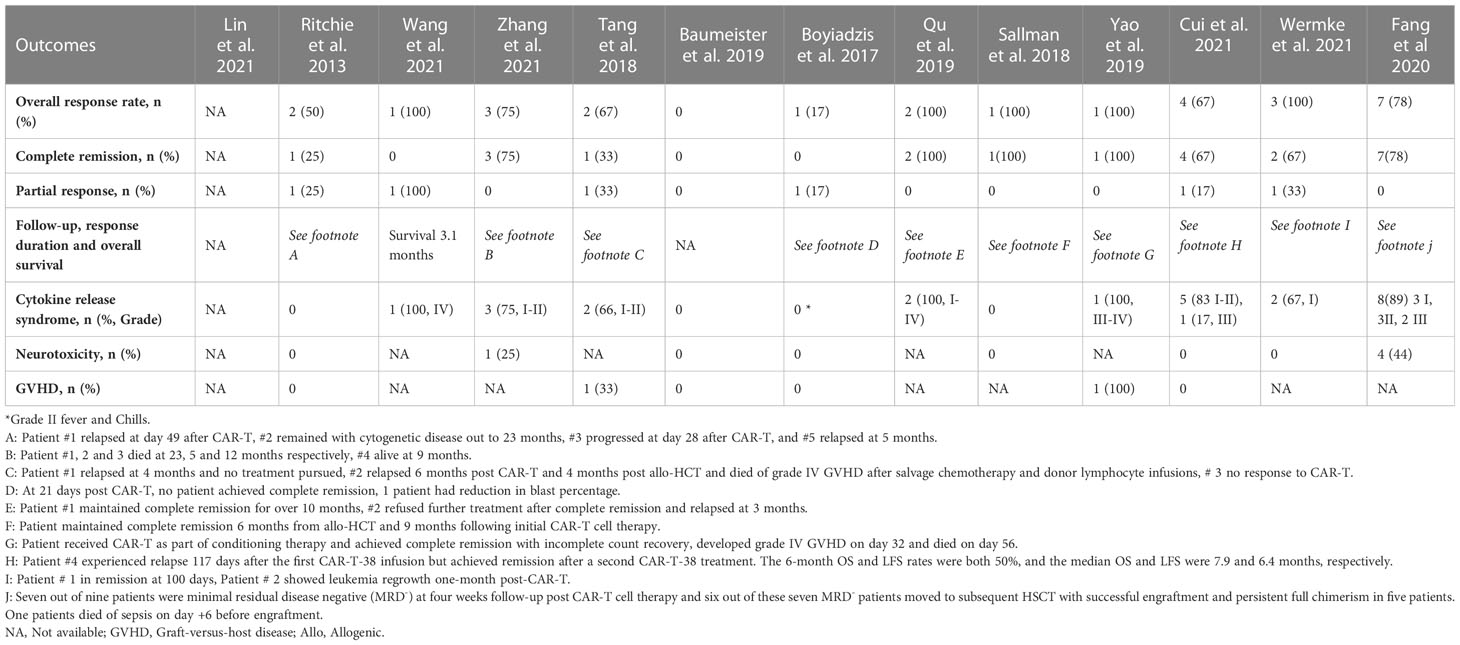
Table 2 Outcomes with Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Acute Myeloid Leukemia (n=57).
