Haematopoietic Stem Cell Transplantation in Adolescents and Young Adults With Acute Lymphoblastic Leukaemia: Special Considerations and Challenges
- 1Pediatric Hematology and Immunology Department, Robert Debré Academic Hospital, GHU APHP Nord - Université de Paris, Paris, France
- 2Department of Pediatric Hematology and Oncology, St. Anna Children's Hospital, Medical University of Vienna, Vienna, Austria
- 3Hematology for Adolescents and Young Adults, Saint-Louis Academic Hospital GHU APHP Nord - Université de Paris, Paris, France
- 4Department of Pediatric Hematology and Oncology, Oslo University Hospital, Oslo, Norway
- 5Department of Pediatric Hematology, Oncology and Stem Cell Transplantation, University Hospital of Regensburg, Regensburg, Germany
Adolescents and young adults (AYAs) represent a challenging group of acute lymphoblastic leukaemia (ALL) patients with specific needs. While there is growing evidence from comparative studies that this age group profits from intensified paediatric-based chemotherapy, the impact and optimal implementation of haematopoietic stem cell transplantation (HSCT) in the overall treatment strategy is less clear. Over recent years, improved survival rates after myeloablative allogeneic HSCT for ALL have been reported similarly for AYAs and children despite differences in transplantation practise. Still, AYAs appear to have inferior outcomes and an increased risk of treatment-related morbidity and mortality in comparison with children. To further improve HSCT outcomes and reduce toxicities in AYAs, accurate stratification and evaluation of additional or alternative targeted treatment options are crucial, based on specific molecular and immunological characterisation of ALL and minimal residual disease (MRD) assessment during therapy. Age-specific factors such as increased acute toxicities and poorer adherence to treatment as well as late sequelae might influence treatment decisions. In addition, educational, social, work, emotional, and sexual aspects during this very crucial period of life need to be considered. In this review, we summarise the key findings of recent studies on treatment approach and outcomes in this vulnerable patient group after HSCT, turning our attention to the different approaches applied in paediatric and adult centres. We focus on the specific needs of AYAs with ALL regarding social aspects and supportive care to handle complications as well as fertility issues. Finally, we comment on potential areas of future research and concisely debate the capacity of currently available immunotherapies to reduce toxicity and further improve survival in this challenging patient group.
Introduction
Adolescents and young adults (AYAs) with acute lymphoblastic leukaemia (ALL) are a unique group of patients at the interface between childhood and adulthood (1). There is no consensus on the definition of AYAs: the World Health Organisation defines adolescents as individuals of 10–19 years old, while the National Cancer Institute defines the AYA population as 15–39 years old (2, 3). In Europe, patients are considered an AYA if they are aged 15–29 years (2). These differences in age definition affect access to different types of care structure, clinical trials and treatment protocols (2).
Although the survival rate now approaches 90% for childhood ALL, the prognosis remains poorer in AYAs (2, 3). In fact, survival of ALL is triphasic during adulthood, with survival rates of 75% when treated at 17 years, 48% at 20 years, and 15% at 70 years—also known as the “survival cliff” (4, 5).
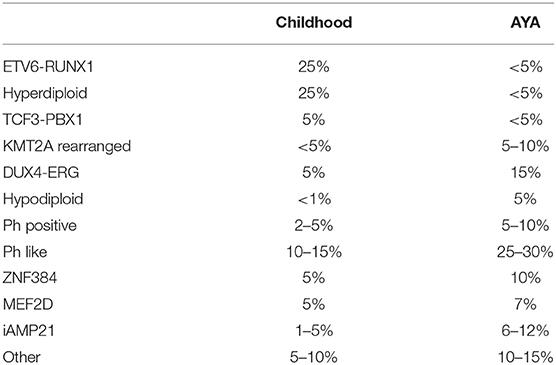
The inferior prognosis of ALL in AYAs compared to in children can be explained in part by the age-related variations in the molecular subtypes of ALL (Table 1) (6). The frequency of ALL with a T-cell phenotype is about twice higher in AYA compared to younger children (<15 years old) (7). The prevalence of hyperdiploidy and ETV6-RUNX1-positive ALL—both of which are associated with good prognosis—declines from 25 to 30% in children to <3% in young adults (aged 21–39 years) (4, 6, 7). Conversely, the prevalence of Philadelphia chromosome (Ph)-positive ALL—which is associated with poor prognosis—is markedly increased in patients aged 21–39 years vs. in children (4, 6, 7). Also, the prevalence of Ph-like ALL (poor prognosis) rises with age, from 10 to 15% in children with B-cell ALL to over 25% in young adults with ALL (5, 6, 8). Some genomic abnormalities have a peak incidence in the AYA population e.g., iAMP21, DUX4 rearrangement, ZNF384 rearrangement, and MEF2D rearrangement (4). Age-related variation of genomic abnormalities from childhood to AYA B-cell precursor ALL (BCP-ALL) are represented in Table 1.
The survival cliff between the ages of 17 and 21 years has also been attributed to the transition of patients from paediatric to adult treatment sites and protocols (5). Superior survival has been consistently demonstrated in various countries when AYA patients are treated using paediatric chemotherapy protocols rather than adult ones, with differences in type and intensity of anti-leukaemic drugs vs. adult protocols (1, 2, 5, 7, 9, 10). In fact, more extensive use of glucocorticoids, vincristine and pegylated asparaginase with intensive and prolonged central nervous system (CNS) prophylaxis results in survival benefit for AYA patients with ALL (10, 11). Indeed, in the largest published cohort of patients 1–45 years of age treated with the same ALL frontline protocol (NOPHO ALL-2008), event-free survival (EFS) and overall survival (OS) for 18–45-year-old patients with Ph-negative ALL were 74 and 78%, respectively (12). For high-risk patients with or without an indication for haematopoietic stem cell transplantation (HSCT), no significant difference in 5-year EFS was seen between the three age groups: 1–9, 10–17, and 18–45 years (12). In keeping with these findings, Wieduliwilt et al. concluded from their study that post-remission therapy with paediatric-style chemotherapy was superior to myeloablative conditioning followed by allogeneic HSCT in AYA patients with Ph-negative ALL in first complete remission (CR1) in terms of OS (66 vs. 45%, respectively), disease-free survival (DFS; 58 vs. 44%, respectively), and non-relapse mortality (NRM; 8 vs. 29%, respectively) (13).
However, access to clinical trials and paediatric regimens is not readily available to all AYA patients (4).
The better OS and lower relapse rate associated with treatment of AYAs on paediatric wards may be due to higher therapy intensity and stricter adherence to chemotherapy schedules. Conversely, being treated on adult wards may lead to better and earlier access to novel therapies not yet available in paediatric centres in patients with refractory disease.
The use of paediatric-inspired or fully paediatric strategies has improved outcomes in AYAs with Ph-negative ALL and, as a consequence, has led experts to question allogeneic HSCT indications in this population (2). Because of its associated short- and long-term toxicities, progress in chemotherapy management, and the advent of immunotherapy, HSCT could be reserved for AYA patients with ALL exhibiting early resistance to chemotherapy assessed by predefined evaluations of minimal residual disease (MRD), as recommended in paediatric protocols (2, 12, 14–16). At least in CR1, in AYA as in other patient-age subgroups MRD represents one of the most important point for identifying patients requesting treatment intensification (2, 12, 14–16). In addition, with the use of combined tyrosine kinase inhibitors (TKIs) and chemotherapy treatment, a further reduction in the proportion of patients eligible for HSCT has been achieved, even in the high-risk group (17–19).
Many adult patients with ALL treated on an adult regimen receive HSCT during first complete remission (CR1) if a matched donor is available. However, the ability to avoid the toxicities, late adverse effects, and financial costs of HSCT substantially favours paediatric regimens (5).
Toxicities represent a major issue in AYA patients with ALL. The use of intensified regimens raises the need for monitoring and preventing acute and late side effects that can affect survival and quality of life (2). Toxicities also represent a significant problem in AYA patients who undergo HSCT, with studies reporting 10–30% treatment-related mortality (TRM), which is higher than reported for younger patients and is mostly due to graft-vs.-host disease (GvHD) and infection (3). Hypofertility/infertility is a particularly relevant late side effect in AYAs (2). Many studies have shown the higher risk of non-adherence during therapy or follow-up in the AYA population vs. their younger counterparts (2).
Therefore, AYA patients undergoing HSCT to treat ALL represent a unique group with medical, psychological, and social issues requiring diligent care and follow-up. We try to address these points in this review. We fully acknowledge that the published literature uses different age definitions for AYAs, which makes comparisons cumbersome; but at least when reporting our own experience, we consider AYA patients to be aged between 15 and 29 years.
Indication for HSCT
Beyond discussions about the definition of the AYA age group, we were not able to identify any papers specifically dedicated to allogeneic HSCT in an AYA ALL population. Published ALL studies reported overall results of ALL therapy in this population but did not necessarily disclose how an AYA group was defined, state whether there were any adjustments in the HSCT indications for this group compared with indications for children or adults, or address HSCT results specifically in AYAs (15, 20). In paediatric studies, AYAs are often limited to 18–21-year-old patients and results are not provided for older AYAs (20). In adult studies, AYAs are usually included in the overall cohort along with 40–60 years old patients; this probably worsen their outcome vs. if the older patients were studied separately.
