Emerging Challenges of Radiation-Associated Cardiovascular Dysfunction (RACVD) in Modern Radiation Oncology: Clinical Practice, Bench Investigation, and Multidisciplinary Care
- 1Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Taiwan
- 2School of Medicine, Tzu Chi University, Hualien, Taiwan
- 3Department of Radiation Oncology, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan
- 4Cancer Centre, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Taiwan
- 5Department of Biomedical Sciences, National Chung Cheng University, Chia-Yi, Taiwan
- 6Department of Anatomic Pathology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Taiwan
Radiotherapy (RT) is a crucial treatment modality in managing cancer patients. However, irradiation dose sprinkling to tumor-adjacent normal tissues is unavoidable, generating treatment toxicities, such as radiation-associated cardiovascular dysfunction (RACVD), particularly for those patients with combined therapies or pre-existing adverse features/comorbidities. Radiation oncologists implement several efforts to decrease heart dose for reducing the risk of RACVD. Even applying the deep-inspiration breath-hold (DIBH) technique, the risk of RACVD is though reduced but still substantial. Besides, available clinical methods are limited for early detecting and managing RACVD. The present study reviewed emerging challenges of RACVD in modern radiation oncology, in terms of clinical practice, bench investigation, and multidisciplinary care. Several molecules are potential for serving as biomarkers and therapeutic targets. Of these, miRNAs, endogenous small non-coding RNAs that function in regulating gene expression, are of particular interest because low-dose irradiation, i.e., 200 mGy (one-tenth of conventional RT daily dose) induces early changes of pro-RACVD miRNA expression. Moreover, several miRNAs, e.g., miR-15b and miR21, involve in the development of RACVD, further demonstrating the potential bio-application in RACVD. Remarkably, many RACVDs are late RT sequelae, characterizing highly irreversible and progressively worse. Thus, multidisciplinary care from oncologists and cardiologists is crucial. Combined managements with commodities control (such as hypertension, hypercholesterolemia, and diabetes), smoking cessation, and close monitoring are recommended. Some agents show abilities for preventing and managing RACVD, such as statins and angiotensin-converting enzyme inhibitors (ACEIs); however, their real roles should be confirmed by further prospective trials.
Introduction
Radiotherapy (RT) is an essential treatment modality in managing cancer patients (1, 2). Biologically, RT delivers ionizing radiation (IR) to eradicate cancer cells mainly through reacting with H2O to generate reactive oxygen species (ROS) to target multiple intra-cellular organelles, such as nucleus (mainly DNA), mitochondria, and cell membrane (3–5). Many IR-associated normal tissue damages are acute toxicities, characterizing potentially reversible and self-limited; however, some types of damages develop late sequelae, which are highly irreversible and progressively worse (4–6). For example, though the incidence is rare, irradiated cancer patients who had IR dose sprinkling to the cardiovascular system may encounter radiation-associated cardiovascular dysfunctions (RACVDs) (7–9), including blood pressure reduction (10), carotid stenosis (11), pericardial disease (12), myocardial infarction (13), pericardial/myocardial fibrosis (14, 15), valvular heart disease (16), arrhythmia (17), and subsequent heart failure (18–20). On clinical presentation, many RACVDs are late RT sequelae, developing a few years later after RT (21). Notably, as time elapsed, the risk of RACVD is larger in the third decade than that of the first two decades after IR exposure (22).
RACVD is a well-known treatment-related toxicity in the field of cardio-oncology (23–25). Other anti-cancer therapies, such as chemotherapy (26–29), targeted therapy (30–33), and immunotherapy (34–36), may also induce cardiovascular dysfunctions (37–39). As a result, when these therapies are prescribed concurrently or sequentially with RT, the risk of RACVD is increased substantially, especially in vulnerable pediatric (40, 41) or elderly cancer patients (42, 43). Besides, other RT-associated adverse events may occur with RACVD, such as ischemic stroke (44, 45) and lung fibrosis (46, 47), which may further impair patients' survival and life quality.
Several cardiovascular pathophysiological dysfunctions are associated with RT, such as late fibrosis/stenosis in the irradiated cardiovascular structures, mainly the endothelium (including endothelial cells and its stroma) and smooth muscle cells (2, 4, 5, 48). Epigenetic dysregulation, e.g., DNA methylation regulating gene expression without changes of sequence, demonstrates profound effects on the development of RACVD. For instance, differentially methylated enhancer of diacylglycerol kinase alpha (DGKA) reduces pro-fibrotic fibroblast activation, involving in radiation-associated tissue fibrosis and vascular stenosis (49). Similarly, microRNAs (miRNAs) also have been found to regulate the innate endothelium response to IR (50).
Clinically, moderate- to high-dose IR to the cardiovascular system increases the risk of RACVD (2, 4, 5). More notably, low-dose IR with a single 200 mGy (i.e., one-tenth of conventional RT daily dose of 200 cGy) has been observed to induce early damage of RACVD, demonstrating expression changes of miRNAs, e.g., miR-21 and miR-146b, and their regulated proteins in primary human coronary artery endothelial cells (51). This finding suggests that miRNAs as potential biomarkers for early detecting RACVD. Furthermore, some miRNAs have been reported as potential targets in managing RACVD, e.g., miR-15b (52), miR-21 (51–54), and miR-126-5p (55).
Hence, the present study aimed to review clinical challenges, potential biomarkers, and therapeutic targets of RACVD, with a focus on the role of miRNA. Emerging challenges of multidisciplinary care and example agents for prevention are also reviewed.
Clinical Challenges and Emerging Issues for Detecting, Managing, and Preventing RACVD
Clinical Challenges in Improving Detection, Management, and Prevention of RACVD
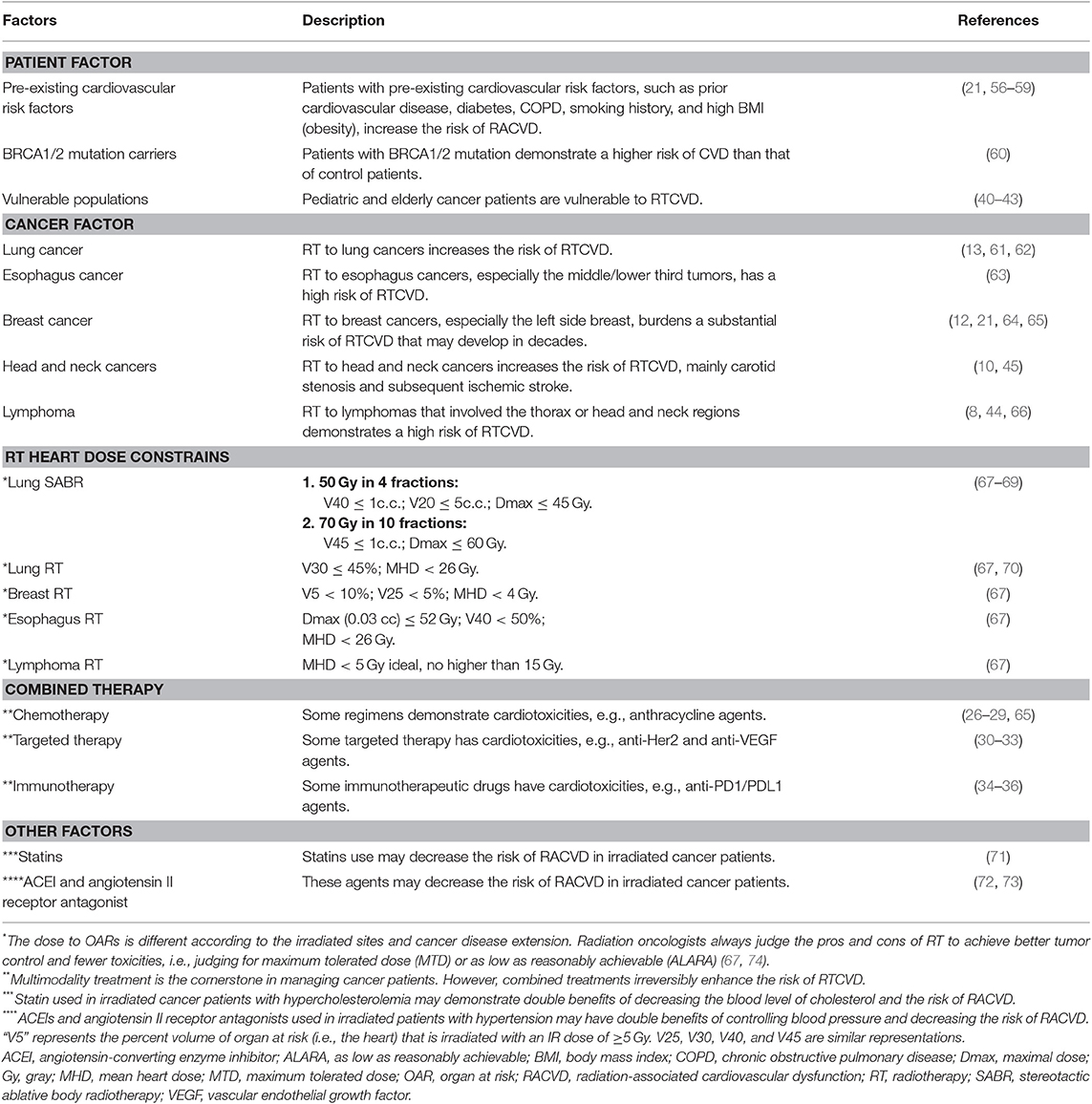
Several factors affect the risk of RACVD (Table 1). As a result, current treatment guidelines recommend several methods to reduce the risk of RACVD (1, 2, 56). For example, in patients at high risk, radiation oncologists always consider alternative treatment choice of deferred RT, adopt rigorous dose constraints on the cardiovascular system, or implement advanced irradiation techniques. However, even implementing advanced techniques, the occurrence of RACVD cannot be avoided totally. Several issues are still challenging in clinical practice.