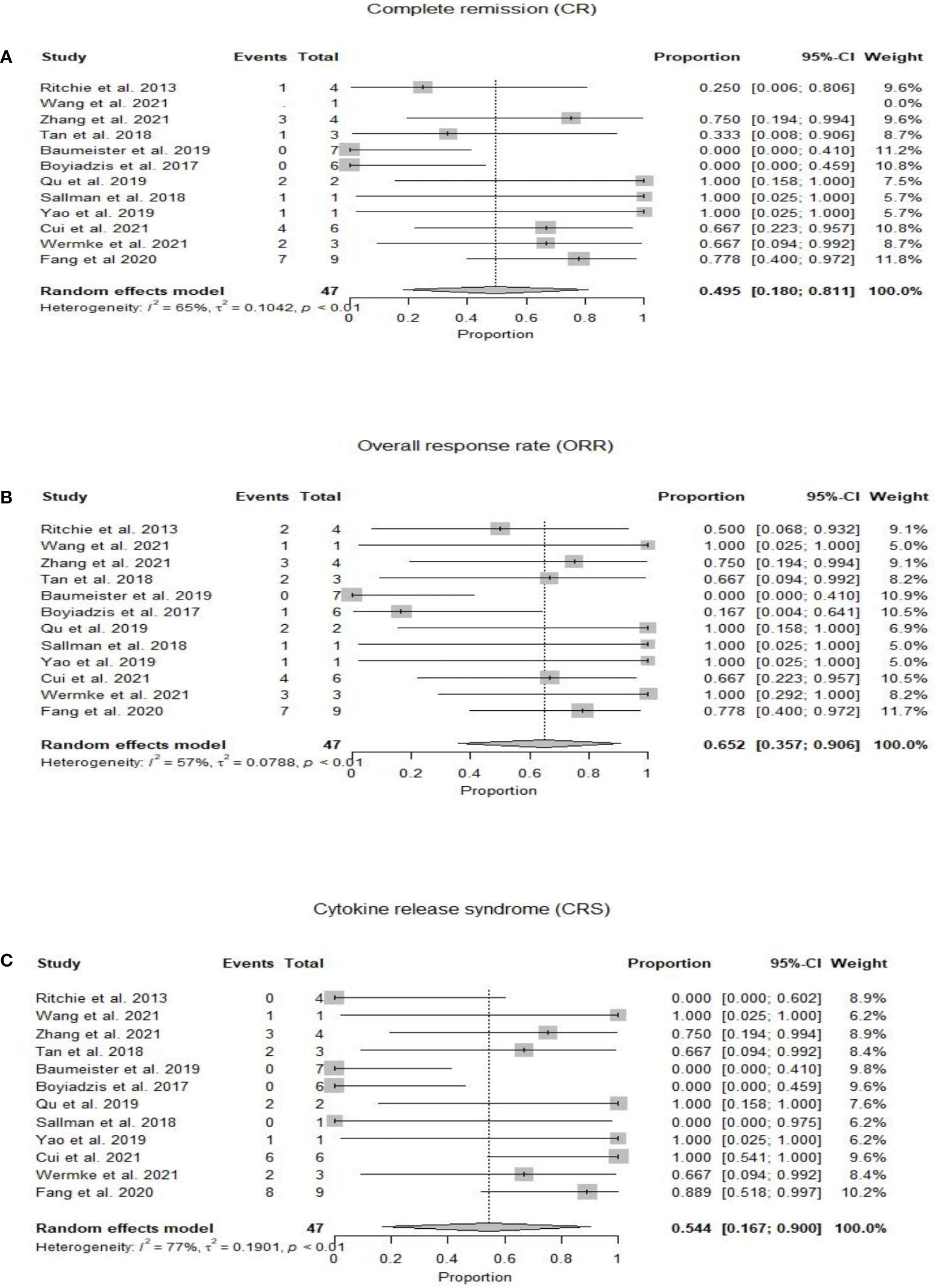
Figure 2 (A) Forest plot of complete response rate (CR) post CAR-T therapy for relapsed refractory acute myeloid leukemia. (B) Forest plot of overall response rate (ORR) post CAR-T therapy for relapsed refractory acute myeloid leukemia. (C) Forest plot of cytokine release syndrome (CRS) post CAR-T therapy for relapsed refractory acute myeloid leukemia.
In 10 patients from 3 studies, CD33 target antigen was used (16, 17, 19). Boyiazis et al. used CD34, CD45, and CD117 in addition to CD33. Four out of 10 patients showed ORR (40%) and one patient had CR (10%). In two studies, four patients were treated with CAR-T targeting CD123 antigen (21, 22). These four patients showed ORR (100%) while three of them achieved CR (75%). NKG2D target antigen was used in eight patients from two studies, and only one out of eight patients showed CR (12.5%) (14, 20). CLL-1 was used in two studies, however, only four patients reported outcomes and three of these patients had CR (75%) (13, 18). CD33+-CLL1+ was the target antigen in Fang et al. and seven out of nine patients achieved MRD- while two patients did not respond (25).