Despite continuous improvement, overall results of ALL treatment in AYAs (including the results of initial chemotherapy, not only HSCT) appear to be worse than those obtained in younger children with the same protocols (3, 15). Differences in OS may relate to a higher incidence of TRM, especially from infections and GvHD (3, 15). However, none of the published registry or centre-based analyses reported in detail potential differences in HSCT indications and donor choice between the paediatric and AYA populations, leading to possible bias in the interpretation of results. Many studies report results based on donor allocation, i.e., a matched sibling donor (MSD) or matched unrelated donor (MUD) matched at 10 out of 10 human-leukocyte antigen (HLA) loci, rather than based on the final treatment received (chemotherapy alone vs. chemotherapy followed by HSCT, regardless of donor type) (3, 11). For patients in CR1, most current paediatric and adult protocols reserve HSCT for AYAs with high-risk/very -high-risk ALL defined by disease characteristics such as ALL cell origin (B cell or T cell), initial leukocyte count, cytogenetics and molecular profile at diagnosis, and response after induction with or without consolidation courses evaluated by MRD measurement using real-time polymerase chain reaction (RQ-PCR) or flow cytometry (3, 11, 21). In some adult protocols, the transplant indication is based on only the persistence of positive MRD at defined time points regardless of other disease characteristics (21). For children or adults, definitions of high-risk cytogenetics and molecular profiles differ by protocol. Since the molecular profile of paediatric and AYA patients differs, with AYAs patients having a poorer-risk profile (see above), a higher percentage of AYAs are allocated to HSCT, logically (3, 11). In addition, the anticipated toxicities of chemotherapy are not identical for the AYA and paediatric population (3). Acute toxicity is more prevalent in AYAs as compared with in paediatric patients. Therefore, clinicians are often quick to propose HSCT for AYA patients in CR1 in order to avoid the risk of death related to second-line chemotherapy if relapse occurs. In contrast, long-term sequalae of HSCT in paediatric patients represent a major concern leading to the avoidance of HSCT in CR1 in as many patients as possible. Paediatric protocols propose HSCT in <5–8% of patients in CR1, while adult protocols enrolling AYA propose HSCT in about 30% of patients in CR1 (22). Adult protocols also propose that patients in second complete remission (CR2) undergo HSCT from any available donor as long as the patient is <40 years old.
In the future, the use of immunotherapy such as inotuzumab ozogamicin and blinatumomab as well as chimeric antigen receptor (CAR) T-cell therapy may change the current HSCT algorithm in the AYA population (23–25).
Choice of Conditioning Regimen
In allogeneic HSCT for ALL, older age is associated with poorer survival (3, 15). In most studies, age does not impact risk of relapse but is associated with increased TRM and GvHD: incidence of toxic death is more frequent in adults compared with in AYAs, and in AYAs compared with in children (3, 15, 26, 27). Thus, in the study of the Centre for International Blood and Marrow Transplant Research (CIBMTR) including patients transplanted for ALL between 2002 and 2007, 5-year TRM was 19% in children, 31% in AYAs, and 41% in older adults (15).
Reduced-intensity conditioning (RIC) regimens offer the advantage of decreased TRM but are often associated with a higher relapse rate (28). This option is being evaluated for patients over 45 years old in the Group for Research on Adult Acute Lymphoblastic Leukaemia (GRAALL) 2014 protocol. To date, there are no prospective studies comparing outcomes of patients with ALL who undergo myeloablative conditioning vs. RIC, but several large retrospective cohorts report the above-mentioned observations.
In a CIBMTR study, Marks et al. examined the role of the RIC regimens in adults over 35 years old transplanted in 1995–2006 for Ph-negative ALL. The age-adjusted OS, TRM and relapse rates were not statistically different between patients undergoing myeloablative conditioning vs. those undergoing RIC (29). In a European Group for Blood and Marrow Transplantation (EBMT) study, including patients over 45 years, RIC was associated with lower TRM and higher relapse rate than was myeloablative conditioning but this did not translate into a significant difference in OS (28). However, results of these retrospective studies should be interpreted with caution due to the heterogeneity of populations in terms of age at transplant, comorbidities, status at transplant, donor source, GvHD strategies, and RIC regimens used. In summary, although RIC could be a suitable alternative to myeloablative conditioning for older adults with ALL, there are no strong data to support a recommendation of this approach in the AYA population.
Total body irradiation (TBI) is widely used in myeloablative conditioning regimens for patients with ALL. Because TBI is associated with early and late adverse effects, transplant with TBI-free conditioning regimens has been evaluated in ALL patients. A small, randomised controlled trial found significantly higher EFS with TBI, etoposide and cyclophosphamide vs. busulfan, etoposide, and cyclophosphamide conditioning in paediatric ALL patients (30). Superiority of TBI over busulfan-based conditioning regimens has been also reported in retrospective studies both in children and in adults (31–34). Recently, the randomised, international, multicentre, Phase III For Omitting Radiation Under Majority age (FORUM) study investigated whether preparative combination chemotherapy could replace TBI in paediatric patients with ALL (35). The study randomised 417 patients aged 4–21 years at transplantation and in complete remission of ALL to myeloablative conditioning with either fractionated 12 Gy TBI and etoposide or with fludarabine, thiotepa and either busulfan or treosulfan. In the intention-to-treat population, 2-year OS was significantly higher following TBI (0.91; 95% confidence interval [CI], 0.86–0.95; p < 0.001) vs. chemo-conditioning (0.75; 95% CI, 0.67–0.81). A major difference was seen in the relapse rate, which was strongly decreased using TBI with a 2-year cumulative incidence of relapse of 0.12 (95% CI, 0.08–0.17) vs. 0.33 (95% CI, 0.25–0.40) following chemo-conditioning (p < 0.001). TRM was low in both arms, with a significant advantage for the TBI group: 2-year cumulative incidence of TRM was 0.02 (95% CI, 0.01–0.05) after TBI vs. 0.09 (95% CI, 0.05–0.14) after chemo-conditioning (P = 0.02). The superiority of TBI over chemotherapy regarding OS was observed both in patients aged 6–10 years and in patients aged 11–21 years (35).
Although the advantage of TBI has not been investigated or demonstrated specifically in an AYA population aged 15–29 years of age, the data above are in favour of TBI-based myeloablative conditioning for AYAs.
Donor Source
Peripheral Blood Stem Cells vs. Bone Marrow
In adults, the source of haematopoietic stem cells is mostly the peripheral blood (PBSC) in both MRD and MUD transplantation (36). Several prospective randomised studies comparing PBSC and bone marrow transplants following myeloablative conditioning have shown that PBSCs were associated with a decreased relapse rate in haematological malignancies and improved OS and DFS in patients with advanced-stage disease but not in those with early-stage disease. However, PBSC transplantation was also associated with a significant risk of extensive chronic GvHD (cGvHD) (37, 38).
In contrast, bone marrow remains the most common source used as an allograft in children with hematologic malignancies. Data regarding the association between HSCT outcome and stem-cell source in paediatric patients are limited and the role of PBSCs is debated. In a retrospective study on behalf of the EBMT Paediatric Diseases Working Party comparing HSCT outcomes either after bone marrow or PBSC allograft in children and adolescents <18 years transplanted for ALL in CR1 or CR2, the OS was significantly poorer after PBSC allograft compared with after bone marrow allograft due to a higher incidence of cGvHD and higher risk of NRM without improvement of relapse risk (38).
To date, it has been difficult to definitively conclude which is the best stem-cell source in AYAs transplanted for ALL but the studies cited above lead to a preference for bone marrow in most transplantations for early-stage disease (i.e., mainly in CR1).
Alternative Donors: Unrelated Cord Blood or Haploidentical Donor
Unmanipulated haploidentical HSCT, other haploidentical HSCT technics and unrelated cord blood (UCB) transplantation may be alternative options to treat patients with high-risk ALL who do not have an HLA-matched donor (39, 40). To date, UCB transplantation has been used mostly in children, while T-cell repleted haploidentical transplant with high-dose post-transplant cyclophosphamide (PTCy) for GvHD prophylaxis has been widely used in adults. The EBMT conducted a retrospective study comparing outcomes in ALL adults patients after transplantation with UCB (n = 370) or an unmanipulated haploidentical graft with PTCy (n = 158) (41). In the multivariate analysis, UCB transplantation was associated with a lower incidence of cGvHD (hazard ratio [HR], 0.58; P = 0.01 for ALL) vs. an unmanipulated haploidentical graft. No difference was observed for relapse incidence (HR, 0.82; P = 0.31 for ALL), NRM (HR, 1.23; P = 0.23 for ALL) and leukaemia-free survival (HR, 1.00; P = 0.84 for ALL) between groups (41). In 2016, Michel et al. reported a randomised prospective study comparing the results of HSCT from either one or two UCB units in 137 paediatric and AYA patients (<35 years) with either ALL or acute myeloid leukaemia (39). Two-year post-transplant survival, DFS and TRM were 68.8% (CI 95%, ± 6.0%), 67.6% (CI 95%, ± 6.0%), and 5.9% (CI 95%, ± 2.9%), respectively, after single-unit transplantation compared with 74.8% (CI 95%, ± 5.5%, 68.1% (CI 95%, ± 6.0%), and 11.6% (CI 95%, ± 3.9%), respectively after double-unit transplantation (P = not significant).