Clinical Challenges of Decreasing the Risk of RACVD in Modern Radiation Oncology
Clinically, the overall incidence of RACVDs is rare but substantially encountered in irradiated patients with mediastinum lymphoma (8, 44, 66), head and neck (10, 45), esophagus (63), lung (13, 61, 62), and breast (12, 21, 56, 64) cancers. High-risk features of RACVD development are as follows: left-side breast irradiation (21, 65), combination with anthracycline-based chemotherapy (65), and vulnerable patient populations [e.g., pre-existing cardiac risk factors/heart disease (21, 57) and BRCA1/2 mutation carriers (60)]. For example, for a typical 50-year-old woman with pre-existing cardiac risk factors, an estimated 20-year risk of death from ischemic heart disease after breast RT is up to 1.6%, which is higher than that of those patients with no RT (i.e., 0.9%) (21, 56, 75). Moreover, in irradiated left breast cancer patients, each additional Gray (Gy) of the mean heart dose (MHD) increases the relative risk of major cardiac events by 7.4% (21).
Radiation oncologists implement several methods to decrease IR dose to the heart for minimizing the risk of RACVD (76), such as prone positioning (77), heart block with electronic compensation (57), heart-sparing three-dimensional printing technique (78), continuous positive airway pressure (CPAP) (79), real-time position management (RPM) inspiration gating (80, 81), proton-beam irradiation (82–85), and deep-inspiration breath-hold technique (DIBH) (86–90). However, even with the highly recommended visual-guided DIBH technique, residual variations of the heart position are still noticeable (91). As a result, the actual heart dose may be underestimated, burdening a higher risk of cardiac toxicities than that of estimation from the RT treatment planning.
For reducing the risk of cardiac toxicities, modern irradiation techniques aim to decrease irradiation dose to the heart. Diminishing the mean heart dose (MHD) is the main goal based on data estimated from conventional tangential technique (21, 92–94). Nevertheless, attenuating IR doses to coronary artery (95–97) and other cardiac substructures, such as left anterior descending artery (LAD) and left ventricle (LV), are more reasonable and suitable in modern precise RT departments (2, 66, 95, 98). However, long-term results investigated dose effects on these cardiac substructures are pending.
Another emerging challenge in clinical radiation oncology is the concept-shifting on treatment consideration. Previously, radiation oncologists always apply IR dose to organs at risk (OARs) according to the principle of “as low as reasonably achievable (ALARA) (99).” However, in some patient populations that required very aggressive managements, the treatment concept frequently shifts to maximum tolerated dose (MTD) for gaining the ultimate tumor control (67, 74). Undoubtedly, adopting MTD increases the heart dose and then burdens a higher risk of RTCVD than that of ALARA.
Challenges of Clinical Detection for RACVD
Early detection of RACVD is challenging. Some clinical predictors have been reported for stratifying patients at risk, such as dosimetric parameters of RT (61), cardiac risk index (100), and coronary calcium score (101). Moreover, biomarkers are clinically helpful for detecting RACVD (102), such as cardiac troponins (e.g., troponin I or T) and natriuretic peptides (e.g., B-type natriuretic peptide (BNP) or pro-BNP) (103). On imaging, echocardiography is the pivotal method to detect cardiac anatomic and functional changes of RACVD (104–106). Profound RACVD may show a reduction of LV ejection fraction, and subclinical disease may reveal early signs of decreased global longitudinal strain (107–109). Recently, other advanced imaging modalities are attractive for detecting RACVD (110), such as cardiac computed tomography (111–113) and cardiovascular magnetic resonance (CMR) (114–116).
In recent precision cardio-oncology, it is a promise direction that applies combined omic-data and metabolic-function nuclear images (117), such as single-photon emission computed tomography (SPECT) (118) and positron emission tomography (PET) (119–122). Of these, PET that demonstrated metabolic changes of the heart is the most expecting image marker for detecting RACVD. However, identifying suitable isotopes of PET for early detecting RACVD is still challenging.
Challenge of Clinical Managements for RACVD
Unfortunately, there is still no effective method to restore RT-associated late sequelae, including RACVD, because their disease courses are generally irreversible (2, 4, 6, 56). However, several pre-clinic studies have suggested potential targets for therapeutic interventions, such as HMGB1 (123) and miR-212 (124). Moreover, selective irradiation to the heart induces early overexpression of pro-hypertrophic miR-212, leading the miR-212 intervention as a reasonable approach for RACVD (124).
Clinical Prevention for RACVD and Future Challenges
Some clinical agents may be used to prevent the occurrence of RACVD. For instance, statins, HMG-CoA reductase inhibitors prescribed for managing hypercholesterolemia, significantly reduces the risk of stroke [hazard ratio (HR) = 0.68; 95% confidence interval (CI), 0.48–0.98; P = 0.0368] and demonstrates a trend to decrease the risk of RACVD (HR = 0.85; 95% CI, 0.69–1.04; P = 0.0811) in irradiated cancer patients (71).
The detailed mechanism of statin in protecting the cardiovascular system is unclear. Some potential mechanisms are proposed. Firstly, statin inhibits RhoA GTPase (125), which is essential to mediate the irradiation inhibition of endothelial cell migration (126–128). Secondly, statin decreases cardiac endothelial cell permeability via activating ERK5 (129). Thirdly, statin enhances the release of Nitric Oxide (NO), which is crucial for improving endothelial function via regulating miR-221/222 (130). Fourthly, statin diminishes IR-induced responses of cardiac Connexin-43 and miR-21 (53) that involves in the process of cardiac fibrosis (52).
Clinical strategies, such as close monitoring, smoking cessation (58, 131), prescribing angiotensin-converting enzyme inhibitors (ACEIs), and β-blockers, are useful to prevent anthracycline-associated cardiac toxicities (132, 133). In the literature, ACEIs also showed a potential for preventing RACVD. For example, Captopril, one of ACEIs prescribed for hypertension or heart failure, has been found to decrease pulmonary endothelial dysfunction in irradiated rats (72). Similarly, Candesartan, an Angiotensin II Receptor Antagonist, has been reported to reduce the risk of RACVD in left breast irradiated patients (73). Thus, a potential mechanism of ACEI for cardioprotection may be demarcated reasonably by inhibiting angiotensin II to decrease the expression of TGF-β, a well-known pro-fibrogenic factor of post-IR late fibrosis (134, 135). However, these methods required further data support to demarcate their real roles in preventing the development of RACVD.
Future Challenge: Mixed-Agent-Associated Cardiotoxicity in Combined Treatments
The major clinical problem is that many cancer patients were managed with multimodality treatments. As a result, the incidence of multi-treatment-associated CVDs, such as combined anthracycline-based chemotherapy and RT (136), is much higher than that of isolated RACVD. This phenomenon increases the difficulty of prevention and management, mostly requiring combined care from multidisciplinary team members, including radiation oncologists, medical oncologists, and cardiologists.
Emerging Challenge of Bench Studies to Improve Early Detection, Management, and Prevention of RACVD, Focusing on the Role of miRNA in Acting as a Biomarker and Therapeutic Target
As mentioned above, in addition to currently clinical use biomarkers, such as cardiac troponins (e.g., troponin I) and natriuretic peptides (e.g., BNP) (103), several pre-clinical studies have been investigated to explore underlying mechanisms of RACVD, such as TGF-β and PPAR-α signaling pathways (137, 138), damage-associated molecular patterns (DAMPs) (139), and miRNA modulations (138). Of these, endogenous small non-coding miRNAs that function in regulating gene expression (140) grasp more interest in terms of biomarkers (141–143) and therapeutic targets (144–146) (Table 2).