Twenty-five patients developed CRS with a pooled incidence of 54.4% (95% CI 0.17-0.90, I2 =77%) (14–25) (Figure 2C). Only five patients in eight studies developed ICANS, with a pooled incidence of 3.9% (95% CI 0.00-0.19, I2 =22%) (14, 15, 18–21, 23, 25). GVHD was reported by six studies and developed in two patients who had prior and subsequent HSCT after CAR-T therapy; one of them developed grade IV GVHD and died, in the setting of salvage therapy with donor lymphocyte infusions for relapsed disease 6 months post-CAR-T and 4 months post second HSCT (16), while the other patient received CAR-T as part of conditioning therapy and developed grade IV GVHD on day 32 post-HSCT (22). CAR-T cell products were allogeneic in both cases who had GVHD (16, 22). Qu et al. and Fang et al. reported grade IV cytopenias in all enrolled patients (24, 25) while Ritchie et al. reported grade II neutropenia in only 25% of the patients (15). Zhang et al. observed grade III anemia and grade IV neutropenia in four and three patients, respectively (18). Fang et al. reported non-hematologic toxicity as liver function test elevation (56%), coagulation disorder (44%), diarrhea (44%), skin rash (11%), and renal insufficiency (11%) (25). Fang et al. also reported incidence of pneumonia (33%), sepsis (33%), and fungal infection (22%) (25) while only one out of six patients had infection in Cui et al (23).
Discussion
In this meta-analysis, we focused on outcomes of relapsed or refractory AML patients who underwent experimental chimeric antigen receptor T cell therapies. CAR-T cell therapy is a recent breakthrough that has shown promising results in hematological malignancies including acute lymphoblastic leukemia (7). Presently, there are 14 ongoing clinical trials in the United States (Table 3) and 20 active international clinical trials studying various target antigens for