Studies comparing manipulated haploidentical stem cell grafts (T-cell depleted) with unmanipulated haploidentical grafts and/or UCB grafts have not been done in adults or children. To date, no data are available on which to base a recommendation regarding which one of these two donor types (haplo-identical donor or UCB) is preferable in AYAs with ALL.
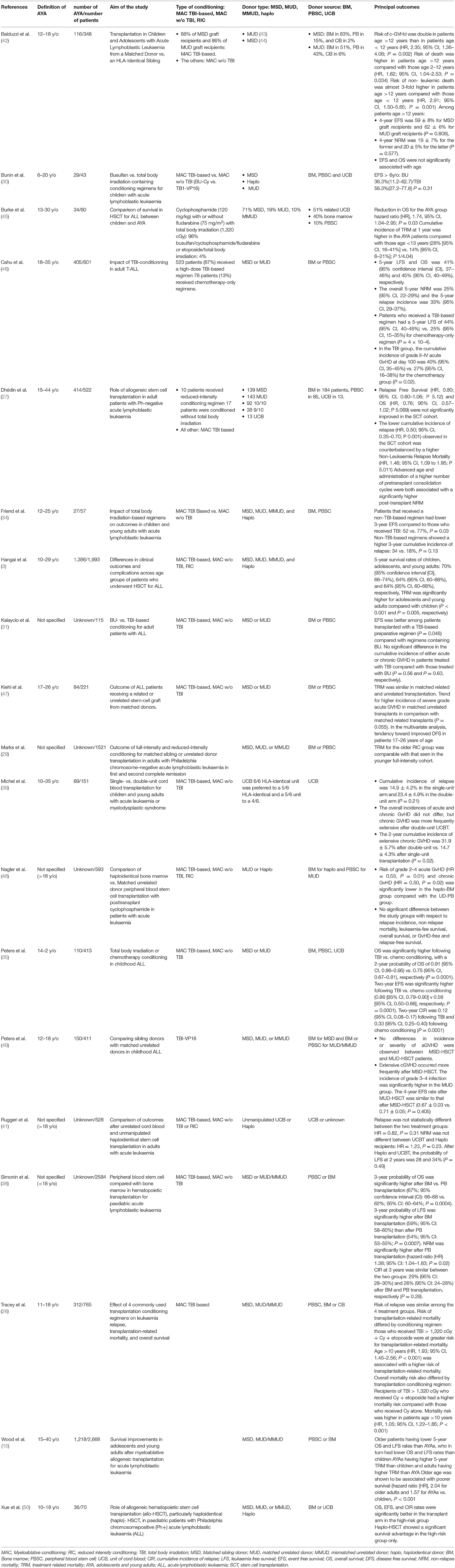
Table 2 summarises these results.
HSCT-Associated Complications and Supportive Care
Early toxicities associated with HSCT in AYAs remain a major issue. According to several studies, TRM in CR1 ranges from 10 to 30%, mostly due to GvHD and infection (12, 14–16, 45, 51).
A retrospective cohort study performed by the Japan Society for Hematopoietic Stem Cell Transplantation (JSHSCT) in 1,993 patients in Japan found a greater risk of NRM in AYAs with ALL after allogeneic HSCT (19%; 95% CI, 16–22%) compared to children (11%; 95% CI, 8.8–14%); p < 0.001 with infectious complications (24 vs. 4.2%, respectively) being the most common cause of death in AYAs (3). Similarly, Burke et al. identified significantly lower 5-year OS in the AYA group (n = 57; HR 1.74; 95% CI, 1.04–2.95; P = 0.03) after myeloablative allogeneic HSCT for ALL vs. the children group (n = 79). The inferior outcome was due to a 2-fold increase in TRM after 1 year (HR 2.23; 95% CI, 1.01–4.90; P = 0.05) in AYA patients compared to <13 years old patients, with rates being particularly high when bone marrow was used as graft source, while no age-related difference in relapse rate or acute GvHD was noted (45).
In a retrospective analysis of the CIBMTR, the outcome of ALL in children (n = 981), AYAs (n = 1,218), and older adults (n = 469) after myeloablative conditioning and allogeneic HSCT over almost two decades in paediatric and adult transplant centres was compared. The researchers noted parallel survival improvements in AYAs and children over time, yet survival remained inversely correlated with age (HR of 2.04, 95% CI 1.75–2.39, for older adults and 1.57, 95% CI 1.40–1.77, for AYA compared with children; p < 0.001). Again, TRM following both MSD or MUD transplantations was higher in AYAs compared with in children (HR, 1.66; 95% CI, 1.42–1.95; p < 0.001). The cause of this age-dependent increase in TRM remains unclear; it was speculated that disease- and age-related biology may play a role. Time-dependent effects seen during an observation period of 27 years were attributed to improved supportive care leading to a lower rate of early TRM over time (15).
Based on these findings, the reduction of TRM and use of attentive supportive care appear critical for successful HSCT in AYAs. Optimised peri-transplantation care with diligent infectious disease work-up and monitoring as well as infectious prophylaxis appear mandatory, particularly in this vulnerable age group.
Special attention should be paid also to immunosuppressive treatment after HSCT in these patients to reduce toxicity and infectious complications. Data in adult patients undergoing MSD HSCT, for example, suggest that the combination of mycophenolate mofetil and cyclosporine A is less toxic but similarly effective to methotrexate and cyclosporine A (52).
Both the Berlin-Frankfurt-Münster (BFM) 2002 and International BFM 2007 HSCT studies in patients with ALL <21 years old demonstrated the safety of less-intensive GvHD prophylaxis with Cyclosporine-A alone for patients transplanted from an MSD with bone marrow as the stem cell source, whereas Cyclosporine-A plus short-duration methotrexate remains the gold standard for GvHD prophylaxis in the adult HSCT setting regardless of donor type (42, 49).
Obesity has been identified as a risk factor for an adverse outcome in AYAs treated for ALL, with inferior DFS observed when BMI ≥ 30 kg/m2 (HR, 1.97; 95% CI, 1.51–2.57; p < 0.001) (13). Both relapse rate and NRM appear to be higher in obese AYAs—a fact that suggests an influence of metabolic parameters and altered pharmacokinetics on outcome (13). Obesity in adults undergoing allogeneic HSCT for hematologic malignancies has not been found to be a deleterious effect on OS or relapse, while some studies have shown increased NRM in adult obese patients (13). Thus, the negative effect of obesity on OS and relapse might be specific to the AYA population. underlining again the specific needs of this group of patients.
Treatment intensity and cumulative toxicity burden before the HSCT procedure must be considered by clinicians as it impacts on overall results. Therefore, the development of novel treatment strategies with fewer toxic side effects than standard chemotherapy may improve outcomes for AYAs with ALL in the future.
Late Effects After Oncological Treatment and Allogeneic HSCT in AYAs
Given that there are very few studies focusing on allogeneic HSCT in AYAs and intensive treatment is usually required to achieve remission, the late effects after HSCT in this age group remain partially obscure. A retrospective Childhood Cancer Survivor Study comparing 10,397 survivors with 3,034 siblings revealed a cumulative incidence of chronic health conditions of up to 73.4% in adults 30 years after their cancer diagnosis (53). Compared to other childhood cancer survivors, those who received an MSD or MUD bone marrow transplant for haematologic malignancies demonstrated a significantly elevated risk of poor general health (relative risk [RR], 3.2 and 2.0, respectively; p < 0.01), functional impairment (RR, 7.8 and 8.4, respectively; p < 0.01) and activity limitations (RR, 5.9 and 10.1, respectively; p < 0.01) (54). With improvements in survival over the years and increasing numbers of younger survivors, this disparity becomes progressively more relevant.
In a retrospective study published by Burke et al. comparing outcomes after HSCT in children vs. AYA B-ALL patients, no correlation between the risk of acute GvHD and age was noted (45). Nevertheless, a non-significant trend toward a higher RR of cGvHD in AYA vs. in children was found in a multivariate analysis (RR, 2.73; 95% CI, 0.93–7.96; P = 0.07) (45). Similarly, Hangai et al. compared outcomes after HSCT for ALL among children (age 1–9 years; n = 607), adolescents (age 10–19 years; n = 783), and young adults (age 20–29 years old, n = 603) retrospectively (3). They found significant age-dependent differences in the 1-year incidence rate of cGvHD following HSCT in children (24%; 95% CI, 21–27%) adolescents (28%; 95% CI, 24–31%) and young adults (32%; 95% CI, 29–36%; P < 0.001), but not in the rate of aGvHD (3).
The potentially heightened risk of cGvHD in AYAs is of utmost importance, as cGvHD is generally associated with considerable mortality and morbidity resulting in a significantly poorer quality of life and functional impairment. This poses a special challenge in AYA patients, who are in a critical phase of development, and raises physical, psychological, and social challenges that need particular attention. Vigorous screening for risk factors and regular function testing after allogeneic HSCT are required to detect such chronic health issues early (44, 55).
This pre-emptive approach also applies to other late sequelae observed in AYA patients treated with allogeneic HSCT, similarly to the approach for late toxicities seen in the oncology patient population; it involves equivalent follow-up and screening programmes as those suggested by the National Comprehensive Cancer Network. Studies on long-term outcome in paediatric patients and AYAs treated with chemotherapy and radiation have revealed an increased incidence of secondary malignancies of 4–17% 10 years after allogeneic HSCT for their primary malignancy (53, 56, 57). As these treatment modalities are part of the conditioning regimen, close monitoring for occurrence of secondary malignancies is mandatory during long-term follow-up.