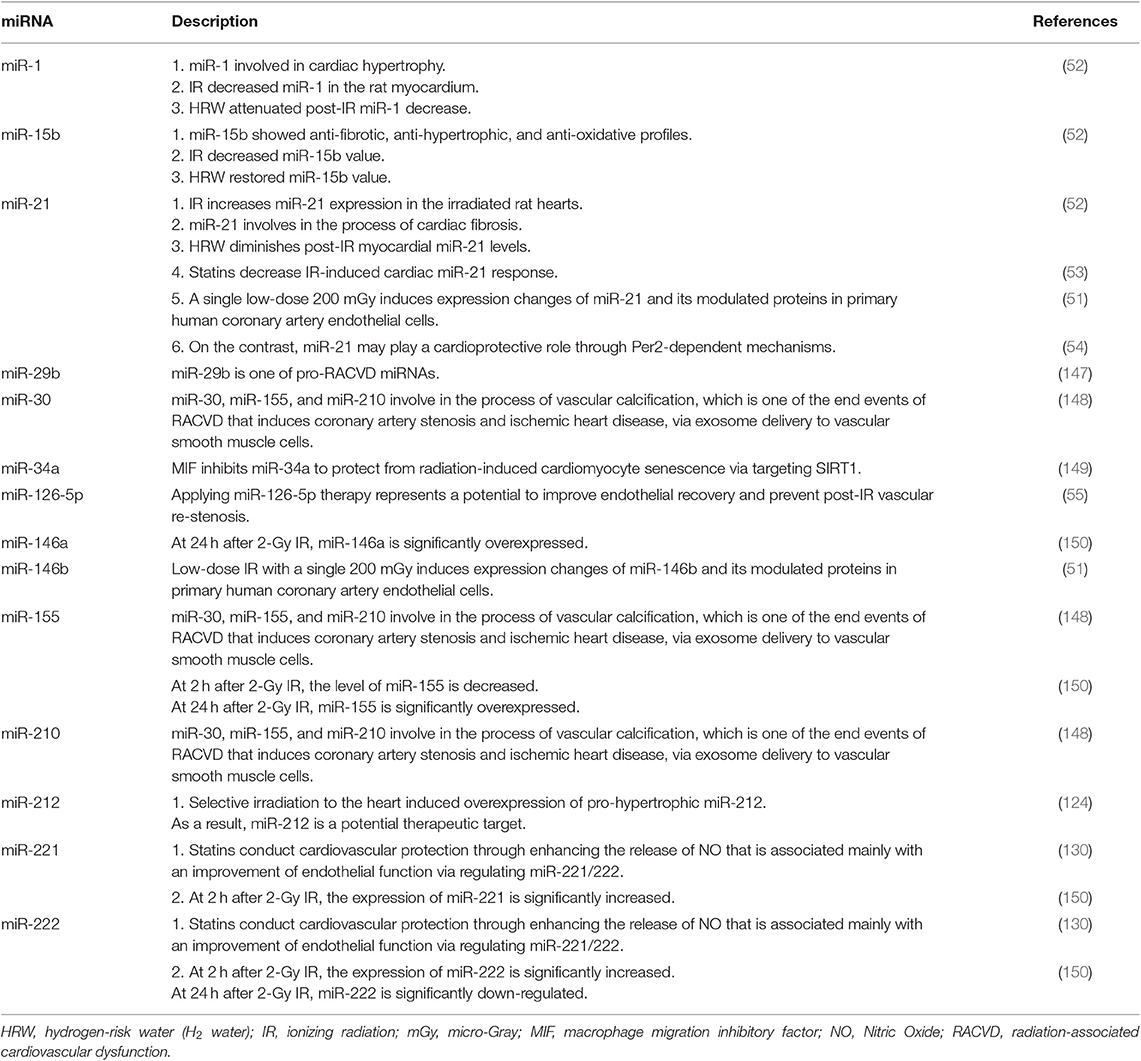
Table 2. Examples of miRNAs involved in the process of RACVD that are potential for severing as biomarkers or therapeutic targets.
Emerging Challenges for Investigating Biological Mechanisms of RACVD
Detail mechanisms of RACVD are not well-recognized. Some potential mechanisms and pathways have been proposed. For example, IR may impair corin function and inhibit natriuretic peptides to accelerate senescence of cardiac and endothelial cells, contributing to the development of RACVD (151). Besides, several pathways have been identified with involvement into the process of RACVD, such as the 5-lipoxygenase (5-LO)/leukotriene pathway (152), the miRNA-34a/sirtuin-1 signaling pathway (149), the Reactive Oxygen Species (ROS)-mediated p16 pathway (153), and the TGF-β-associated signaling (154).
Moreover, some molecules may play roles in the process of RACVD, such as peroxisome proliferator-activated receptor gamma (PPAR-γ) (155), Growth differentiation factor 15 (GDF15) (153), and RhoA GTPase (125) that is essential to mediate the irradiation inhibition of endothelial cell migration. More recently, by using RNA-seq, differential gene-expression profiles have been identified in mice models, such as Nrf2, PDK1, and sirtuins (156). However, despite these lines of evidence, the whole picture of RACVD development is still not well-demarcated.
Another emerging challenge of investigating bio-mechanisms of RACVD comes from the difference of biological effects among different irradiation sources, e.g., proton vs. photon beams. Although proton and photon beams activate similar canonical radiation response pathways, distinct vascular genomic responses have been observed in the murine aorta (157). That is, models established according to photon radiation may not accurately predict the risk of RACVD associated with proton radiation.
Emerging Challenge of Bench Studies for Early Detecting and Managing RACVD, Focusing on the Example Role of miRNA
In the literature, many clinical studies assessed circulating miRNA levels in peripheral blood for diagnosing, predicting, and monitoring human diseases (158–163), including cardiac and vascular disease (CVD) (164–169). For example, the combination of miR-34a-5p and fibrinogen levels have been reported as a useful tool in differentiating pre-thrombotic status in patients with stable coronary artery disease (165). Moreover, the plasma expression level of miR-423-5p has been reported to serve as a promising biomarker for stratifying patients with coronary artery disease (168).
Similarly, several miRNAs have been found to involve in the process of RACVD (147, 170, 171). For example, via exosomes-based delivery to vascular smooth muscle cells, miR-30, miR-210, and miR-155 play roles in developing vascular calcification, which is one of the end events of RACVD that induces coronary artery stenosis and ischemic heart disease (148). Remarkably, IR-induced miRNAs expression behaves in a dose- and time-depended manner (150, 172). For instance, at 2 h after 2-Gy IR, the expression of miRNA-221 and miRNA-222 are significantly increased, but the level of miRNA-155 is decreased. On the other hand, at 24 h after 2-Gy IR, miRNA-146a and miRNA-155 are significantly overexpressed, but miRNA-222 is down-regulated (150). These patterns of miRNA expression changes require attention in further prospective studies that intend to demarcate the role of miRNAs in association with RACVD.
Although it requires further efforts to bridge miRNAs from bench to bedside, some miRNAs are attractive in early detecting and managing RACVD (124). For instance, applying miR-126-5p therapy potentially improves endothelial recovery and prevents post-irradiation vascular re-stenosis (55). Besides, inhibiting miR34a by macrophage migration inhibitory factor (MIF) has been reported to reduce radiation-induced cardiomyocyte senescence via targeting SIRT1, implicating a novel strategy for managing RACVD (149). Moreover, molecular hydrogen, i.e., hydrogen-rich water (HRW; H2 water), shows protective effects on IR-induced heart damage via regulating miRNA-1, -15b, and -21 (52).
In conjunction with miRNAs, circular RNAs (circRNAs) have been identified to involve in the regulatory network of the cardiovascular system. In biological function, circular RNAs may interact with RNA-binding proteins and act as miRNA sponges that inhibit the function of correspondingly matched miRNAs (173), demonstrating an ability for serving as novel biomarkers to early detect cardiovascular disease (174).
Applying circulating miRNA levels of peripheral blood is an immediately translatable mean for screening/monitoring RACVD. When researchers selected their miRNA targets by a literature review (such as targets that listed in the present study), miRNA database search, or miRNA-specific sequencing, they can subsequently conduct prospective clinical studies to validate their targets of interest under the pre-defined purpose of detecting, screening, or monitoring RACVD by using blood samples. However, testing details of circulating miRNAs (such as measuring methods, timing, and cut-off point values) are still required to be validated by prospectively clinical trials.
In the ClinicalTrials.gov (175), two actively recruiting trials integrate circulating miRNA as predicting biomarkers to detect RACVD in irradiated breast cancer patients, entitled: (1), Pre- or Postoperative Accelerated Radiotherapy (POP-ART; Identifier: NCT03783364) and (2), Breast Cancer and Cardiotoxicity Induced by Radiotherapy: the BACCARAT Study (Identifier: NCT02605512). Of these, the BACCARAT study investigates the role of several types of circulating biomarkers in detecting RACVD, including B-type natriuretic peptide, TGF-β1, and several miRNAs (e.g., miR-1, miR-34, miR-126, and miR-155). The results of the two trials are highly anticipated.
One potential limitation of applying miRNA in clinical practice is that the expression level of specific miRNAs would be varied in different tissues and testing time points. Therefore, the studies proceeding on the ClinicalTrials.gov may be very informative. Before the information of these clinical trials is available, in the authors' consensus opinion, integrating miRNAs as a component of circulating biomarkers for detecting RACVD may be critically considered in future clinical trials and practice that apply RT. Several measuring time points that similar to the protocol of the BACCARAT study are suggested as follows: before RT, the middle term of the RT course, and five time points after RT (i.e., 1 day, 6 months, 2, 5, and 10 years).
Why the time points of 2, 5, and 10 years should be considered testing and measuring? The main reason is that RACVD is a well-known RT toxicity; it characterizes not only acute cardiovascular damage but also late sequelae of cardiovascular dysfunction that may be encountered a few years or decades after RT (21, 56, 75). Thus, long-term series measuring (i.e., 2-, 5-, and 10-years after RT) of target miRNA levels is useful for early detecting and monitoring the occurrence and severity altering of RACVD.
Emerging Challenge: Novel Agents and Managements for Treating RACVD
As mentioned above, TGF-β-associated signaling gains a substantial interest in investigating the process of RACVD. For example, reducing irradiation-induced TGF-β1 production through blocking the NF-kB signaling pathway has been reported to provide a new insight in inhibiting irradiation-induced myocardial fibrosis (154). Besides, Protein Kinase C (PKC) has been reported to play a role in the process of RACVD (48). Remarkably, inhibiting PKC, such as applying RNA-interference techniques (176), could be a reasonable approach for managing IR-induced vascular dysfunction (48).
Some radioprotection agents, such as L-arginine, show protection effects on blood vessels of urinary bladder wall in patients treated with pelvic RT (177). Furthermore, IR-damaged vascular dysfunction has been observed to be restored by quercetin-filled phosphatidylcholine liposomes and mesenchymal stem cell injection (48). However, the real clinical roles of these agents and interventions on the cardiovascular system require further evidence to define.