relapsed/refractory AML (6, 26) (Table 4). Examples of target antigens being investigated include CD33, CD38, CD123, UCART123, CD123/CLL1, CD33/CLL1, WT1, CD7/NK92, and NKG2D (26). Our meta-analysis included 13 studies with a total of 57 patients. Most patients were males with a median age of 41 years, and a total of 38 patients had reported outcomes. The pooled overall and complete response rate was 65.2% and 49.5%, respectively. The pooled incidence of CRS, ICANS, and GVHD was 54.4%, 3.9%, and 1.6%, respectively.
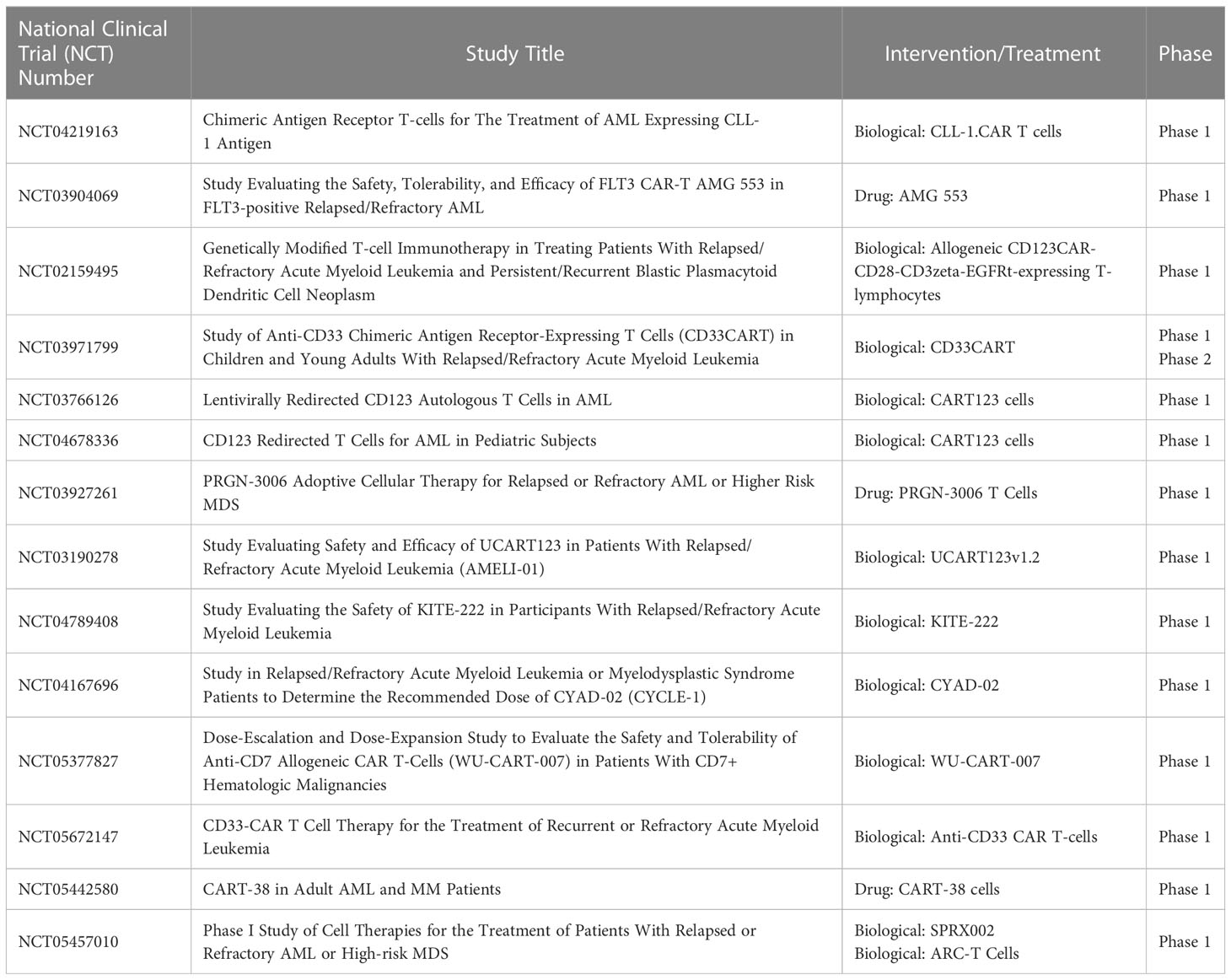
Table 3 Ongoing CAR-T therapy clinical trials in the United States for the treatment of relapsed or refractory acute myeloid leukemia.
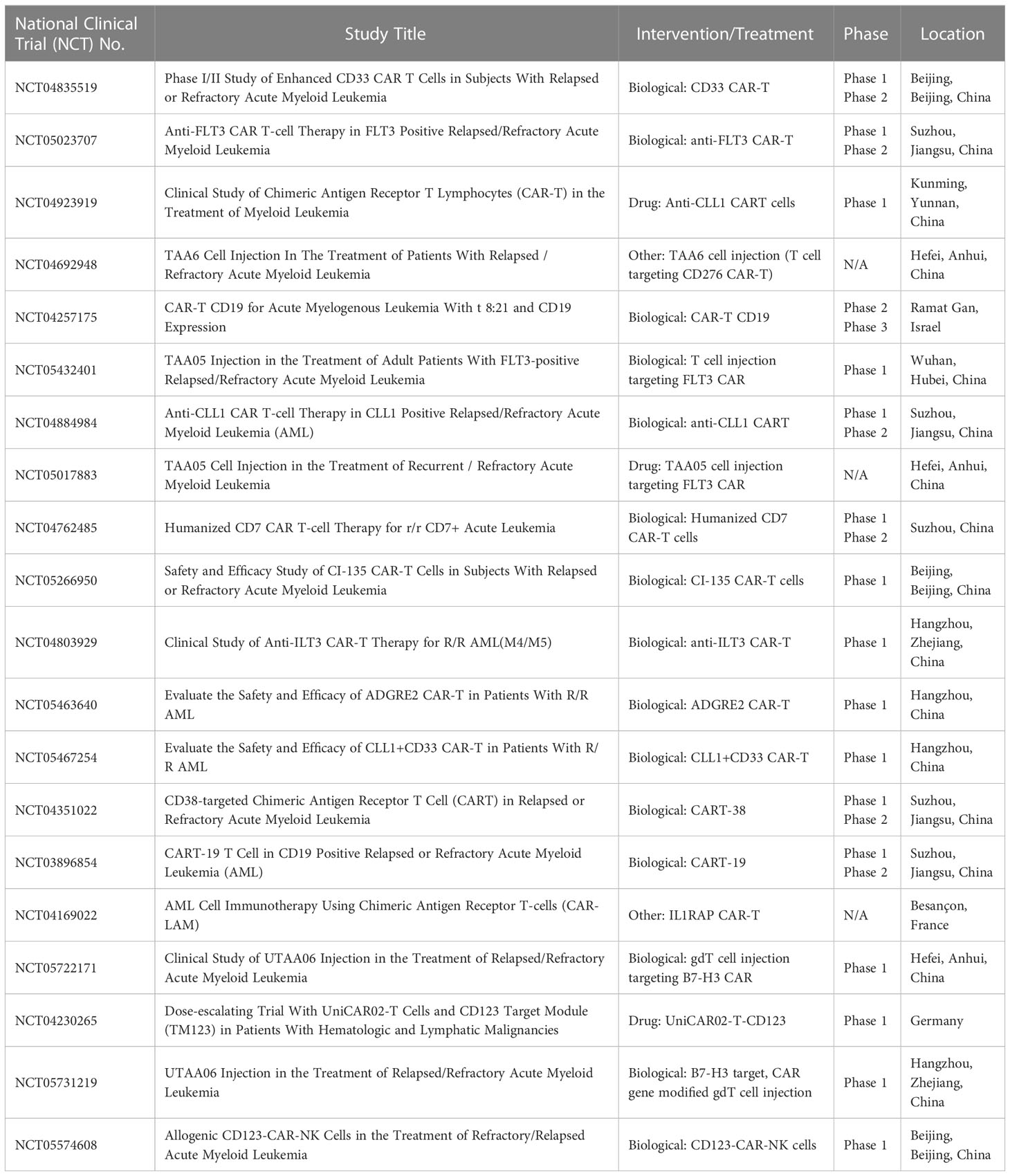
Table 4 Ongoing international CAR-T therapy clinical trials for the treatment of relapsed or refractory acute myeloid leukemia.
The most prominent feature of CAR-T in AML is the heterogeneity of targets used for AML. Four of the studies included in this meta-analysis had patients who underwent HSCT before CAR-T cell therapy. CD33 antigen was used in 10 of the included patients (16, 17, 19), while Boyiazis et al. used CD34, CD45, and CD117 in addition to CD33 (19). The reported ORR and CR were 40% and 10%, respectively. CD33 is a myeloid differentiation antigen that is present on myeloid blasts and has been used for antibody-based targeted therapies like gemtuzumab-ozogomycin for many years (27), so naturally, it is a common target for CAR-T development. Two studies used CD123 antigen and the reported ORR and CR were 100% and 75%, respectively (21, 22). CD123 is an alpha subunit of interleukin 3 receptor (IL3R) and is highly expressed in the AML blast cells differentially. It is expressed at a low level in hematopoietic stem cells, making it an ideal target for pharmacotherapy and cellular therapies (28). NKG2D (natural-killer