Besides haematologic diseases such as myelodysplastic syndrome and secondary leukaemia (cumulative incidence of 3.8 at 6 years), solid tumours (cumulative risk of 11% after 15 years) are more prevalent after allogeneic HSCT, in children than in adults (57–59). Therefore, screening for cutaneous malignancies (cumulative incidence of 3.4–6.5% at 20 years) as well as lung, breast (HR of 10.8 10 years after HSCT, compared to United States Surveillance Epidemiology End Results (SEER) for expected rates) and thyroid cancer (RR of 4.8 in AYAs, compared to general population) is mandatory during long-term follow-up after HSCT. An even higher incidence of solid cancer is observed in patients who received TBI-based conditioning vs. those who received chemotherapy based-conditioning (56, 60, 61). The cumulative incidence of post-transplant lymphoproliferative disorder at 10 years after allogeneic HSCT is 1% and varies depending on risk factors such as a mismatched donor, T-cell depletion, GvHD and irradiation (62).
Endocrine effects are another special consideration after allogeneic HSCT in AYA, particularly when TBI is part of the conditioning regimen. An increased rate of thyroid and gonadal dysfunction with >90% risk of infertility, growth impairment and skeletal complications occur after chemotherapy and irradiation (63–65). Therefore, preventive measures and timely replacement therapy are important.
In addition, regular testing of pulmonary, renal, and cardiovascular parameters is advisable as these organs may exhibit chronic dysfunction after allogeneic HSCT detectable as abnormal pulmonary function test results, renal insufficiency, hypertension, abnormal electrocardiogram readings, and impaired cardiac function. Although these adverse effects are not exclusively observed in AYAs as a consequence of chemotherapy or irradiation therapy, early detection is crucial in order to mitigate disabilities (54).
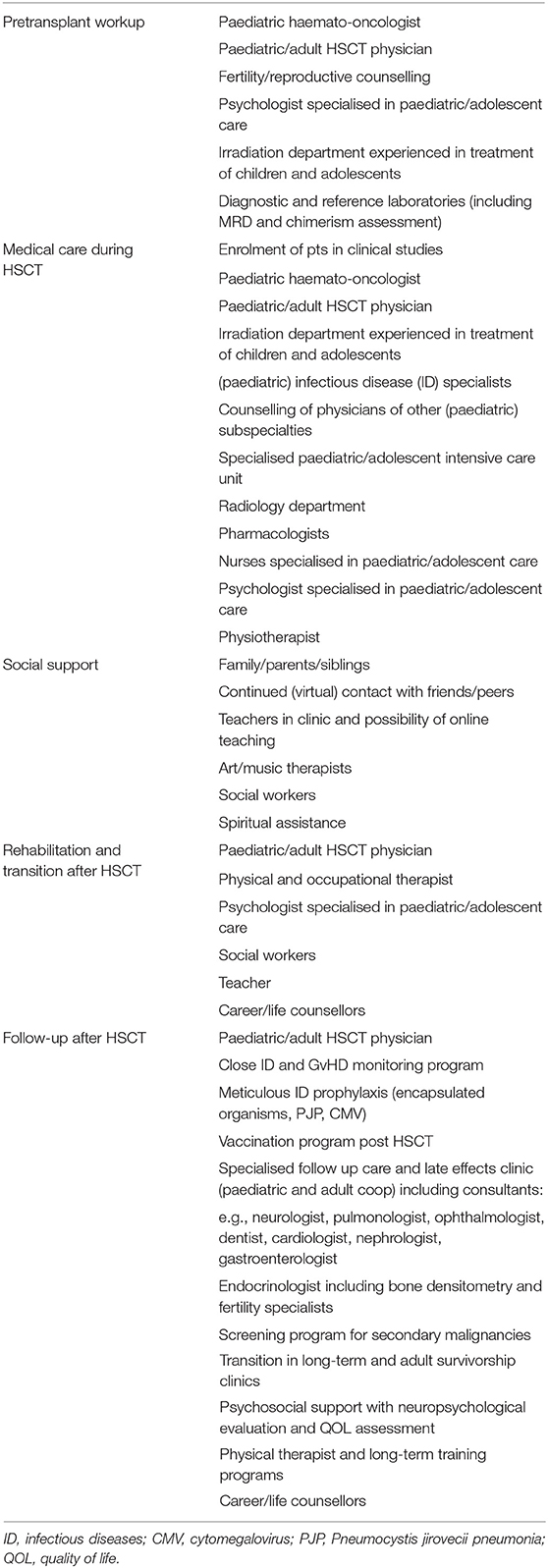
Table 3 propose recommendations for follow-up examination after allegeneic HSCT in AYA patients in the context of ALL treatment [adapted from (55)].
Social and Psychological Issues
A difficulty of treating AYA patients is that the cancer diagnosis and treatment occur in a critical stage of life. Treatment side effects such as weight changes, hair loss, and growth disturbances may be more difficult to cope with for patients in this age group than for younger or older patients. Adolescence and young adulthood is a period of developmental processes and psychosocial and hormonal challenges, where major aspects of life and future plans (developmental tasks of emerging adulthood) become more important (66).
The attainment of social, financial, and physical independence are three major aspects in the transition to adulthood—all of which can be affected by anti-cancer treatment including HSCT. This may hinder successful transition to adulthood and compromise individuals' long-term quality of life (67, 68).
AYAs with cancer are generally more dependent on family than are other AYAs, which makes it difficult to “cut the cord” from the parents (69, 70). They can have difficulties in continuing their education and occupation, with alterations needed to educational or career plans (71).
The feeling of social and medically imposed isolation due to absence from school and separation from friends can lead to loneliness, developmental discrepancy, and social disruption (67). The loss of friendships during therapy aggravates patients' reintegration into “normal life” (43).
This disruption of everyday life and patients' confrontation with their own mortality can bring fear, distress, and uncertainty (66, 71). Several studies have shown higher levels of depression, anxiety and distress in AYA patients with oncological disease compared with healthy peers (66, 72).
Non-compliance to treatments in this age group is a major problem (73). It is important to help AYAs to continue to live as normally as possible, have as much information as they need, and to be involved in treatment decisions and their own care, in order to support autonomy and to promote a trustful relationship (74, 75). Clinicians must consider that each patient is at a unique developmental point and, therefore, their needs differ (76).
During adolescence and young adulthood, intimate emotional, and sexual relationships are often formed, which can be complicated by a cancer diagnosis and treatment due to altered body image, social isolation, and the fear of rejection. Patients may consider themselves as unattractive and undesirable leading to decreased libido (77). Furthermore, the fear of infertility can have a negative influence on intimacy (75). Early management of sexual dysfunction can improve the situation and patients' quality of life.
Clinicians should consider all of the aspects above when treating AYAs, although there are no specific recommendations for those with ALL undergoing HSCT. In general, only a specialised, multidisciplinary team of health professionals (including specialised physicians, nurses, psychologists, and social workers) who can provide age-appropriate information and address the fears of this age group should treat AYA patients (78, 79).
Various evidence-based psychosocial interventions exist to reduce distress and help patients to cope with exceptional circumstances. Multidisciplinary programmes include peer-to-peer support to encourage relationships and skill-based and technology-based interventions tailored to the unique needs of AYAs facing cancer and HSCT (75, 79). Various studies emphasise that securing adequate social support is the most important coping strategy and resource for AYAs when facing cancer (74).
Moreover, to achieve the best outcomes, treatment should be administered in specialised centres with the highest expertise and the opportunity to enrol patients in clinical trials (73, 80).
Fertility Issues
As described above, compromised reproductive function is a major and severe late complication in cancer survivors, which can lead to psychosocial distress and depression and has a significant influence on quality of life (1, 81–84).
Studies have shown that infertility caused by cytostatic drugs is dependent on the dosage and type of drug given and also on the patient's age at treatment (85–87).
Younger age (<10 years old) at the time of exposure to cytostatic drugs or radiotherapy is associated with a lower risk of premature ovarian insufficiency (POI) (87, 88).
The risk of infertility is high in patients receiving HSCT following a conditioning regimen of TBI, high-dose cyclophosphamide, melphalan, and busulfan (81, 82, 85). More than two thirds of patients who receive allogeneic HSCT develop gonadal dysfunction (85). In transplanted patients, the detrimental effects of HSCT and of previously received frontline chemotherapy play a synergistic role (87).
Although long-term recovery of gonadal dysfunction after HSCT has been reported, female patients who receive TBI have a high risk of a later-onset POI (87, 89). The ovarian damage is irreversible in most cases (90). Female patients who received TBI or high-dose cyclophosphamide prior to HSCT have a much higher miscarriage rate and increased risks of preterm delivery and delivery of low-birth-weight infants (84, 91, 92). Chemotherapy and/or TBI as conditioning typically lead to disruption of the hypothalamic-pituitary-gonadal axis and direct gonadal damage (77, 93).
There are a few studies showing a recovery of spermatogenesis after TBI prior to HSCT (86, 93). Nevertheless, TBI plays a central role in infertility in male as well as female patients.
Considering this high risk of infertility and the impact it might have on a patient's life, comprehensive age-appropriate counselling about the risks, options for fertility preservation and why certain interventions cannot be performed in some situations (for example, coelioscopy or transvaginal puncture in cases of severe neutropenia or thrombopenia) is absolutely essential to reduce distress and is recommended in several guidelines (81, 82, 93–95).