Emerging Challenge: Further Multidisciplinary Cooperation Among Radiation Oncologists, Cardiologists, and Molecular Biologists
Multidisciplinary care is required for preventing, detecting, and managing RACVD in irradiated cancer patients (178). In conjunction with the improvement of detection methods, increasing awareness and integrating works between oncologists and cardiologists are essential (179). Managing comorbidities adequately [e.g., hypertension, hypercholesterolemia, and diabetes control (180)], exercise therapy (181), and smoking cessation (58, 131) are all useful to decrease the risk of anti-cancer-treatment-related CVD (182), including RACVD. For multidisciplinary management, standard recommendation and structure/infra-structure requirements for patient care are ongoing established (183–188). For example, establishing consensus guidance to train RT staffs to delineate cardiac substructures decreases inter-observer variation and increases the accuracy of dose estimation, helping in implementing further randomized clinical trials and then daily clinical practice (189, 190).
Remarkably, several radiation-associated toxicities, including RACVDs, are diagnosed by a ruling-out—not ruling-in—way (2, 6). That is, diagnosing RACVD requires excluding other heart diseases, such as infectious disease or prior-existing subclinical cardiovascular dysfunctions. This work requires tight cooperation and interaction among multidisciplinary team members, such as radiation oncologists, medical oncologists, and cardiologists. Further consensus and recommendations are encouraged to establish in a multidisciplinary manner.
Conclusion
Overall, the incidence of RACVD is rare in irradiated cancer patients. When it happened, however, RACVD may significantly impair patients' survival and life quality, particularly in vulnerable patient populations. Radiation oncologists implement many clinical efforts to reduce the risk; the incidence of RACVD is decreased but still substantial.
Further efforts from bench studies are emergently required to improve early detection, management, and prevention. For example, miRNAs play active roles in serving as biomarkers and therapeutic targets. Remarkably, integrating cooperation among multidisciplinary team members, such as oncologists and cardiologists, is encouraged and ongoing.
In the ClinicalTrials.gov (175), more than 20 clinical trials are actively or not yet recruiting for investigating challenging issues of RACVD, mainly focusing on early detection (e.g., circulating and imaging biomarkers) and aggressively avoidance/prevention (e.g., DIBH and proton therapy). Results from these ongoing trials are hopeful for resolving clinical obstacles of RACVD in the future.
Author Contributions
All authors contributed to the brainstorming of the ideas generation. M-SL and D-WL also contributed to first draft writing. S-KH, C-CY, C-LC, W-YC, L-CC, R-IL, L-WH, C-HC, and F-CH also contributed to literature review and interpretation. H-YL and MC also co-corresponded to overall manuscript communication and final approval.
Funding
The present study was supported by the Ministry of Science and Technology, Taiwan (Grant No. 106-2923-B-194-001-MY3), and the Buddhist Tzu Chi Medical Foundation (Grant Nos. TCMMP105-09-02, TCMMP106-02-02, and DTCRD1062-E-18).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. NCCN.org. Clinical Practice Guidelines in Oncology. Available online at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed November 09, 2019).
2. Halperin EC, Wazer DE, Perez CA, Brady LW. Perez & Brady's Principles and Practice of Radiation Oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins (LWW) (2018).
3. Islam MT. Radiation interactions with biological systems. Int J Radiat Biol. (2017) 93:487–93. doi: 10.1080/09553002.2017.1286050
6. Koontz BF. Radiation Therapy Treatment Effects: An Evidence-Based Guide to Managing Toxicity. New York, NY: Springer Publishing Company (2017).
7. Armanious MA, Mohammadi H, Khodor S, Oliver DE, Johnstone PA, Fradley MG. Cardiovascular effects of radiation therapy. Curr Probl Cancer. (2018) 42:433–42. doi: 10.1016/j.currproblcancer.2018.05.008
8. Nolan MT, Russell DJ, Marwick TH. Long-term risk of heart failure and myocardial dysfunction after thoracic radiotherapy: a systematic review. Can J Cardiol. (2016) 32:908–20. doi: 10.1016/j.cjca.2015.12.020
9. Marmagkiolis K, Finch W, Tsitlakidou D, Josephs T, Iliescu C, Best JF, et al. Radiation toxicity to the cardiovascular system. Curr Oncol Rep. (2016) 18:15. doi: 10.1007/s11912-016-0502-4
10. Leibowitz A, Grossman E, Berkovitch A, Levartovski M, Appel S, Sharabi Y, Gluck I. The effect of head and neck radiotherapy on blood pressure and orthostatic hypotension in patients with head and neck tumors. Am J Hypertens. (2018) 31:235–39. doi: 10.1093/ajh/hpx158
11. Mahmood SS, Nohria A. Cardiovascular complications of cranial and neck radiation. Curr Treat Options Cardiovasc Med. (2016) 18:45. doi: 10.1007/s11936-016-0468-4
12. Marinko T. Pericardial disease after breast cancer radiotherapy. Radiol Oncol. (2018) 53:1–5. doi: 10.2478/raon-2018-0035
13. Lee CC, Zheng H, Soon YY, Foo LL, Koh WY, Leong CN, et al. Association between radiation heart dosimetric parameters, myocardial infarct and overall survival in stage 3 non-small cell lung cancer treated with definitive thoracic radiotherapy. Lung Cancer. (2018) 120:54–9. doi: 10.1016/j.lungcan.2018.03.024
14. Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. (2010) 76:656–65. doi: 10.1016/j.ijrobp.2009.09.064
15. Wu SP, Tam M, Vega RM, Perez CA, Gerber NK. Effect of breast irradiation on cardiac disease in women enrolled in BCIRG-001 at 10-year follow-up. Int J Radiat Oncol Biol Phys. (2017) 99:541–8. doi: 10.1016/j.ijrobp.2017.06.018
16. Stewart MH, Jahangir E, Polin NM. Valvular heart disease in cancer patients: etiology, diagnosis, and management. Curr Treat Options Cardiovasc Med. (2017) 19:53. doi: 10.1007/s11936-017-0550-6
17. Viganego F, Singh R, Fradley MG. Arrhythmias and other electrophysiology issues in cancer patients receiving chemotherapy or radiation. Curr Cardiol Rep. (2016) 18:52. doi: 10.1007/s11886-016-0730-0
18. Marangou J, Redfern A, Haddad T, Rankin JM, Dwivedi G. Heart failure following oncological treatment. Curr Opin Cardiol. (2018) 33:237–44. doi: 10.1097/HCO.0000000000000488
19. Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. (2017) 135:1388–96. doi: 10.1161/CIRCULATIONAHA.116.025434
20. Nolan MT, Russell DJ, Negishi K, Marwick TH. Meta-analysis of association between mediastinal radiotherapy and long-term heart failure. Am J Cardiol. (2016) 118:1685–91. doi: 10.1016/j.amjcard.2016.08.050
21. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. (2013) 368:987–98. doi: 10.1056/NEJMoa1209825
22. Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. (2013) 108:179–82. doi: 10.1038/bjc.2012.575
23. Lee Chuy K, Nahhas O, Dominic P, Lopez C, Tonorezos E, Sidlow R, et al. Cardiovascular complications associated with mediastinal radiation. Curr Treat Options Cardiovasc Med. (2019) 21:31. doi: 10.1007/s11936-019-0737-0
24. Steingart R.M, Yadav N, Manrique C, Carver J.R, Liu J. Cancer survivorship: cardiotoxic therapy in the adult cancer patient; cardiac outcomes with recommendations for patient management. Semin Oncol. (2013) 40:690–708. doi: 10.1053/j.seminoncol.2013.09.010
25. Spetz J, Moslehi J, Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr Treat Opt Cardiovasc Med. (2018) 20:31. doi: 10.1007/s11936-018-0627-x
26. Saunders S, Anwar M. Capecitabine-induced myopericarditis - A case report and review of literature. J Oncol Pharm Pract. (2019) 25:1006–10. doi: 10.1177/1078155218774871
27. Nicol M, Baudet M, Cohen-Solal A. Subclinical left ventricular dysfunction during chemotherapy. Card Fail Rev. (2019) 5:31–6. doi: 10.15420/cfr.2018.25.1
28. Kanduri J, More LA, Godishala A, Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. (2019) 37:399–405. doi: 10.1016/j.ccl.2019.07.004
29. Zhang KW, Finkelman BS, Gulati G, Narayan HK, Upshaw J, Narayan V, et al. Abnormalities in 3-dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. JACC Cardiovasc Imaging. (2018) 11:1059–68. doi: 10.1016/j.jcmg.2018.01.015
30. Tsugu T, Nagatomo Y, Nakajima Y, Kageyama T, Akise Y, Endo J, et al. Cancer therapeutics-related cardiac dysfunction in a patient treated with abiraterone for castration-resistant prostate cancer. J Med Ultrason. (2019) 46:239–43. doi: 10.1007/s10396-018-0897-7
31. Shah CP, Moreb JS. Cardiotoxicity due to targeted anticancer agents: a growing challenge. Ther Adv Cardiovasc Dis. (2019) 13:1753944719843435. doi: 10.1177/1753944719843435
32. Hussain Y, Drill E, Dang CT, Liu JE, Steingart RM, Yu AF. Cardiac outcomes of trastuzumab therapy in patients with HER2-positive breast cancer and reduced left ventricular ejection fraction. Breast Cancer Res Treat. (2019) 175:239–46. doi: 10.1007/s10549-019-05139-6