group 2, member D) target antigen was used in eight patients (14, 20). NKG2D is an activating receptor that is mostly expressed on cells of the cytotoxic arm of the immune system, and NKG2D ligands are differentially expressed on malignant or stressed cells as compared to healthy tissue, making it a promising CAR candidate (29, 30). Only one out of eight patients showed CR (12.5%). CLL-1 (C-type lectin-like molecule-1) was used in two studies (13, 18); however, only four patients reported outcomes and three of these patients had CR (75%). CD33+-CLL1+ was used as target antigen in one study with 78% CR (25). Although the patient population is limited, a good response rate appears to have been observed with CD33, CLL-1, and CD-123 as target antigens. Most of the included studies did not report the type of relapse. Ritchie et al. demonstrated that LeY T cells were detectable in all patients by PCR until relapse, which ranged from 28 days to 23 months. Despite demonstrable CAR–T-cell persistence, the AML progression could be due to lost LeY expression on residual AML blasts (15).
The cumulative incidence of GVHD in CAR-T trials patient population is 5%. Twelve patients had allogeneic HSCT before CAR-T cell therapy (31.5%). Two patients in our meta-analysis who developed GVHD post-CAR-T cell therapy already had HSCT before CAR-T therapy and CAR-T cell products were allogeneic in both patients (16, 22). The contribution of CAR-T in the development of GVHD is not clear given the previous history of HSCT, and previous studies in B-ALL patients showed that GVHD is mild after allogeneic CAR-T administration (31). In the study by Liu et al., the cumulative incidence of acute GVHD after allogeneic HSCT was 39.5% (32). Another study by Sandhu et al. shows an incidence of GVHD of 43.8% (33); however, further studies are needed to better understand this.