Numerous cancer survivors report a lack of information and the fear of infertility often leads to maladaptive coping strategies (94, 96). Therefore, ALL patients should receive proactive counselling even though their options for fertility preservation (especially for pre-pubertal or female patients) are quite limited, patients are frequently acutely ill at diagnosis, and the severity of the disease may not permit a delay of anti-cancer therapy.
Frontline therapy for ALL usually does not cause permanent gonadal dysfunction (93, 97). The crux of treating patients with ALL is that the indication for HSCT is not clear at diagnosis in most patients; therefore, patients have been treated already with several cytostatic drugs before fertility protective procedures can be performed. In post-pubertal boys, sperm cryopreservation is an effective method for fertility preservation, which can easily be organised in most cases (81). It should ideally be implemented before the start of cancer treatment. Emotional stress, azoospermia, or decreased sperm mobility are some of the reasons why sperm banking is unsuccessful (84). It is common that male patients with an oncological diagnosis have low sperm counts even before starting cytostatic treatment (93). For post-pubertal girls, fertility preservation options are very limited. The two options are embryo or oocyte cryopreservation for which hormonal hyperstimulation is necessary. As this causes a delay of cancer treatment of ~2 weeks, it is often not feasible in acutely ill patients. High oestrogen levels need to be avoided due to their potential side effects in cancer patients. In addition, invasive procedures are associated with a higher rate of complications, mostly due to infections and bleeding in pancytopenic patients (93). Ovarian tissue or ovarian cortex cryopreservation are still experimental in ALL or AML because low levels of leukaemic cells have been found in the ovarian tissue of mice and humans in studies, which might possibly increase the relapse risk after re-transplantation of the tissue (77, 93, 98, 99). Whether these methods will be available in the future after testing for MRD remains to be determined (93). In pre-pubertal patients, gonadal tissue conservation is the only recommended option. However, due to the high relapse risk after re-transplantation, there are currently no recommendations in clinical guidelines for this technique in patients with ALL (82).
Another difficulty in standardised, international recommendations for all patients undergoing HSCT for ALL is the significantly different access to fertility protecting procedures between countries and the financial coverage through different healthcare systems. Financial coverage should be mandatory for fertility protection in treatment-associated infertility. Many AYA patients with cancer describe a high financial burden. Affording fertility preservation procedures for future family planning often causes massive emotional distress (93). The costs of preservation, banking and of further processing when pregnancy is desired are often obstacles for cancer survivors (77).
Immunotherapeutic Concepts to Reduce Toxicity and Improve Outcomes in AYA Patients
Wood et al. noted a higher rate of late relapse (>12 months from HSCT) in AYAs who received HSCT for ALL vs. the relapse rate in children (HR, 2.1, 95%CI 1.59–2.75; p < 0.001); this increased risk did not significantly change with time in long-term studies. This increased risk of late relapse may be associated with the unfavourable disease biology observed in AYA and adult patients and raises the question of how to optimise pre-transplantation therapy, immunosuppressive strategies and consolidation treatment in certain patient groups (15).
With the advent of targeted immunotherapies such as the bispecific anti-CD3/anti-CD19 T-cell engager blinatumomab, the toxin-conjugated anti-CD22 antibody inotuzumab ozogamicin, and CAR T-cell therapies, novel strategies have emerged to: (1) induce deeper remissions prior to HSCT; (2) substitute toxic chemotherapy elements in vulnerable patient groups; and (3) substitute the entire HSCT procedure by use of long-lasting CAR T-cell products. The companion papers in this supplement by Krauss et al. and Buechner with colleagues discuss these topics in detail for the paediatric ALL population. No studies with any of the abovementioned drugs have been performed solely in an AYA cohort; however, information regarding AYAs can be retrieved from combined paediatric/AYA studies and isolated adult studies.
Blinatumomab
Blinatumomab is approved in both adults and children for several indications in B-cell precursor (BCP)-ALL patients. Common to both groups is the approval in patients with relapsed (second or higher relapse) or refractory (R/R) CD19+ Ph-negative BCP-ALL. Additionally, adults with Ph-positive ALL who have failed treatment with at least two TKIs and adults with Ph-negative ALL in CR1 or CR2 with persistent MRD >0.1% have an approved indication for blinatumomab. In children, blinatumomab has additional approval for first high-risk relapse as part of consolidation therapy. It is currently being investigated by several collaborative study groups as part of paediatric ALL frontline protocols.
In a systematic meta-analysis including 628 R/R ALL patients from six clinical trials, response rates to blinatumomab did not significantly differ between paediatric and adult patients (100). The multinational, randomised, Phase 3 TOWER study (ClinicalTrials.gov identifier: NCT02013167) examined the outcomes of 405 adults with Ph-negative R/R BCP-ALL (age range 18–80 years) randomised to either standard-of-care chemotherapy or blinatumomab (24). Blinatumomab induced deep remission in responding patients, which was associated with a significantly longer OS (median OS 7.7 months, 95% CI 5.6–9.6 in blinatumomab group vs. 4.0 months,95% CI 2.9–5.3 in the chemotherapy group; HR for death, 0.71; 95% CI 0.55–0.93; P = 0.01). The trial was prematurely stopped due to a significant OS benefit in the blinatumomab arm regardless of subsequent HSCT. In the pivotal Phase 2 ALCANTARA study (NCT02000427) of adult patients (n = 45, range 23–78 years) with Ph-positive BCP-ALL who were intolerant to TKIs, response to blinatumomab was observed in 36% of patients, with 86% of the responders being MRD negative (101). In the Phase 2 BLAST study (NCT01207388, N = 116 patients range, 18–76 years), 78% of adults with BCP-ALL in CR but with persistent MRD (≥10−3) achieved complete MRD response within one blinatumomab treatment cycle (102).
Very similar results were obtained in children age <18 years with R/R BCP-ALL in a pivotal Phase II trial (NCT01471782) (103) and the blinatumomab expanded-access RIALTO trial (ClinicalTrials.gov: NCT02187354) (104), including in toxicity-prone patients with Down syndrome (104). In two recent studies in children with a first high-risk relapse of BCP-ALL, the superiority of blinatumomab compared to standard chemotherapy was impressively demonstrated; both trials stopped prematurely due to significantly better outcomes in the blinatumomab arm, including the fraction of patients eligible for subsequent HSCT and a lower rate of severe toxicity (105, 106).
Taking these findings together, blinatumomab induces MRD-negative responses in a substantial fraction of patients with MRD persistence or R/R disease, both in children and adults. Although distinct toxicities related to blinatumomab occur (immune-effector-cell–associated neurotoxicity syndrome and cytokine-release syndrome), blinatumomab is generally (and compared to alternative intensive chemotherapy) well-tolerated, with fewer serious adverse events compared to intensified chemotherapy. There are no data indicating that the AYA group would respond differently. Blinatumomab may be an alternative or supplement to current chemotherapy approaches to reduce toxicity before HSCT in high-risk AYA patients.
Inotuzumab Ozogamicin
In the Phase 3 INO-VATE trial (NCT01564784), adult patients (age range 18–78 years) with R/R CD22+ BCP-ALL were randomised to either receive inotuzumab ozogamicin or standard-of-care chemotherapy (23). Patients treated in the inotuzumab ozogamicin arm had a significantly higher response rate (CR, 80.7 vs. 29.4%, respectively; p < 0.001), higher rate of MRD-negative remission (78.4 vs. 28.1%, respectively; p < 0.001) and were more likely to proceed to HSCT directly after their treatment course (48 vs. 32%, respectively; P = 0.12) than were patients in the chemotherapy arm. Non-haematological toxicities were mainly related to the liver, with veno-occlusive disease occurring in 15% of patients; the vast majority (90%) in patients proceeding to HSCT.
Inotuzumab ozogamicin has been used sporadically in children with R/R BCP-ALL in compassionate-use programs, as recently published (107, 108). CR was achieved in 67% of patients, with the majority (71%) of responders being MRD negative. Of note, responses were observed irrespective of cytogenetic subtype or number or type of prior treatment regimens. However, 52% of the patients who proceeded to HSCT post inotuzumab ozogamicin developed veno-occlusive disease. The efficacy and safety of inotuzumab ozogamicin as monotherapy or in combination with chemotherapy is currently being systematically investigated in children in a Phase I/II study (ITCC-059; EudraCT Number: 2016-000227-71) (109).
Similarly to the pattern observed with blinatumomab, the efficacy and toxicity profile of inotuzumab ozogamicin in adults and children are similar and do not indicate that AYAs would have distinct outcomes or profiles. However, veno-occlusive disease is one of the major complications seen after inotuzumab ozogamicin use and needs to be monitored closely and considered early in AYA patients, especially in the context of TBI-containing conditioning as used in ALL.
