- 1Department of Neurosurgery and Neurotechnology, Eberhard Karls University Tübingen, Tübingen, Germany
- 2Department of Neurosurgery, Alfried Krupp Hospital, Essen, Germany
Background: Despite the ongoing debate on the risk–benefit ratio of vestibular schwannoma (VS) treatment options, watchful observation and radiation are usually favored in the elderly (>65 years). If surgery is inevitable, a multimodal approach after deliberate subtotal resection has been described as a valid option. The relationship between the extent of resection (EOR) of surgical and functional outcomes and recurrence-free survival (RFS) remains unclear. This present study aims to evaluate the functional outcome and RFS of the elderly in relation to the EOR.
Methods: This matched cohort study analyzed all consecutive elderly VS patients treated at a tertiary referral center since 2005. A separate cohort (<65 years) served as a matched control group (young). Clinical status was assessed by the Charlson Comorbidity Index (CCI), the Karnofsky Performance (KPS), and the Gardner and Robertson (GR) and House & Brackmann (H&B) scales. RFS was evaluated by Kaplan–Meier analysis using contrast-enhanced magnetic resonance imaging to identify tumor recurrence.
Results: Among 2,191 patients, 296 (14%) patients were classified as elderly, of whom 133 (41%) were treated surgically. The elderly were characterized by a higher preoperative morbidity and worse gait uncertainty. Postoperative mortality (0.8% and 1%), morbidity (13% and 14%), and the functional outcome (G&R, H&B, and KPS) did not differ between the elderly and the young. There was a significant benefit in regard to the preoperative imbalance. Gross total resection (GTR) was accomplished in 74% of all cases. Lower grades of the EOR (subtotal and decompressive surgery) raised the incidence of recurrence significantly. Mean time to recurrence in the surgELDERLY was 67.33 ± 42.02 months and 63.2 ± 70.98 months in the surgCONTROL.
Conclusions: Surgical VS treatment aiming for complete tumor resection is feasible and safe, even in advanced age. A higher EOR is not associated with cranial nerve deterioration in the elderly compared to the young. In contrast, the EOR determines RFS and the incidence of recurrence/progression in both study cohorts. If surgery is indicated in the elderly, GTR can be intended safely, and if only subtotal resection is achieved, further adjuvant therapy, e.g., radiotherapy, should be discussed in the elderly, as the incidence of recurrence is not significantly lower compared to the young.
Introduction
Among benign nerve sheath tumors, the vast majority are vestibular schwannomas (VSs) with a reported incidence rate of 1.52 per 100,000 (1, 2). They account for 80%–90% of all tumors in the cerebellopontine angle and approximately 6%–8% of all primary intracranial neoplasms (3, 4). Historically, surgical removal has been an appreciated treatment of choice as complete resection represents maximal tumor control (5). However, due to the anatomical relationship of the VS to multiple cranial nerves (CNs), a great deal of precision and delicacy is required in surgical management (1, 6). Several studies have documented severe clinical consequences of postoperative CN function decline for VS patients (1, 7). Thus, other treatment options including watchful observation (i.e., wait-and-scan, WaS) and radiation (i.e., stereotactic radiosurgery, SRS) have been claimed (8). Ever since, there is an ongoing debate on the risk–benefit ratio of these treatment options on tumor control and function preservation considering different factors such as tumor size, initial CN function, and/or patient’s age (9).
In contrast to other patient cohorts, there seems to be a general agreement that watchful observation and radiation treatment should be favored in the elderly (>65 years of age) assuming a higher operative morbidity and lower life expectancy (10, 11). However, surgical intervention is sometimes inevitable, even in this patient cohort (e.g., large VS compressing the brainstem). In these particular cases, a multimodal therapy approach [i.e., deliberate subtotal resection (STR) with adjuvant SRS] has been suggested as a valid option recently in order to reduce perioperative morbidity (9). The level of evidence, however, is remarkably low (9).
However, creating clinical evidence in VS management, the elderlies (>65 years of age) is of paramount importance, as an incidence peak is described in this specific patient group (9). In detail, there are three evidence gaps for the elderly patient cohort. Firstly, the few existing small-sized studies on surgical morbidity in the elderly describe the outcome of surgeries performed several decades ago (10–12) and surgical techniques have evolved reducing surgery time and improving functional outcome since then (8, 13). Thus, it remains unclear whether the surgical morbidity of the elderly differs from the young in the environment of contemporary neurosurgery. Second, as deliberate STR or decompressive surgery (DS) could be a valid option in this specific subgroup, the relation of surgical morbidity to the extent of resections (EORs) in the elderly should be investigated. Third, there is no data on the relation between the EOR on tumor control as in long-term recurrence-free-survival (RFS) (i.e. progress-free-survival in subtotal resection) in the elderly. Notably, most studies provide follow-up data of <5 years (10, 11).