The two major known toxicities of CAR-T cells, cytokine release syndrome and immune effector cell associated neurotoxicity syndrome (ICANS) were observed, but grade III-IV toxicities were rarely reported; only five patients had grade III-IV CRS, and none had grade III-IV ICANS. No patient died due to treatment-related toxicities. The durability of response remains a concern after CAR-T therapy and long-term follow-up data are lacking. The median duration of response for patients after CAR-T was in the range of a few months. However, Sallman et al. showed the most promising results with a patient in complete remission 6 months post-HSCT and 9 months post-CAR-T cell therapy (20). Of note, Sallman et al. also demonstrated that NKG2DL CAR-T cell therapy is not associated with significant adverse effects due to the selective up-regulation of NKG2DL on transformed cells, skipping normal hematopoietic stem cells. Ritchie et al. established that CAR-T cell persistence in blood and marrow until the time of relapse is an indication of CAR-T cell immunotherapy as a long-lasting treatment option (15).
Currently, several factors that limit the use of CAR-T therapy for AML patients, including biological barriers, manufacturing issues, limitations in the delivery of therapy, and patient-related factors (34). Most important is the lack of a target antigen that is specific to leukemic that avoids hematopoietic stem cell depletion (34). Relapsed or refractory AML patients are usually of advanced age, with limited tolerability to intensive treatment and co-morbidities restricting CAR-T therapy (34). Hostile and tolerogenic tumor microenvironment in AML can also limit the anti-tumor effectiveness of CAR-T cells resulting in immune exhaustion and relapse. Designing CAR-T cells that target antigens shared by AML blast cells and suppressive immune cells such as B7-H3 can lead to enhanced anti-leukemic activity (35, 36). Incorporating stimulatory domains such as CD28, 4-1BB, CD3, CD27, and CD3z can lead to enhanced efficacy and persistence (37, 38).
To our knowledge, this is the first meta-analysis on the safety and efficacy of CAR-T therapy for relapsed refractory AML. Study limitations include small sample size and the heterogeneous nature of the included studies. Several factors make it challenging to conduct this review including biologic heterogeneity of AML with prognostic significance, paucity of the available literature, conditioning regimens/dosing differences, and autologous versus allogeneic CAR-T constructs with a variety of targets. Additionally, due to a small number of patients and included studies, we were not able to investigate factors affecting the outcomes, such as CAR-T targets, age, prognostic markers like genetic mutations, lines of prior therapy, and prior allogeneic hematopoietic cell transplantation. Nevertheless, this seminal review will be useful to design and prioritize future clinical trials for CAR-T cell therapy in AML patients.
Conclusion
Chimeric antigen receptor T cell therapy has shown favorable responses in relapsed/refractory acute myeloid leukemia patients with a complete remission rate observed in over one-third of patients with acceptable toxicity profile. The conclusions reached by this meta-analysis should be taken with caution given the small sample size and heterogeneous nature of the included studies. Improved CAR-T constructs will hopefully overcome current challenges, including the heterogeneous biology of AML, lack of a unique targetable antigen expression on malignant cells, and immune exhaustion, and improve the outcomes in this therapeutically challenging patient population. Therefore, further prospective clinical trials are needed to evaluate the utility of CAR-T cell therapy in this therapeutically challenging patient population.
Author contributions
All authors contributed to the manuscript and fulfilled criteria per the uniform requirements set forth by the International Committee of Medical Journal Editors (ICJME) guidelines. All authors have reviewed and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
JM has speaking, consulting and advisory role in Kite, Juno Therapeutics, Allovir, Magenta Therapeutics, EcoR1 Capital, and has research funding from Novartis, Fresenius Biotech, Astellas Pharma, Bellicum Pharmaceuticals, Gamida Cell, Pluristem Therapeutics, Kite and AlloVir.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1152457/full#supplementary-material
References
1. Stats NC. Acute myeloid leukemia - cancer stat facts. (2022). Available at: https://seer.cancer.gov/statfacts/html/amyl.html.
2. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med (2015) 373(12):1136–52. doi: 10.1056/NEJMra1406184
3. Koenig K, Mims A, Levis MJ, Horowitz MM. The changing landscape of treatment in acute myeloid leukemia. Am Soc Clin Oncol Educ Book (2020) 40):343–54. doi: 10.1200/edbk_279129
4. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA (2009) 301(22):2349–61. doi: 10.1001/jama.2009.813
5. Koenig K, Mims A. Relapsed or primary refractory AML: moving past MEC and FLAG-ida. Curr Opin Hematol (2020) 27(2):108–14. doi: 10.1097/MOH.0000000000000561
6. DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood (2020) 136(9):1023–32. doi: 10.1182/blood.2019001982
7. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med Feb 1 (2018) 378(5):439–48. doi: 10.1056/NEJMoa1709866
8. Roschewski M, Longo DL, Wilson WH. CAR T-cell therapy for Large b-cell lymphoma — who, when, and how? New Engl J Med (2021) 386(7):692–6. doi: 10.1056/NEJMe2118899
9. Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New Engl J Med (2021) 384(8):705–16. doi: 10.1056/NEJMoa2024850
10. Madduri D, Berdeja JG, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. CARTITUDE-1: phase 1b/2 study of ciltacabtagene autoleucel, a b-cell maturation antigen-directed chimeric antigen receptor T cell therapy, in relapsed/refractory multiple myeloma. Blood (2020) 136:22–5. doi: 10.1182/blood-2020-136307
11. He X, Feng Z, Ma J, Ling S, Cao Y, Gurung B, et al. Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood Mar (2020) 5135(10):713–23. doi: 10.1182/blood.2019002779
12. Fiorenza S, Turtle CJ. CAR-T cell therapy for acute myeloid leukemia: preclinical rationale, current clinical progress, and barriers to success. BioDrugs (2021) 35(3):281–302. doi: 10.1007/s40259-021-00477-8
13. Lin G, Zhang Y, Yu L, Wu D. Cytotoxic effect of CLL−1 CAR−T cell immunotherapy with PD−1 silencing on relapsed/refractory acute myeloid leukemia. Mol Med Rep (2021) 23(3):1. doi: 10.3892/mmr.2021.11847
14. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res (2019) 7(1):100–12. doi: 10.1158/2326-6066.Cir-18-0307
15. Luo Y, Chang LJ, Hu Y, Dong L, Wei G, Huang H, et al. First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood (2015) 126(23):3778.
16. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res (2018) 8(6):1083–9.
17. Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther (2015) 23(1):184–91. doi: 10.1038/mt.2014.164
18. Zhang H, Wang P, Li Z, He Y, Gan W, Jiang H. Anti-CLL1 chimeric antigen receptor T-cell therapy in children with Relapsed/Refractory acute myeloid leukemia. Clin Cancer Res (2021) 27(13):3549–55. doi: 10.1158/1078-0432.Ccr-20-4543
19. Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou JZ, et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy (2017) 19(10):1225–32. doi: 10.1016/j.jcyt.2017.07.008
20. Sallman DA, Brayer J, Sagatys EM, Lonez C, Breman E, Agaugué S, et al. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica (2018) 103(9):e424–6. doi: 10.3324/haematol.2017.186742
21. Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler ME, Middeke JM, et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood (2021) 137(22):3145–8. doi: 10.1182/blood.2020009759
22. Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, et al. Donor-derived CD123-targeted CAR T cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front Oncol (2019) 9:1358. doi: 10.3389/fonc.2019.01358
23. Cui Q, Qian C, Xu N, Kang L, Dai H, Cui W, et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol (2021) 14(1):82. doi: 10.1186/s13045-021-01092-4
24. Qu C, Li Z, Kang L, Wang Y, Dai H, Yin J, et al. Successful treatment of two relapsed/refractory t () acute myeloid leukemia patients by CD19-directed chimeric antigen receptor T cells. Bone Marrow Transplant Jul (2019) 54(7):1138–40. doi: 10.1038/s41409-018-0423-y
25. Liu F, Zhang H, Sun L, Li Y, Zhang S, He G, et al. First-in-human CLL1-CD33 compound CAR (cCAR) T cell therapy in relapsed and refractory acute myeloid leukemia. In Proceedings of the 25th EHA Annual Congress, Frankfurt, Germany (2020) 12.