Chimeric Antigen Receptor T-Cell Therapy
Most published studies investigating CAR T-cell therapy in BCP-ALL included patients up to the age of 25 years, or older patients. The companion paper by Buechner and colleagues in this supplement discusses CAR T-cell trials in detail. The pivotal ELIANA Phase II trial (NCT02435849) (25), which led to the approval by the Food and Drug Administration and European Medicines Agency of tisagenlecleucel as the currently only CD19 CAR T-cell therapy for R/R BCP-ALL, enrolled 75 patients in the age range 3–23 years (25). Remission rate by month 3 after infusion did not differ across age groups and was 81% (overall remission rate) for the entire cohort. In a combined analysis of two similar tisagenlecleucel trials (ENSIGN [NCT02228096] and ELIANA) focusing specifically on AYA patients aged 18–25 years old (n = 20), rates of adverse events were comparable to younger age cohorts and did not indicate that AYA patients are at higher risk of adverse events after a single infusion of tisagenlecleucel (110). A recent meta-analysis on CAR T-cell therapy in ALL did not find significant differences in outcomes when paediatric and adult data were compared (111). Indeed, the current approval of tisagenlecleucel includes AYA ≼ 25 years of age with either a second or higher relapse, a relapse post HSCT or who have a refractory BCP-ALL.
Beside its efficacy in inducing remissions across all age groups, all cytogenetic risk groups and in patients with previous HSCT, tisagenlecleucel may also have the potential to induce sustained remissions without consolidative HSCT because it can persist for months and years in patients and thereby provide disease control, at least against CD19+ disease. Toxicities in AYAs are well-documented (110). Still, long-term data, both on outcomes and toxicities, are sparse and further focus is needed on the AYA group. Moreover, antigen loss or lineage switch (in patients with KMT2A-rearrangements) are currently intrinsic limitations of targeted immunotherapies (see the review by Buechner et al. in this Frontiers supplement).
Discussion and Conclusion
The data reviewed here reveal the special and dedicated attention that AYA patients require. Precisely defining the indication for allogeneic HSCT in this age group is fundamental, particularly because ALL in AYA patients is often associated with high-risk genetic abnormalities and refractory disease. Therefore, currently the percentage of AYA patients requiring HSCT in CR1 remains higher than that in paediatric patients <15 years old.
Careful monitoring and management of early toxicities associated with intensive chemotherapy and subsequent allogeneic HSCT is fundamental. AYA patients appear especially prone to developing fatal infectious complications. Reduced compliance with infectious prophylaxis regimens and neglect in reporting the clinical signs of infections in this age group may contribute to the dismal outcome and should be considered and addressed with the patient while on immunosuppressive treatment and during regular follow-up visits. In addition, late effects on functional impairment also remain major issues in this cohort. In fact, given the higher incidence of secondary malignancies and organ dysfunctions seen after irradiation, careful and systematic follow-up of these patients should be provided (Table 3).
For most patients with malignant haematological diseases, there is neither an optimal timing nor method for fertility preservation. All patients should be proactively counselled about their infertility risk and possible fertility protective options both before and after HSCT. A referral to reproductive specialists after HSCT is strongly recommended, especially if no fertility protection was performed before the start of therapy. All interventions must be provided with careful psychological support to try to avoid depressive crisis and feelings of loneliness with a dramatic loss of social links.
The development of less-toxic transplantation modalities associated with novel treatment strategies before HSCT associated with fewer adverse effects should be thoroughly investigated in the future. Indeed, the severe adverse events that characterise the treatment of high-risk ALL in this fragile cohort of patients might be mitigated by the introduction of new immunotherapies. Both inotuzumab ozogamicin and blinatumomab are promising drugs to induce MRD negativity in patients with chemo-refractory BCP-ALL clones and may help to put AYA patients into “transplantable” deep remissions with less toxicity than conventional chemotherapy.
Given the severity of acute and chronic adverse events and long-term physical and psychological sequelae detected in AYA patients, dedicated prospective, and comparative studies are urgently needed. The increasing accessibility of new immunotherapeutic approaches allows evaluation of their significance in the treatment of AYA patients. Of particular interest will be the question of whether these agents are able to induce long and stable CRs without HSCT, as is currently being investigated in the CASSIOPEIA trial (NCT03876769) of CAR T-cell therapy for patients aged 1–25 years with high-risk de novo BCP-ALL, with the goal to see whether HSCT can be substituted.
In conclusion, in light of their unique needs, we strongly recommend that AYA patients receive treatment in dedicated centres with multidisciplinary expert teams. Such multidisciplinary approaches require different specialised physicians working beside one another, including the haematologist, stem transplantation expert, psychologists, physiotherapists and social workers familiar with the requirements of AYAs as outlined in Table 4 [adapted from (47)].
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study received funding from the St. Anna Children's Cancer Research Institute, Vienna, Austria. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of Interest
JB has participated in advisory boards organised by Novartis, Janssen and Gilead, and has received consulting/speaker's bureau fees from Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared consortium with the authors at time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Editorial support in the preparation of this manuscript was provided by Hannah Bridges of HB Health Comms Limited.
References
1. Mehta PA, Rotz SJ, Majhail NS. Unique challenges of hematopoietic cell transplantation in adolescent and young adults with hematologic malignancies. Biol Blood Marrow Transplant. (2018) 24:e11–9. doi: 10.1016/j.bbmt.2018.09.011
2. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. (2018) 132:351–61. doi: 10.1182/blood-2018-02-778530
3. Hangai M, Urayama KY, Tanaka J, Kato K, Nishiwaki S, Koh K, et al. Allogeneic stem cell transplantation for acute lymphoblastic leukemia in adolescents and young adults. Biol Blood Marrow Transplant. (2019) 25:1597–602. doi: 10.1016/j.bbmt.2019.04.014
4. Roberts KG. Genetics and prognosis of ALL in children vs adults. Hematol Am Soc Hematol Educ Program. (2018) 2018:137–45. doi: 10.1182/asheducation-2018.1.137
5. Siegel SE, Stock W, Johnson RH, Advani A, Muffly L, Douer D, et al. Pediatric-Inspired treatment regimens for adolescents and young adults with philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol. (2018) 4:725–34. doi: 10.1001/jamaoncol.2017.5305
6. Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. (2015) 12:344–57. doi: 10.1038/nrclinonc.2015.38
7. Boissel N, Auclerc M-F, Lhéritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. (2003) 21:774–80. doi: 10.1200/JCO.2003.02.053
8. Tran TH, Loh ML. Ph-like acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. (2016) 2016:561–6. doi: 10.1182/asheducation-2016.1.561
9. Gupta S, Pole JD, Baxter NN, Sutradhar R, Lau C, Nagamuthu C, et al. The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs adult centers: an IMPACT Cohort study. Cancer Med. (2019) 8:2095–103. doi: 10.1002/cam4.2096
10. Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. (2019) 133:1548–59. doi: 10.1182/blood-2018-10-881961
11. Carobolante F, Chiaretti S, Skert C, Bassan R. Practical guidance for the management of acute lymphoblastic leukemia in the adolescent and young adult population. Ther Adv Hematol. (2020) 11:2040620720903531. doi: 10.1177/2040620720903531
12. Toft N, Birgens H, Abrahamsson J, Griškevičius L, Hallböök H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. (2018) 32:606–15. doi: 10.1038/leu.2017.265
13. Wieduwilt MJ, Stock W, Advani A, Luger S, Larson RA, Tallman M, et al. Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia. (2021) 35:2076–85. doi: 10.1038/s41375-021-01213-5
14. Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. (2009) 27:911–8. doi: 10.1200/JCO.2008.18.6916
15. Wood WA, Lee SJ, Brazauskas R, Wang Z, Aljurf MD, Ballen KK, et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2014) 20:829–36. doi: 10.1016/j.bbmt.2014.02.021
16. Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. (2008) 111:1827–33. doi: 10.1182/blood-2007-10-116582
17. Millot F, Guilhot J, Baruchel A, Petit A, Leblanc T, Bertrand Y, et al. Growth deceleration in children treated with imatinib for chronic myeloid leukaemia. Eur J Cancer Oxf Engl. (2014) 50:3206–11. doi: 10.1016/j.ejca.2014.10.007
18. Aricò M, Schrappe M, Hunger SP, Carroll WL, Conter V, Galimberti S, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. (2010) 28:4755–61. doi: 10.1200/JCO.2010.30.1325
19. Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi X, Pulsipher MA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with philadelphia chromosome-positive acute lymphoblastic Leukemia: results of children's Oncology Group Trial AALL0622. J Clin Oncol. (2018) 36:2306–14. doi: 10.1200/JCO.2017.76.7228
20. Rajendra A, Jain H, Bonda VNA, Nayak L, Tembhare P, Shetty D, et al. Outcomes and prognostic factors in adolescents and young adults with ALL treated with a modified BFM-90 protocol. Blood Adv. (2021) 5:1178–93. doi: 10.1182/bloodadvances.2020003526
21. Saadeh SS, Litzow MR. Hematopoietic stem cell transplant in adults with acute lymphoblastic leukemia: the present state. Expert Rev Hematol. (2018) 11:195–207. doi: 10.1080/17474086.2018.1433030
22. Muffly L, Li Q, Alvarez E, Kahn J, Winestone L, Cress R, et al. Hematopoietic cell transplantation in young adult acute lymphoblastic leukemia: a United States population-level analysis. J Adolesc Young Adult Oncol. (2019) 8:254–61. doi: 10.1089/jayao.2018.0140
23. Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. (2016) 375:740–53. doi: 10.1056/NEJMoa1509277
24. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. (2017) 376:836–47. doi: 10.1056/NEJMoa1609783
25. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
26. Tracey J, Zhang M-J, Thiel E, Sobocinski KA, Eapen M. Transplantation conditioning regimens and outcomes after allogeneic hematopoietic cell transplantation in children and adolescents with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2013) 19:255–9. doi: 10.1016/j.bbmt.2012.09.019
27. Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. (2015) 125:2486–96; quiz 2586. doi: 10.1182/blood-2014-09-599894
28. Mohty M, Labopin M, Volin L, Gratwohl A, Socié G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. (2010) 116:4439–43. doi: 10.1182/blood-2010-02-266551
29. Marks DI, Wang T, Pérez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. (2010) 116:366–74. doi: 10.1182/blood-2010-01-264077
30. Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. (2003) 32:543–8. doi: 10.1038/sj.bmt.1704198
31. Kalaycio M, Bolwell B, Rybicki L, Absi A, Andresen S, Pohlman B, et al. BU- vs TBI-based conditioning for adult patients with ALL. Bone Marrow Transplant. (2011) 46:1413–7. doi: 10.1038/bmt.2010.314
32. Granados E, de La Cámara R, Madero L, Díaz MA, Martín-Regueira P, Steegmann JL, et al. Hematopoietic cell transplantation in acute lymphoblastic leukemia: better long term event-free survival with conditioning regimens containing total body irradiation. Haematologica. (2000) 85:1060–7.