33. Guha A, Armanious M, Fradley MG. Update on cardio-oncology: novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med. (2019) 29:29–39. doi: 10.1016/j.tcm.2018.06.001
34. Ball S, Ghosh RK, Wongsaengsak S, Bandyopadhyay D, Ghosh GC, Aronow WS, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:1714–27. doi: 10.1016/j.jacc.2019.07.079
35. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. (2018) 19:1579–89. doi: 10.1016/S1470-2045(18)30608-9
36. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. (2018) 19:e447–58. doi: 10.1016/S1470-2045(18)30457-1
37. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. (2019) 280:163–75. doi: 10.1016/j.ijcard.2019.01.038
38. Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol. (2019) 13:1179546819866445. doi: 10.1177/1179546819866445
39. Zheng PP, Li J, Kros JM. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: critical research-practice gaps, challenges, and insights. Med Res Rev. (2018) 38:325–76. doi: 10.1002/med.21463
40. Ryan TD, Nagarajan R, Godown J. Pediatric cardio-oncology: development of cancer treatment-related cardiotoxicity and the therapeutic approach to affected patients. Curr Treat Options Oncol. (2019) 20:56. doi: 10.1007/s11864-019-0658-x
41. Mulcahy Levy JM, Tello T, Giller R, Wilkening G, Quinones R, Keating AK, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer. (2013) 60:700–4. doi: 10.1002/pbc.24252
42. Findlay SG, Gill JH, Plummer R, DeSantis C, Plummer C. Chronic cardiovascular toxicity in the older oncology patient population. J Geriatr Oncol. (2019) 10:685–9. doi: 10.1016/j.jgo.2019.01.018
43. Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. (2014) 32:2654–61. doi: 10.1200/JCO.2013.55.0459
44. Huang R, Zhou Y, Hu S, Ren G, Cui F, Zhou PK. Radiotherapy exposure in cancer patients and subsequent risk of stroke: a systematic review and meta-analysis. Front Neurol. (2019) 10:233. doi: 10.3389/fneur.2019.00233
45. Chu CN, Chen PC, Bai LY, Muo CH, Sung FC, Chen SW. Young nasopharyngeal cancer patients with radiotherapy and chemotherapy are most prone to ischaemic risk of stroke: a national database, controlled cohort study. Clin Otolaryngol. (2013) 38:39–47. doi: 10.1111/coa.12064
46. Omer H, Sulieman A, Alzimami K. Risks of lung fibrosis and pneumonitis after postmastectomy electron radiotherapy. Radiat Prot Dosimetry. (2015) 165:499–502. doi: 10.1093/rpd/ncv111
47. Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst. (1996) 88:918–22. doi: 10.1093/jnci/88.13.918
48. Soloviev AI, Kizub IV. Mechanisms of vascular dysfunction evoked by ionizing radiation and possible targets for its pharmacological correction. Biochem Pharmacol. (2019) 159:121–39. doi: 10.1016/j.bcp.2018.11.019
49. Weigel C, Veldwijk MR, Oakes CC, Seibold P, Slynko A, Liesenfeld DB, et al. Epigenetic regulation of diacylglycerol kinase alpha promotes radiation-induced fibrosis. Nat Commun. (2016) 7:10893. doi: 10.1038/ncomms10893
50. Wagner-Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol. (2010) 5:25. doi: 10.1186/1748-717X-5-25
51. Barjaktarovic Z, Anastasov N, Azimzadeh O, Sriharshan A, Sarioglu H, Ueffing M, et al. Integrative proteomic and microRNA analysis of primary human coronary artery endothelial cells exposed to low-dose gamma radiation. Radiat Environ Biophys. (2013) 52:87–98. doi: 10.1007/s00411-012-0439-4
52. Kura B, Kalocayova B, LeBaron TW, Frimmel K, Buday J, Surovy J, et al. Regulation of microRNAs by molecular hydrogen contributes to the prevention of radiation-induced damage in the rat myocardium. Mol Cell Biochem. (2019) 457:61–72. doi: 10.1007/s11010-019-03512-z
53. Viczenczova C, Kura B, Egan Benova T, Yin C, Kukreja RC, Slezak J, et al. Irradiation-induced cardiac Connexin-43 and miR-21 responses are hampered by treatment with atorvastatin and aspirin. Int J Mol Sci. (2018) 19:E1128. doi: 10.3390/ijms19041128
54. Bartman CM, Oyama Y, Brodsky K, Khailova L, Walker L, Koeppen M, et al. Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PLoS ONE. (2017) 12:e0176243. doi: 10.1371/journal.pone.0176243
55. Zhou Z, Schober A, Nazari-Jahantigh M. Dicer promotes endothelial recovery and limits lesion formation after vascular injury through miR-126–5p. Int J Cardiol. (2018) 273:199–202. doi: 10.1016/j.ijcard.2018.09.006
56. Wright JL. Toxicities of Radiation Treatment for Breast Cancer: Risks and Management Strategies. Cham: Springer Nature Switzerland (2019). doi: 10.1007/978-3-030-11620-0
57. Kang HJ, Kim SW, Son SH. The feasibility of a heart block with an electron compensation as an alternative whole breast radiotherapy technique in patients with underlying cardiac or pulmonary disease. PLoS ONE. (2017) 12:e0184137. doi: 10.1371/journal.pone.0184137
58. The American Cancer Society. The American Cancer Society's Principles of Oncology: Prevention to Survivorship. Oxford, UK; Hoboken, NJ; Chichester: Wiley (2018).
59. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. (1999) 282:1523–9. doi: 10.1001/jama.282.16.1523
60. van Westerop LL, Arts-de Jong M, Hoogerbrugge N, de Hullu JA, Maas AH. Cardiovascular risk of BRCA1/2 mutation carriers: a review. Maturitas. (2016) 91:135–9. doi: 10.1016/j.maturitas.2016.06.012
61. Borkenhagen JF, Bergom C, Rapp CT, Klawikowski SJ, Rein LE, Gore EM. Dosimetric predictors of cardiotoxicity in thoracic radiotherapy for lung cancer. Clin Lung Cancer. (2019) 20:435–41. doi: 10.1016/j.cllc.2019.05.014
62. Ming X, Feng Y, Yang C, Wang W, Wang P, Deng J. Radiation-induced heart disease in lung cancer radiotherapy: a dosimetric update. Medicine. (2016) 95:e5051. doi: 10.1097/MD.0000000000005051
63. Frandsen J, Boothe D, Gaffney DK, Wilson BD, Lloyd S. Increased risk of death due to heart disease after radiotherapy for esophageal cancer. J Gastrointest Oncol. (2015) 6:516–23.
64. Piroth MD, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R, et al. Heart toxicity from breast cancer radiotherapy: current findings, assessment, and prevention. Strahlenther Onkol. (2019) 195:1–12. doi: 10.1007/s00066-018-1378-z
65. Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C, Darby SC, et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol. (2017) 123:299–305. doi: 10.1016/j.radonc.2017.03.012
66. Hoppe BS, Bates JE, Mendenhall NP, Morris CG, Louis D, Ho MW, et al. The meaningless meaning of mean heart dose in mediastinal lymphoma in the modern radiation therapy era. Pract Radiat Oncol. (2019) doi: 10.1016/j.prro.2019.09.015. [Epub ahead of print].
67. Ozyigit G, Selek U. Radiation Oncology: A Case-Based Review. Cham: Springer Nature Switzerland (2019). doi: 10.1007/978-3-319-97145-2
68. Chang JY, Bezjak A, Mornex F, IASLC Advanced Radiation Technology Committee. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. (2015) 10:577–85. doi: 10.1097/JTO.0000000000000453
69. Zhao L, Zhou S, Balter P, Shen C, Gomez DR, Welsh JD, et al. Planning target volume D95 and mean dose should be considered for optimal local control for stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. (2016) 95:1226–35. doi: 10.1016/j.ijrobp.2016.01.065
70. Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. (2010) 76:S10–9. doi: 10.1016/j.ijrobp.2009.07.1754
71. Boulet J, Pena J, Hulten EA, Neilan TG, Dragomir A, Freeman C, et al. Statin use and risk of vascular events among cancer patients after radiotherapy to the thorax, head, and neck. J Am Heart Assoc. (2019) 8:e005996. doi: 10.1161/JAHA.117.005996
72. Ward WF, Kim YT, Molteni A, Solliday NH. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. (1988) 15:135–40. doi: 10.1016/0360-3016(88)90357-4
73. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. (2016) 37:1671–80. doi: 10.1093/eurheartj/ehw022
74. Ohri N. Radiotherapy dosing for locally advanced non-small cell lung carcinoma: “MTD” or “ALARA”? Front Oncol. (2017) 7:205. doi: 10.3389/fonc.2017.00205
75. Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol. (2015) 27:621–9. doi: 10.1016/j.clon.2015.06.007
76. Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc Med. (2019) 30:22–8. doi: 10.1016/j.tcm.2019.01.006
77. Freedman GM, Lin L. Cardiac-sparing radiation therapy for breast cancer. Appl Radiat Oncol. (2016) 5:6–11.
78. Yang K, Park W, Ju SG, Chung Y, Choi DH, Cha H, et al. Heart-sparing radiotherapy with three-dimensional printing technology after mastectomy for patients with left breast cancer. Breast J. (2019) 25:682–6. doi: 10.1111/tbj.13304
79. Kil WJ, Pham T, Kim K. Heart sparing breast cancer radiotherapy using continuous positive airway pressure (CPAP) and conventional supine tangential fields: an alternative method for patients with limited accessibility to advanced radiotherapy techniques. Acta Oncol. (2019) 58:105–9. doi: 10.1080/0284186X.2018.1503711
80. Kaplinsky A, Pyatigorskaya V, Granot H, Gelernter I, Ben-Ayun M, Alezra D, et al. RPM inspiration gating: improving radiotherapy for left breast cancer patients with anterior heart position. Isr Med Assoc J. (2018) 20:548–52.