26. Marofi F, Rahman HS, Al-Obaidi ZMJ, Jalil AT, Abdelbasset WK, Suksatan W, et al. Novel CAR T therapy is a ray of hope in the treatment of seriously ill AML patients. Stem Cell Res Ther (2021) 12(1):465. doi: 10.1186/s13287-021-02420-8
27. Walter RB. The role of CD33 as therapeutic target in acute myeloid leukemia. Expert Opin Ther Targets (2014) 18(7):715–8. doi: 10.1517/14728222.2014.909413
28. Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematology (2013) 161(3):389–401. doi: 10.1111/bjh.12282
29. Wensveen FM, Jelenčić V, Polić B. NKG2D: a master regulator of immune cell responsiveness. mini review. Front Immunol (2018) 9: 441. doi: 10.3389/fimmu.2018.00441
30. Curio S, Jonsson G, Marinović S. Societies yEFoI. a summary of current NKG2D-based CAR clinical trials. Immunotherapy Advances (2021) 1(1). doi: 10.1093/immadv/ltab018
31. Brudno JN, Somerville RPT, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of b-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-Versus-Host disease. J Clin Oncol (2016) 34(10):1112–21. doi: 10.1200/jco.2015.64.5929
32. Liu W, Li Y, Qiu ZX, Yin Y, Sun YH, Xu WL, et al. [Clinical outcome of allogeneic hematopoietic stem cell transplantation with FLAG sequential busulfan/cyclophosphamide conditioning regimen for refractory/relapsed acute myeloid leukemia]. Zhonghua Nei Ke Za Zhi (2018) 57(8):576–81. doi: 10.3760/cma.j.issn.0578-1426.2018.08.008
33. Sandhu KS, Dadwal S, Yang D, Mei M, Palmer J, Salhotra A, et al. Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant (2020) 26(12):e322–7. doi: 10.1016/j.bbmt.2020.08.027
34. Cummins KD, Gill S. Will CAR T cell therapy have a role in AML? promises and pitfalls. Semin Hematol (2019) 56(2):155–63. doi: 10.1053/j.seminhematol.2018.08.008
35. Lichtman EI, Du H, Shou P, Song F, Suzuki K, Ahn S, et al. Preclinical evaluation of B7-H3–specific chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Clin Cancer Res (2021) 27(11):3141–53. doi: 10.1158/1078-0432.CCR-20-2540
36. Epperly R, Gottschalk S, Velasquez MP. A bump in the road: how the hostile AML microenvironment affects CAR T cell therapy. Front Oncol (2020) 10:262. doi: 10.3389/fonc.2020.00262
37. Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res (2006) 66(22):10995–1004. doi: 10.1158/0008-5472.CAN-06-0160
38. Song D-G, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood J Am Soc Hematology (2012) 119(3):696–706. doi: 10.1182/blood-2011-03-344275
Keywords: acute myeloid leukemia, relapsed or refractory AML, chimeric antigen receptor T cell therapy, outcomes, immunotherapy
Citation: Shahzad M, Nguyen A, Hussain A, Ammad-Ud-Din M, Faisal MS, Tariq E, Ali F, Butt A, Anwar I, Chaudhary SG, Lutfi F, Ahmed N, Singh AK, Hematti P, McGuirk JP and Mushtaq MU (2023) Outcomes with chimeric antigen receptor t-cell therapy in relapsed or refractory acute myeloid leukemia: a systematic review and meta-analysis. Front. Immunol. 14:1152457. doi: 10.3389/fimmu.2023.1152457
Received: 27 January 2023; Accepted: 11 April 2023;
Published: 24 April 2023.
Edited by:
Giovanni Marconi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Dina Schneider, Lentigen Technology, United StatesChiara F. Magnani, University of Zurich, Switzerland
Jie Sun, Zhejiang University, China
Copyright © 2023 Shahzad, Nguyen, Hussain, Ammad-Ud-Din, Faisal, Tariq, Ali, Butt, Anwar, Chaudhary, Lutfi, Ahmed, Singh, Hematti, McGuirk and Mushtaq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Umair Mushtaq, mushtaqmu@gmail.com
 Moazzam Shahzad
Moazzam Shahzad Andrea Nguyen1
Andrea Nguyen1 Muhammad Salman Faisal
Muhammad Salman Faisal Ezza Tariq
Ezza Tariq Atif Butt
Atif Butt Nausheen Ahmed
Nausheen Ahmed Anurag K. Singh
Anurag K. Singh Peiman Hematti
Peiman Hematti Joseph P. McGuirk
Joseph P. McGuirk Muhammad Umair Mushtaq
Muhammad Umair Mushtaq