33. Gupta T, Kannan S, Dantkale V, Laskar S. Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. (2011) 4:17–29. doi: 10.5144/1658-3876.2011.17
34. Friend BD, Bailey-Olson M, Melton A, Shimano KA, Kharbanda S, Higham C, et al. The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. (2020) 67:e28079. doi: 10.1002/pbc.28079
35. Peters C, Dalle J-H, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority Phase III study. J Clin Oncol. (2021) 39:295–307. doi: 10.1200/JCO.20.02529
36. Giebel S, Boumendil A, Labopin M, Seesaghur A, Baron F, Ciceri F, et al. Trends in the use of hematopoietic stem cell transplantation for adults with acute lymphoblastic leukemia in Europe: a report from the acute leukemia working party of the European Society for Blood and Marrow Transplantation (EBMT). Ann Hematol. (2019) 98:2389–98. doi: 10.1007/s00277-019-03771-2
37. Stem Cell Trialists' Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. (2005) 23:5074–87. doi: 10.1200/JCO.2005.09.020
38. Simonin M, Dalissier A, Labopin M, Willasch A, Zecca M, Mouhab A, et al. More chronic GvHD and non-relapse mortality after peripheral blood stem cell compared with bone marrow in hematopoietic transplantation for paediatric acute lymphoblastic leukemia: a retrospective study on behalf of the EBMT Paediatric Diseases Working Party. Bone Marrow Transplant. (2017) 52:1071–3. doi: 10.1038/bmt.2017.66
39. Michel G, Galambrun C, Sirvent A, Pochon C, Bruno B, Jubert C, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. (2016) 127:3450–7. doi: 10.1182/blood-2016-01-694349
40. Nagler A, Labopin M, Dholaria B, Angelucci E, Afanasyev B, Cornelissen JJ, et al. Comparison of haploidentical bone marrow versus matched unrelated donor peripheral blood stem cell transplantation with posttransplant cyclophosphamide in patients with acute leukemia. Clin Cancer Res. (2021) 27:843–51. doi: 10.1158/1078-0432.CCR-20-2809
41. Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. (2015) 29:1891–900. doi: 10.1038/leu.2015.98
42. Balduzzi A, Dalle J-H, Wachowiak J, Yaniv I, Yesilipek A, Sedlacek P, et al. Transplantation in children and adolescents with acute lymphoblastic leukemia from a matched donor versus an HLA-identical sibling: is the outcome comparable? Results from the international BFM ALL SCT 2007 study. Biol Blood Marrow Transplant. (2019) 25:2197–210. doi: 10.1016/j.bbmt.2019.07.011
43. Andrés-Jensen L, Larsen HB, Johansen C, Frandsen TL, Schmiegelow K, Wahlberg A. Everyday life challenges among adolescent and young adult survivors of childhood acute lymphoblastic leukemia: an in-depth qualitative study. Psychooncology. (2020) 29:1630–7. doi: 10.1002/pon.5480
44. Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. (2006) 12:138–51. doi: 10.1016/j.bbmt.2005.09.012
45. Burke MJ, Gossai N, Wagner JE, Smith AR, Bachanova V, Cao Q, et al. Survival differences between adolescents/young adults and children with B precursor acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2013) 19:138–42. doi: 10.1016/j.bbmt.2012.08.020
46. Cahu X, Labopin M, Giebel S, Aljurf M, Kyrcz-Krzemien S, Socié G, et al. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. (2016) 51:351–7. doi: 10.1038/bmt.2015.278
47. Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. (2004) 22:2816–25. doi: 10.1200/JCO.2004.07.130
48. Nagler A, Baron F, Labopin M, Polge E, Esteve J, Bazarbachi A, et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant. (2021) 56:218–24. doi: 10.1038/s41409-020-01005-y
49. Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol. (2015) 33:1265–74. doi: 10.1200/JCO.2014.58.9747
50. Xue Y-J, Cheng Y-F, Lu A-D, Wang Y, Zuo Y-X, Yan C-H, et al. Allogeneic hematopoietic stem cell transplantation, especially haploidentical, may improve long-term survival for high-risk pediatric patients with philadelphia chromosome-positive acute lymphoblastic leukemia in the tyrosine kinase inhibitor era. Biol Blood Marrow Transplant. (2019) 25:1611–20. doi: 10.1016/j.bbmt.2018.12.007
51. Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. Lancet Oncol. (2016) 17:896–906. doi: 10.1016/S1470-2045(16)00162-5
52. Piñana JL, Valcárcel D, Fernández-Avilés F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transplant. (2010) 45:1449–56. doi: 10.1038/bmt.2009.362
53. Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. (2004) 54:208–36. doi: 10.3322/canjclin.54.4.208
54. Armenian SH, Sun C-L, Kawashima T, Arora M, Leisenring W, Sklar CA, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. (2011) 118:1413–20. doi: 10.1182/blood-2011-01-331835
55. Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2012) 18:348–71. doi: 10.1016/j.bbmt.2011.12.519
56. Cohen A, Rovelli A, Merlo DF, van Lint MT, Lanino E, Bresters D, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. (2007) 25:2449–54. doi: 10.1200/JCO.2006.08.9276
57. Pui CH, Ribeiro RC, Hancock ML, Rivera GK, Evans WE, Raimondi SC, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. (1991) 325:1682–7. doi: 10.1056/NEJM199112123252402
58. Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation–a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. (2005) 90:969–75.
59. Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socíe G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. (1997) 336:897–904. doi: 10.1056/NEJM199703273361301
60. Leisenring W, Friedman DL, Flowers MED, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. (2006) 24:1119–26. doi: 10.1200/JCO.2005.02.7052
61. Friedman DL, Rovo A, Leisenring W, Locasciulli A, Flowers MED, Tichelli A, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-late effect working party. Blood. (2008) 111:939–44. doi: 10.1182/blood-2007-07-099283
62. Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. (2009) 113:4992–5001. doi: 10.1182/blood-2008-09-178046
63. Dvorak CC, Gracia CR, Sanders JE, Cheng EY, Baker KS, Pulsipher MA, et al. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, & reproductive risks. Biol Blood Marrow Transplant. (2011) 17:1725–38. doi: 10.1016/j.bbmt.2011.10.006
64. Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. (2004) 89:5981–6. doi: 10.1210/jc.2004-0836
65. Ghavamzadeh A, Larijani B, Jahani M, Khoshniat M, Bahar B, Tabatabaei O. Thyroid, parathyroid, gonadal, and pancreatic beta-cell function after bone marrow transplantation with chemotherapy-only conditioning. Transplant Proc. (2003) 35:3101–4. doi: 10.1016/j.transproceed.2003.10.030
66. Pulewka K, Wolff D, Herzberg PY, Greinix H, Heussner P, Mumm FHA, et al. Physical and psychosocial aspects of adolescent and young adults after allogeneic hematopoietic stem-cell transplantation: results from a prospective multicenter trial. J Cancer Res Clin Oncol. (2017) 143:1613–9. doi: 10.1007/s00432-017-2424-4
67. Brauer E, Pieters HC, Ganz PA, Landier W, Pavlish C, Heilemann MV. Coming of age with cancer: physical, social, and financial barriers to independence among emerging adult survivors. Oncol Nurs Forum. (2018) 45:148–58. doi: 10.1188/18.ONF.148-158
68. Walsh CA, Yi JC, Rosenberg AR, Crouch M-LV, Leisenring WM, Syrjala KL. Factors associated with social functioning among long-term cancer survivors treated with hematopoietic stem cell transplantation as adolescents or young adults. Psychooncology. (2020) 29:1579–86. doi: 10.1002/pon.5460
69. Kim B, Patterson P, White K. Developmental considerations of young people with cancer transitioning to adulthood. Eur J Cancer Care. (2018) 27:e12836. doi: 10.1111/ecc.12836
70. McDonald FEJ, Patterson P, Kim B, White K. Working beyond the patient and cancer for adolescents and young adults. Eur J Cancer Care. (2018) 27:e12967. doi: 10.1111/ecc.12967
71. Livestrong. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. (2016). Available online at: https://www.livestrong.org/content/closing-gap-research-and-care-imperatives-adolescents-and-young-adults-cancer (accessed August 29, 2021)
72. Cupit MC, Duncan C, Savani BN, Hashmi SK. Childhood to adult transition and long-term follow-up after blood and marrow transplantation. Bone Marrow Transplant. (2016) 51:176–81. doi: 10.1038/bmt.2015.228
73. Rytting ME, Jabbour EJ, O'Brien SM, Kantarjian HM. Acute lymphoblastic leukemia in adolescents and young adults. Cancer. (2017) 123:2398–403. doi: 10.1002/cncr.30624
74. Kyngäs H, Mikkonen R, Nousiainen EM, Rytilahti M, Seppänen P, Vaattovaara R, et al. Coping with the onset of cancer: coping strategies and resources of young people with cancer. Eur J Cancer Care. (2001) 10:6–11. doi: 10.1046/j.1365-2354.2001.00243.x
75. Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol. (2012) 30:1221–6. doi: 10.1200/JCO.2011.39.5467
76. Morgan S, Davies S, Palmer S, Plaster M. Sex, drugs, and rock “n” roll: caring for adolescents and young adults with cancer. J Clin Oncol. (2010) 28:4825–30. doi: 10.1200/JCO.2009.22.5474
77. Murphy J, McKenna M, Abdelazim S, Battiwalla M, Stratton P. A practical guide to gynecologic and reproductive health in women undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant. (2019) 25:e331–43. doi: 10.1016/j.bbmt.2019.07.038
78. Lie N-EK, Larsen TMB, Hauken MA. Coping with changes and uncertainty: a qualitative study of young adult cancer patients' challenges and coping strategies during treatment. Eur J Cancer Care. (2018) 27:e12743. doi: 10.1111/ecc.12743
79. Cooke L, Chung C, Grant M. Psychosocial care for adolescent and young adult hematopoietic cell transplant patients. J Psychosoc Oncol. (2011) 29:394–414.
80. Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. (2006) 107:1645–55. doi: 10.1002/cncr.22102
81. Lambertini M, Del Mastro L, Pescio MC, Andersen CY, Azim HA, Peccatori FA, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. (2016) 14:1. doi: 10.1186/s12916-015-0545-7
82. Dalle J-H, Lucchini G, Balduzzi A, Ifversen M, Jahnukainen K, Macklon KT, et al. State-of-the-art fertility preservation in children and adolescents undergoing haematopoietic stem cell transplantation: a report on the expert meeting of the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT) in Baden, Austria, 29-30 September 2015. Bone Marrow Transplant. (2017) 52:1029–35. doi: 10.1038/bmt.2017.21
83. Lawrenz B, Jauckus J, Kupka MS, Strowitzki T, von Wolff M. Fertility preservation in >1,000 patients: patient's characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. (2011) 283:651–6. doi: 10.1007/s00404-010-1772-y
84. Joshi S, Savani BN, Chow EJ, Gilleece MH, Halter J, Jacobsohn DA, et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant. (2014) 49:477–84. doi: 10.1038/bmt.2013.211
85. Borgmann-Staudt A, Rendtorff R, Reinmuth S, Hohmann C, Keil T, Schuster FR, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant. (2012) 47:271–6. doi: 10.1038/bmt.2011.78
86. Rovó A, Tichelli A, Passweg JR, Heim D, Meyer-Monard S, Holzgreve W, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. (2006) 108:1100–5. doi: 10.1182/blood-2006-01-0176
87. Cattoni A, Parissone F, Porcari I, Molinari S, Masera N, Franchi M, et al. Hormonal replacement therapy in adolescents and young women with chemo- or radio-induced premature ovarian insufficiency: practical recommendations. Blood Rev. (2021) 45:100730. doi: 10.1016/j.blre.2020.100730
88. Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. (1992) 166:788–93. doi: 10.1016/0002-9378(92)91335-8
89. Pfitzer C, Orawa H, Balcerek M, Langer T, Dirksen U, Keslova P, et al. Dynamics of fertility impairment and recovery after allogeneic haematopoietic stem cell transplantation in childhood and adolescence: results from a longitudinal study. J Cancer Res Clin Oncol. (2015) 141:135–42. doi: 10.1007/s00432-014-1781-5
90. Alexandroni H, Shoham G, Levy-Toledano R, Nagler A, Mohty M, Duarte R, et al. Fertility preservation from the point of view of hematopoietic cell transplant specialists-a worldwide-web-based survey analysis. Bone Marrow Transplant. (2019) 54:1747–55. doi: 10.1038/s41409-019-0519-z
91. Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. (2006) 37:1023–9. doi: 10.1038/sj.bmt.1705364
92. Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. (1996) 87:3045–52. doi: 10.1182/blood.V87.7.3045.bloodjournal8773045
93. Loren AW, Senapati S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood. (2019) 134:746–60. doi: 10.1182/blood.2018846790
94. Hohmann C, Borgmann-Staudt A, Rendtorff R, Reinmuth S, Holzhausen S, Willich SN, et al. Patient counselling on the risk of infertility and its impact on childhood cancer survivors: results from a national survey. J Psychosoc Oncol. (2011) 29:274–85. doi: 10.1080/07347332.2011.563344
95. Quinn GP, Murphy D, Knapp CA, Christie J, Phares V, Wells KJ. Coping styles of female adolescent cancer patients with potential fertility loss. J Adolesc Young Adult Oncol. (2013) 2:66–71. doi: 10.1089/jayao.2012.0038
96. Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. (2002) 20:1880–9. doi: 10.1200/JCO.2002.07.175
97. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. (2006) 24:2917–31. doi: 10.1200/JCO.2006.06.5888
98. Chevillon F, Clappier E, Arfeuille C, Cayuela J-M, Dalle JH, Kim R, et al. Minimal residual disease quantification in ovarian tissue collected from patients in complete remission of acute leukemia. Blood. (2021) 137:1697–701. doi: 10.1182/blood.2020007782
99. Silvestris E, Cormio G, Skrypets T, Dellino M, Paradiso AV, Guarini A, et al. Novel aspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit Rev Oncol Hematol. (2020) 151:102981. doi: 10.1016/j.critrevonc.2020.102981
100. Yu J, Wang W, Huang H. Efficacy and safety of bispecific T-cell engager (BiTE) antibody blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin's lymphoma: a systemic review and meta-analysis. Hematol Amst Neth. (2019) 24:199–207. doi: 10.1080/16078454.2018.1549802
101. Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Rambaldi A, et al. Long-term follow-up of blinatumomab in patients with relapsed/refractory Philadelphia chromosome-positive B-cell precursor acute lymphoblastic leukaemia: final analysis of ALCANTARA study. Eur J Cancer Oxf Engl. (2021) 146:107–14. doi: 10.1016/j.ejca.2020.12.022
102. Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. (2018) 131:1522–31. doi: 10.1182/blood-2017-08-798322
103. von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. (2016) 34:4381–9. doi: 10.1200/JCO.2016.67.3301
104. Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel P-G, et al. Author correction: blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. (2021) 11:28. doi: 10.1038/s41408-021-00413-7
105. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325:833–42. doi: 10.1001/jama.2021.0669
106. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325:843–54. doi: 10.1001/jama.2021.0987
107. Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Correction: inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. (2019) 33:1061–2. doi: 10.1038/s41375-019-0426-8
108. Calvo C, Cabannes-Hamy A, Adjaoud D, Bruno B, Blanc L, Boissel N, et al. Inotuzumab ozogamicin compassionate use for French paediatric patients with relapsed or refractory CD22-positive B-cell acute lymphoblastic leukaemia. Br J Haematol. (2020) 190:e53–6. doi: 10.1111/bjh.16732
109. Brivio E, Locatelli F, Lopez-Yurda M, Malone A, Díaz-de-Heredia C, Bielorai B, et al. A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study). Blood. (2021) 137:1582–90. doi: 10.1182/blood.2020007848
110. Levine JE, Grupp SA, Pulsipher MA, Dietz AC, Rives S, Myers GD, et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J Immunother Cancer. (2021) 9:e002287. doi: 10.1136/jitc-2020-002287
Keywords: acute lymphoblastic leukaemia, adolescent, young adult, haematopoietic stem cell transplant, conditioning regimen, supportive care, transition to adult care
Citation: Calvo C, Ronceray L, Dhédin N, Buechner J, Troeger A and Dalle J-H (2022) Haematopoietic Stem Cell Transplantation in Adolescents and Young Adults With Acute Lymphoblastic Leukaemia: Special Considerations and Challenges. Front. Pediatr. 9:796426. doi: 10.3389/fped.2021.796426
Received: 16 October 2021; Accepted: 02 December 2021;
Published: 11 January 2022.
Edited by:
Adriana Balduzzi, Clinica Pediatrica Università Degli Studi di Milano Bicocca, ItalyReviewed by:
Carsten Heilmann, Juliane Marie Centre, Rigshospitalet, DenmarkLuca Lo Nigro, Azienda Ospedaliero Universitaria Policlinico - San Marco, Italy
Valentino Conter, Fondazione MBBM, Italy
Tomasz Szczepanski, Medical University of Silesia, Poland
Copyright © 2022 Calvo, Ronceray, Dhédin, Buechner, Troeger and Dalle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Hugues Dalle, jean-hugues.dalle@aphp.fr
 Charlotte Calvo
Charlotte Calvo Leila Ronceray
Leila Ronceray Nathalie Dhédin3
Nathalie Dhédin3  Jochen Buechner
Jochen Buechner Jean-Hugues Dalle
Jean-Hugues Dalle