81. Becker-Schiebe M, Stockhammer M, Hoffmann W, Wetzel F, Franz H. Does mean heart dose sufficiently reflect coronary artery exposure in left-sided breast cancer radiotherapy?: influence of respiratory gating. Strahlenther Onkol. (2016) 192:624–31. doi: 10.1007/s00066-016-1011-y
82. Speleers BA, Belosi FM, De Gersem WR, Deseyne PR, Paelinck LM, Bolsi A, et al. Comparison of supine or prone crawl photon or proton breast and regional lymph node radiation therapy including the internal mammary chain. Sci Rep. (2019) 9:4755. doi: 10.1038/s41598-019-41283-1
83. Braunstein LZ, Cahlon O. Potential morbidity reduction with proton radiation therapy for breast cancer. Semin Radiat Oncol. (2018) 28:138–49. doi: 10.1016/j.semradonc.2017.11.009
84. Kammerer E, Guevelou JL, Chaikh A, Danhier S, Geffrelot J, Levy C, et al. Proton therapy for locally advanced breast cancer: a systematic review of the literature. Cancer Treat Rev. (2018) 63:19–27. doi: 10.1016/j.ctrv.2017.11.006
85. Tommasino F, Durante M, D'Avino V, Liuzzi R, Conson M, Farace P, et al. Model-based approach for quantitative estimates of skin, heart, and lung toxicity risk for left-side photon and proton irradiation after breast-conserving surgery. Acta Oncol. (2017) 56:730–6. doi: 10.1080/0284186X.2017.1299218
86. Simonetto C, Eidemuller M, Gaasch A, Pazos M, Schonecker S, Reitz D, et al. Does deep inspiration breath-hold prolong life? Individual risk estimates of ischaemic heart disease after breast cancer radiotherapy. Radiother Oncol. (2019) 131:202–7. doi: 10.1016/j.radonc.2018.07.024
87. Duma MN, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R, et al. Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther Onkol. (2019) 195:861–71. doi: 10.1007/s00066-019-01495-w
88. Wikstrom K, Isacsson U, Nilsson K, Ahnesjo A. Reproducibility of heart and thoracic wall position in repeated deep inspiration breath holds for radiotherapy of left-sided breast cancer patients. Acta Oncol. (2018) 57:1318–24. doi: 10.1080/0284186X.2018.1490027
89. Corradini S, Ballhausen H, Weingandt H, Freislederer P, Schonecker S, Niyazi M, et al. Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease: effects of modern radiotherapy techniques. Strahlenther Onkol. (2018) 194:196–205. doi: 10.1007/s00066-017-1213-y
90. Al-Hammadi N, Caparrotti P, Naim C, Hayes J, Rebecca Benson K, Vasic A, et al. Voluntary deep inspiration breath-hold reduces the heart dose without compromising the target volume coverage during radiotherapy for left-sided breast cancer. Radiol Oncol. (2018) 52:112–20. doi: 10.1515/raon-2018-0008
91. Koivumaki T, Tujunen J, Viren T, Heikkila J, Seppala J. Geometrical uncertainty of heart position in deep-inspiration breath-hold radiotherapy of left-sided breast cancer patients. Acta Oncol. (2017) 56:879–83. doi: 10.1080/0284186X.2017.1298836
92. Niska JR, Thorpe CS, Allen SM, Daniels TB, Rule WG, Schild SE, et al. Radiation and the heart: systematic review of dosimetry and cardiac endpoints. Expert Rev Cardiovasc Ther. (2018) 16:931–50. doi: 10.1080/14779072.2018.1538785
93. Drost L, Yee C, Lam H, Zhang L, Wronski M, McCann C, et al. A systematic review of heart dose in breast radiotherapy. Clin Breast Cancer. (2018) 18:e819–24. doi: 10.1016/j.clbc.2018.05.010
94. Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. (2015) 93:845–53. doi: 10.1016/j.ijrobp.2015.07.2292
95. Milgrom SA, Varghese B, Gladish GW, Choi AD, Dong W, Patel ZS, et al. Coronary artery dose-volume parameters predict risk of calcification after radiation therapy. J Cardiovasc Imaging. (2019) 27:268–79. doi: 10.4250/jcvi.2019.27.e38
96. Tan W, Liu D, Xue C, Xu J, Li B, Chen Z, et al. Anterior myocardial territory may replace the heart as organ at risk in intensity-modulated radiotherapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys. (2012) 82:1689–97. doi: 10.1016/j.ijrobp.2011.03.009
97. Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. (2012) 83:1580–6. doi: 10.1016/j.ijrobp.2011.10.053
98. Jacob S, Camilleri J, Derreumaux S, Walker V, Lairez O, Lapeyre M, et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat Oncol. (2019) 14:29. doi: 10.1186/s13014-019-1234-z
99. Hiniker SM, Donaldson SS. ALARA: in radiation oncology and diagnostic imaging alike. Oncology. (2014) 28:247–8.
100. Sung K, Choi YE, Lee KC. Cardiac risk index as a simple geometric indicator to select patients for the heart-sparing radiotherapy of left-sided breast cancer. J Med Imaging Radiat Oncol. (2017) 61:410–7. doi: 10.1111/1754-9485.12567
101. Tjessem KH, Bosse G, Fossa K, Reinertsen KV, Fossa SD, Johansen S, et al. Coronary calcium score in 12-year breast cancer survivors after adjuvant radiotherapy with low to moderate heart exposure - relationship to cardiac radiation dose and cardiovascular risk factors. Radiother Oncol. (2015) 114:328–34. doi: 10.1016/j.radonc.2015.01.006
102. Riddell E, Lenihan D. The role of cardiac biomarkers in cardio-oncology. Curr Probl Cancer. (2018) 42:375–85. doi: 10.1016/j.currproblcancer.2018.06.012
103. Shah KS, Yang EH, Maisel AS, Fonarow GC. The role of biomarkers in detection of cardio-toxicity. Curr Oncol Rep. (2017) 19:42. doi: 10.1007/s11912-017-0602-9
104. Negishi T, Miyazaki S, Negishi K. Echocardiography and Cardio-Oncology. Heart Lung Circ. (2019) 28:1331–8. doi: 10.1016/j.hlc.2019.04.023
105. Liu J, Banchs J, Mousavi N, Plana JC, Scherrer-Crosbie M, Thavendiranathan P, et al. Contemporary role of echocardiography for clinical decision making in patients during and after cancer therapy. JACC Cardiovasc Imaging. (2018) 11:1122–31. doi: 10.1016/j.jcmg.2018.03.025
106. Bordun KA, Premecz S, daSilva M, Mandal S, Goyal V, Glavinovic T, et al. The utility of cardiac biomarkers and echocardiography for the early detection of bevacizumab- and sunitinib-mediated cardiotoxicity. Am J Physiol Heart Circ Physiol. (2015) 309:H692–701. doi: 10.1152/ajpheart.00172.2015
107. Laufer-Perl M, Arnold JH, Mor L, Amrami N, Derakhshesh M, Moshkovits Y, et al. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin Res Cardiol. (2020) 109:255–62. doi: 10.1007/s00392-019-01508-9
108. Laufer-Perl M, Derakhshesh M, Milwidsky A, Mor L, Ravid D, Amrami N, et al. Usefulness of global longitudinal strain for early identification of subclinical left ventricular dysfunction in patients with active cancer. Am J Cardiol. (2018) 122:1784–9. doi: 10.1016/j.amjcard.2018.08.019
109. Mercurio V, Cuomo A, Della Pepa R, Ciervo D, Cella L, Pirozzi F, et al. What is the cardiac impact of chemotherapy and subsequent radiotherapy in lymphoma patients? Antioxid Redox Signal. (2019) 31:1166–74. doi: 10.1089/ars.2019.7842
110. Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. (2018) 11:1173–86. doi: 10.1016/j.jcmg.2018.06.003
111. Layoun ME, Yang EH, Herrmann J, Iliescu CA, Lopez-Mattei JC, Marmagkiolis K, et al. Applications of cardiac computed tomography in the cardio-oncology population. Curr Treat Options Oncol. (2019) 20:47. doi: 10.1007/s11864-019-0645-2
112. Pitekova B, Ravi S, Shah SV, Mladosievicova B, Heitner S, Ferencik M. The role of imaging with cardiac computed tomography in cardio-oncology patients. Curr Cardiol Rep. (2016) 18:87. doi: 10.1007/s11886-016-0768-z
113. Yang Y, Yam Y, Chen L, Aljizeeri A, Aliyary Ghraboghly S, Al-Harbi I, et al. Assessment of left ventricular ejection fraction using low radiation dose computed tomography. J Nucl Cardiol. (2016) 23:414–21. doi: 10.1007/s12350-015-0123-6
114. Jordan JH, Hundley WG. MRI of cardiotoxicity. Cardiol Clin. (2019) 37:429–39. doi: 10.1016/j.ccl.2019.07.007
115. Jeong D, Gladish G, Chitiboi T, Fradley MG, Gage KL, Schiebler ML. MRI in cardio-oncology: a review of cardiac complications in oncologic care. J Magn Reson Imaging. (2019) 50:1349–66. doi: 10.1002/jmri.26895
116. Loffler AI, Salerno M. Cardiac MRI for the evaluation of oncologic cardiotoxicity. J Nucl Cardiol. (2018) 25:2148–58. doi: 10.1007/s12350-018-1293-9
117. Dreyfuss AD, Bravo PE, Koumenis C, Ky B. Precision cardio-oncology. J Nucl Med. (2019) 60:443–50. doi: 10.2967/jnumed.118.220137
118. Zhang P, Hu X, Yue J, Meng X, Han D, Sun X, et al. Early detection of radiation-induced heart disease using (99m)Tc-MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiother Oncol. (2015) 115:171–8. doi: 10.1016/j.radonc.2015.04.009
119. Zyromska A, Malkowski B, Wisniewski T, Majewska K, Reszke J, Makarewicz R. 15O-H2O PET/CT as a tool for the quantitative assessment of early post-radiotherapy changes of heart perfusion in breast carcinoma patients. Br J Radiol. (2018) 91:20170653. doi: 10.1259/bjr.20170653
120. Tong D, Zaha VG. Metabolic imaging in cardio-oncology. J Cardiovasc Transl Res. (2019). doi: 10.1007/s12265-019-09927-9
121. Soufer A, Liu C, Henry ML, Baldassarre LA. Nuclear cardiology in the context of multimodality imaging to detect cardiac toxicity from cancer therapeutics: established and emerging methods. J Nucl Cardiol. (2019). doi: 10.1007/s12350-019-01671-6
122. Kahanda MG, Hanson CA, Patterson B, Bourque JM. Nuclear cardio-oncology: from its foundation to its future. J Nucl Cardiol. (2019). doi: 10.1007/s12350-019-01655-6
123. Raucci A, Di Maggio S, Scavello F, D'Ambrosio A, Bianchi ME, Capogrossi MC. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci. (2019) 76:211–29. doi: 10.1007/s00018-018-2930-9
124. Sarkozy M, Gaspar R, Zvara A, Kiscsatari L, Varga Z, Kovari B, et al. Selective heart irradiation induces cardiac overexpression of the pro-hypertrophic miR-212. Front Oncol. (2019) 9:598. doi: 10.3389/fonc.2019.00598
125. Rousseau M, Gaugler MH, Rodallec A, Bonnaud S, Paris F, Corre I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun. (2011) 414:750–5. doi: 10.1016/j.bbrc.2011.09.150
126. Oesterle A, Liao JK. The pleiotropic effects of statins - from coronary artery disease and stroke to atrial fibrillation and ventricular tachyarrhythmia. Curr Vasc Pharmacol. (2019) 17:222–32. doi: 10.2174/1570161116666180817155058
127. Tanaka S, Fukumoto Y, Nochioka K, Minami T, Kudo S, Shiba N, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol. (2013) 33:1591–600. doi: 10.1161/ATVBAHA.112.300922
128. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. (2005) 45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748
129. Wilkinson EL, Sidaway JE, Cross MJ. Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells. J Cell Physiol. (2018) 233:186–200. doi: 10.1002/jcp.26064
130. Cerda A, Fajardo CM, Basso RG, Hirata MH, Hirata RD. Role of microRNAs 221/222 on statin induced nitric oxide release in human endothelial cells. Arq Bras Cardiol. (2015) 104:195–201. doi: 10.5935/abc.20140192
131. Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. (2017) 35:1641–9. doi: 10.1200/JCO.2016.72.0722
132. Dlugosz-Danecka M, Gruszka AM, Szmit S, Olszanecka A, Ogorka T, Sobocinski M, et al. Primary cardioprotection reduces mortality in lymphoma patients with increased risk of anthracycline cardiotoxicity, treated by R-CHOP regimen. Chemotherapy. (2018) 63:238–45. doi: 10.1159/000492942
133. Gulati G, Heck SL, Rosjo H, Ree AH, Hoffmann P, Hagve TA, et al. Neurohormonal Blockade and Circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (prevention of cardiac dysfunction during adjuvant breast cancer therapy) study. J Am Heart Assoc. (2017) 6:e006513. doi: 10.1161/JAHA.117.006513
134. Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. (2006) 6:702–13. doi: 10.1038/nrc1950
135. Hahn AW, Resink TJ, Bernhardt J, Ferracin F, Buhler FR. Stimulation of autocrine platelet–derived growth factor AA-homodimer and transforming growth factor beta in vascular smooth muscle cells. Biochem Biophys Res Commun. (1991) 178:1451–8. doi: 10.1016/0006-291X(91)91056-I
136. Armanious MA, Mishra S, Fradley MG. Electrophysiologic toxicity of chemoradiation. Curr Oncol Rep. (2018) 20:45. doi: 10.1007/s11912-018-0691-0
137. Subramanian V, Seemann I, Merl-Pham J, Hauck SM, Stewart FA, Atkinson MJ, et al. Role of TGF beta and PPAR alpha signaling pathways in radiation response of locally exposed heart: integrated global transcriptomics and proteomics analysis. J Proteome Res. (2017) 16:307–18. doi: 10.1021/acs.jproteome.6b00795
138. Slezak J, Kura B, Babal P, Barancik M, Ferko M, Frimmel K, et al. Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury. Can J Physiol Pharmacol. (2017) 95:1190–203. doi: 10.1139/cjpp-2017-0121
139. Klee NS, McCarthy CG, Martinez-Quinones P, Webb RC. Out of the frying pan and into the fire: damage-associated molecular patterns and cardiovascular toxicity following cancer therapy. Ther Adv Cardiovasc Dis. (2017) 11:297–317. doi: 10.1177/1753944717729141
140. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. (2016) 1:15004. doi: 10.1038/sigtrans.2015.4
141. Lamichhane SR, Thachil T, De Ieso P, Gee H, Moss SA, Milic N. Prognostic role of microRNAs in human non-small-cell lung cancer: a systematic review and meta-analysis. Dis Markers. (2018) 2018:8309015. doi: 10.1155/2018/8309015
142. Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: a systematic review and meta-analysis. Oncotarget. (2017) 8:46611–23. doi: 10.18632/oncotarget.16679
143. Asiaf A, Ahmad ST, Arjumand W, Zargar MA. MicroRNAs in breast cancer: diagnostic and therapeutic potential. Methods Mol Biol. (2018) 1699:23–43. doi: 10.1007/978-1-4939-7435-1_2
144. Aghdam AM, Amiri A, Salarinia R, Masoudifar A, Ghasemi F, Mirzaei H. MicroRNAs as diagnostic, prognostic, and therapeutic biomarkers in prostate cancer. Crit Rev Eukaryot Gene Expr. (2019) 29:127–39. doi: 10.1615/CritRevEukaryotGeneExpr.2019025273
145. Wan TM, Iyer DN, Ng L. Roles of microRNAs as non-invasive biomarker and therapeutic target in colorectal cancer. Histol Histopathol. (2020) 35, 225–237. doi: 10.14670/HH-18-171
146. Movahedpour A, Ahmadi N, Ghasemi Y, Savardashtaki A, Shabaninejad Z. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in prostate cancer: current status and future perspectives. J Cell Biochem. (2019) 120:16316–29. doi: 10.1002/jcb.29053
147. Eken SM, Christersdottir T, Winski G, Sangsuwan T, Jin H, Chernogubova E, et al. miR-29b mediates the chronic inflammatory response in radiotherapy-induced vascular disease. JACC Basic Transl Sci. (2019) 4:72–82. doi: 10.1016/j.jacbts.2018.10.006
148. Zhang C, Zhang K, Huang F, Feng W, Chen J, Zhang H, et al. Exosomes, the message transporters in vascular calcification. J Cell Mol Med. (2018) 22:4024–33. doi: 10.1111/jcmm.13692
149. Hu Y, Xia W, Hou M. Macrophage migration inhibitory factor serves a pivotal role in the regulation of radiation-induced cardiac senescencethrough rebalancing the microRNA-34a/sirtuin 1 signaling pathway. Int J Mol Med. (2018) 42:2849–58. doi: 10.3892/ijmm.2018.3838
150. Esplugas R, Belles M, Serra N, Arenas M, Hernandez V, Vallve JC, et al. Effect of radiation on the expression of CVD-related miRNAs, inflammation and endothelial dysfunction of HUVECs. Anticancer Res. (2019) 39:771–80. doi: 10.21873/anticanres.13174
151. Kim EJ, Lee J, Jung YR, Park JJ, Park MJ, Lee JS, et al. Involvement of corin downregulation in ionizing radiation-induced senescence of myocardial cells. Int J Mol Med. (2015) 35:731–8. doi: 10.3892/ijmm.2014.2048
152. Halle M, Christersdottir T, Back M. Chronic adventitial inflammation, vasa vasorum expansion, and 5-lipoxygenase up-regulation in irradiated arteries from cancer survivors. FASEB J. (2016) 30:3845–52. doi: 10.1096/fj.201600620R
153. Park H, Kim CH, Jeong JH, Park M, Kim KS. GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells. Oncotarget. (2016) 7:9634–44. doi: 10.18632/oncotarget.7457
154. Chen ZY, Hu YY, Hu XF, Cheng LX. The conditioned medium of human mesenchymal stromal cells reduces irradiation-induced damage in cardiac fibroblast cells. J Radiat Res. (2018) 59:555–64. doi: 10.1093/jrr/rry048
155. Gao S, Wu R, Zeng Y. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiat Environ Biophys. (2012) 51:53–9. doi: 10.1007/s00411-011-0390-9
156. Schlaak RA, Frei A, Schottstaedt AM, Tsaih SW, Fish BL, Harmann L, et al. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am J Physiol Heart Circ Physiol. (2019) 316:H1267–80. doi: 10.1152/ajpheart.00482.2018
157. Ricciotti E, Sarantopoulou D, Grant GR, Sanzari JK, Krigsfeld GS, Kiliti AJ, et al. Distinct vascular genomic response of proton and gamma radiation-A pilot investigation. PLoS ONE. (2019) 14:e0207503. doi: 10.1371/journal.pone.0207503
158. Shang S, Wang J, Chen S, Tian R, Zeng H, Wang L, et al. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. (2019) 8:7728–40. doi: 10.1002/cam4.2633
159. Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene. (2019) 706:91–6. doi: 10.1016/j.gene.2019.04.082
160. Bascunan KA, Perez-Bravo F, Gaudioso G, Vaira V, Roncoroni L, Elli L, et al. A miRNA-based blood and mucosal approach for detecting and monitoring celiac disease. Dig Dis Sci. (2019). doi: 10.1007/s10620-019-05966-z
161. Wang H, Wang TT, Lv XP. Expression and prognostic value of miRNA-29b in peripheral blood for endometrial cancer. Future Oncol. (2018) 14:1365–76. doi: 10.2217/fon-2017-0594
162. Chen PC, Yu CC, Huang WY, Huang WH, Chuang YM, Lin RI, et al. c-Myc acts as a competing endogenous RNA to sponge miR-34a, in the upregulation of CD44, in urothelial carcinoma. Cancers. (2019) 11:E1457. doi: 10.3390/cancers11101457
163. Soyocak A, Kurt H, Ozgen M, Turgut Cosan D, Colak E, Gunes HV. miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene. (2017) 627:207–11. doi: 10.1016/j.gene.2017.06.027
164. Freitas LS, Silveira AC, Martins FC, Costa-Hong V, Lebkuchen A, Cardozo KHM, et al. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath. (2020). doi: 10.1007/s11325-019-02003-1
165. Gao J, Liu J, Zhang Y, Guan B, Qu H, Chai H, et al. PBMCs-Derived microRNA signature as a prethrombotic status discriminator in stable coronary artery disease. Thromb Haemost. (2020) 120:121–31. doi: 10.1055/s-0039-1700518
166. Zhu GF, Chu T, Ruan Z, Zhang M, Zhou M, Zhang Q, et al. Inflammation-related microRNAs are associated with plaque stability calculated by IVUS in coronary heart disease patients. J Interv Cardiol. (2019) 2019:9723129. doi: 10.1155/2019/9723129
167. Vogel J, Niederer D, Engeroff T, Vogt L, Troidl C, Schmitz-Rixen T, et al. Effects on the profile of circulating miRNAs after single bouts of resistance training with and without blood flow restriction-a three-arm, randomized crossover trial. Int J Mol Sci. (2019) 20:E3249. doi: 10.3390/ijms20133249
168. Rizzacasa B, Morini E, Mango R, Vancheri C, Budassi S, Massaro G, et al. MiR-423 is differentially expressed in patients with stable and unstable coronary artery disease: a pilot study. PLoS ONE. (2019) 14:e0216363. doi: 10.1371/journal.pone.0216363
169. Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y, et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun. (2019) 10:959. doi: 10.1038/s41467-019-08895-7
170. Viczenczova C, Szeiffova Bacova B, Egan Benova T, Kura B, Yin C, Weismann P, et al. Myocardial connexin-43 and PKC signalling are involved in adaptation of the heart to irradiation-induced injury: implication of miR-1 and miR-21. Gen Physiol Biophys. (2016) 35:215–22. doi: 10.4149/gpb_2015038
171. Palayoor ST, John-Aryankalayil M, Makinde AY, Falduto MT, Magnuson SR, Coleman CN. Differential expression of stress and immune response pathway transcripts and miRNAs in normal human endothelial cells subjected to fractionated or single-dose radiation. Mol Cancer Res. (2014) 12:1002–15. doi: 10.1158/1541-7786.MCR-13-0623
172. Babini G, Tanno B, De Stefano I, Giardullo P, Leonardi S, Pasquali E, et al. Bioinformatic analysis of dose- and time-dependent miRNome responses. Radiat Prot Dosimetry. (2019) 183:151–5. doi: 10.1093/rpd/ncy215
173. Wang L, Meng X, Li G, Zhou Q, Xiao J. Circular RNAs in cardiovascular diseases. Adv Exp Med Biol. (2018) 1087:191–204. doi: 10.1007/978-981-13-1426-1_15
174. Zhou Q, Zhang Z, Bei Y, Li G, Wang T. Circular RNAs as novel biomarkers for cardiovascular diseases. Adv Exp Med Biol. (2018) 1087:159–70. doi: 10.1007/978-981-13-1426-1_13
175. ClinicalTrials.gov. Availabe online: https://clinicaltrials.gov/ct2/home (accessed on January 15, 2020).
176. Van Taunay JS, Albelda MT, Frias JC, Lipinski MJ. Biologics and cardiovascular disease. J Cardiovasc Pharmacol. (2018) 72:77–85. doi: 10.1097/FJC.0000000000000595
177. Costa WS, Ribeiro MN, Cardoso LE, Dornas MC, Ramos CF, Gallo CB, et al. Nutritional supplementation with L-arginine prevents pelvic radiation-induced changes in morphology, density, and regulating factors of blood vessels in the wall of rat bladder. World J Urol. (2013) 31:653–8. doi: 10.1007/s00345-012-0938-6
178. Parent S, Pituskin E, Paterson DI. The cardio-oncology program: a multidisciplinary approach to the care of cancer patients with cardiovascular disease. Can J Cardiol. (2016) 32:847–51. doi: 10.1016/j.cjca.2016.04.014
179. Rose-Felker K, Effinger K, Kelleman MS, Sachdeva R, Meacham LR, Border WL. Improving paediatric cardiologists' awareness about the needs of childhood cancer survivors: results of a single-centre directed educational initiative. Cardiol Young. (2019) 29:808–12. doi: 10.1017/S104795111900088X
180. Montazeri K, Unitt C, Foody JM, Harris JR, Partridge AH, Moslehi J. ABCDE steps to prevent heart disease in breast cancer survivors. Circulation. (2014) 130:e157–9. doi: 10.1161/CIRCULATIONAHA.114.008820
181. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. (2018) 137:1176–91. doi: 10.1161/CIRCULATIONAHA.117.024671
182. Kosalka P, Johnson C, Turek M, Sulpher J, Law A, Botros J, et al. Effect of obesity, dyslipidemia, and diabetes on trastuzumab-related cardiotoxicity in breast cancer. Curr Oncol. (2019) 26:e314–21. doi: 10.3747/co.26.4823
183. Michel L, Rassaf T. Cardio-oncology: need for novel structures. Eur J Med Res. (2019) 24:1. doi: 10.1186/s40001-018-0359-0
184. Lancellotti P, Suter TM, Lopez-Fernandez T, Galderisi M, Lyon AR, Van der Meer P, et al. Cardio-Oncology Services: rationale, organization, and implementation. Eur Heart J. (2019) 40:1756–63. doi: 10.1093/eurheartj/ehy453
185. Kounis NG, Koniari I, Plotas P, Soufras GD, Tsigkas G, Davlouros P, et al. Emergence, development, and future of cardio-oncology in China: cardiohypersensitivity, cardiotoxicity and the Kounis syndrome. Chin Med J. (2019) 132:753–4. doi: 10.1097/CM9.0000000000000130
186. Kostakou PM, Kouris NT, Kostopoulos VS, Damaskos DS, Olympios CD. Cardio-oncology: a new and developing sector of research and therapy in the field of cardiology. Heart Fail Rev. (2019) 24:91–100. doi: 10.1007/s10741-018-9731-y
187. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. (2019) 139:e997–1012. doi: 10.1161/CIR.0000000000000679
188. Barish R, Lynce F, Unger K, Barac A. Management of cardiovascular disease in women with breast cancer. Circulation. (2019) 139:1110–20. doi: 10.1161/CIRCULATIONAHA.118.039371
189. Francolini G, Desideri I, Meattini I, Becherini C, Terziani F, Olmetto E, et al. Assessment of a guideline-based heart substructures delineation in left-sided breast cancer patients undergoing adjuvant radiotherapy: quality assessment within a randomized phase III trial testing a cardioprotective treatment strategy (SAFE-2014). Strahlenther Onkol. (2019) 195:43–51. doi: 10.1007/s00066-018-1388-x
190. Lorenzen EL, Taylor CW, Maraldo M, Nielsen MH, Offersen BV, Andersen MR, et al. Inter-observer variation in delineation of the heart and left anterior descending coronary artery in radiotherapy for breast cancer: a multi-centre study from Denmark and the UK. Radiother Oncol. (2013) 108:254–8. doi: 10.1016/j.radonc.2013.06.025
Keywords: radiation, cardiovascular dysfunction, miRNA, late sequelae, toxicity
Citation: Lee M-S, Liu D-W, Hung S-K, Yu C-C, Chi C-L, Chiou W-Y, Chen L-C, Lin R-I, Huang L-W, Chew C-H, Hsu F-C, Chan MWY and Lin H-Y (2020) Emerging Challenges of Radiation-Associated Cardiovascular Dysfunction (RACVD) in Modern Radiation Oncology: Clinical Practice, Bench Investigation, and Multidisciplinary Care. Front. Cardiovasc. Med. 7:16. doi: 10.3389/fcvm.2020.00016
Received: 15 December 2019; Accepted: 31 January 2020;
Published: 21 February 2020.
Edited by:
Carlo Gabriele Tocchetti, University of Naples Federico II, ItalyReviewed by:
Alessandra Cuomo, Federico II University Hospital, ItalySherry-Ann Brown, Mayo Clinic, United States
Copyright © 2020 Lee, Liu, Hung, Yu, Chi, Chiou, Chen, Lin, Huang, Chew, Hsu, Chan and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael W. Y. Chan, biowyc@ccu.edu.tw; Hon-Yi Lin, doc16021@gmail.com
†These authors have contributed equally to this work
 Moon-Sing Lee1,2†
Moon-Sing Lee1,2†  Ru-Inn Lin
Ru-Inn Lin Li-Wen Huang
Li-Wen Huang Michael W. Y. Chan
Michael W. Y. Chan Hon-Yi Lin
Hon-Yi Lin