Thus, the aim of this study is to evaluate (1) the patterns of VS management in a tertiary neurosurgical center, (2) the onco-functional outcome, and (3) the incidence of recurrence in relation to the EOR in a large cohort of elderly patients compared to a matched-control cohort.
Methods
Study design and patient cohort
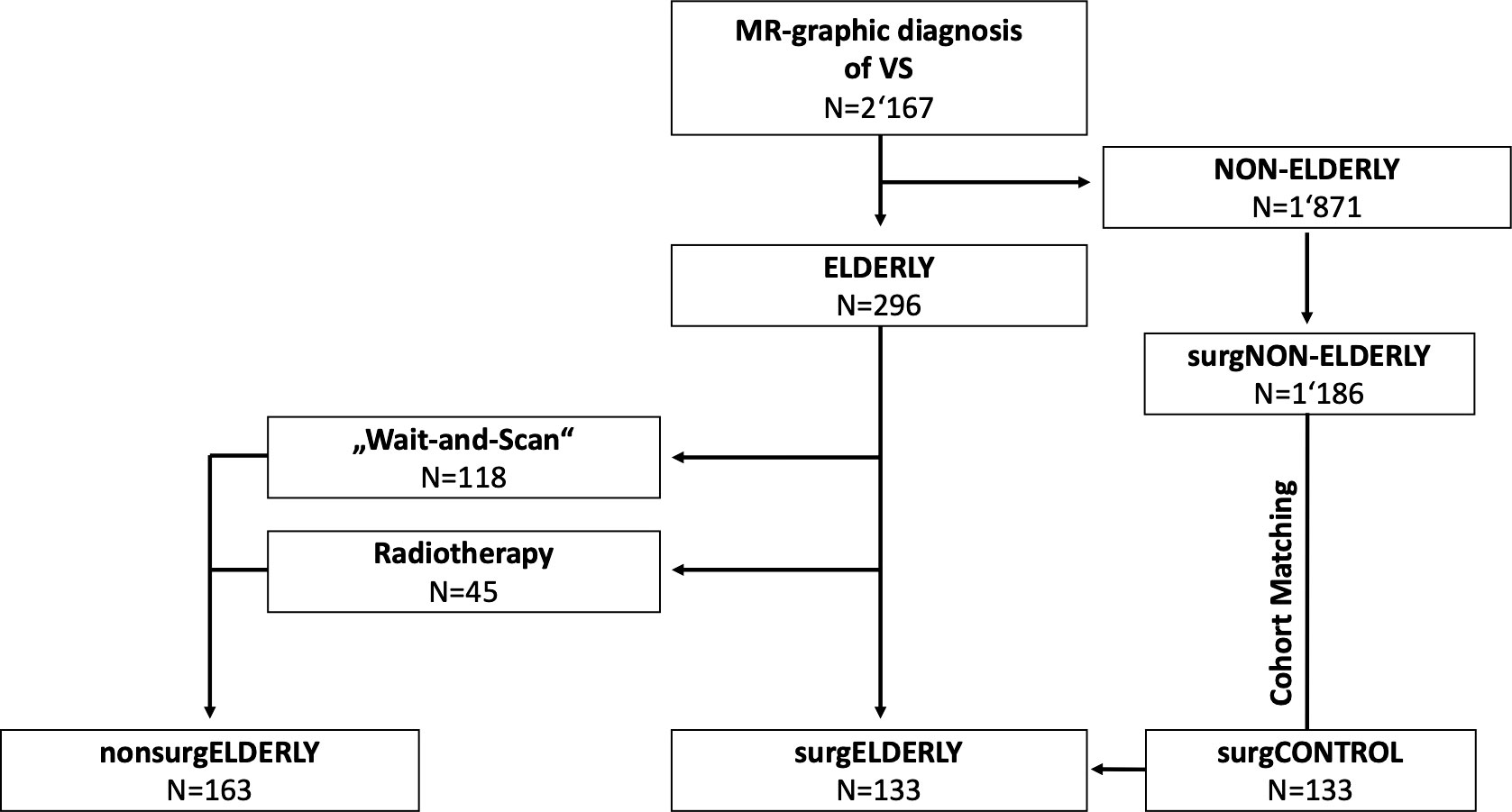
This retrospective blinded cohort study analyzed all consecutive (elderly) patients (>65 years) with unilateral VS treated at a German academic, tertiary referral center between April 2005 and October 2020. Patients were referred to non-surgical (i.e., WaS, stereotactic radiosurgery; nonsurgELDERLY) or surgical treatment (surgELDERLY) depending on tumor size, the presence of hydrocephalus, clinical presentation, and a patient’s individual preference. A separate cohort of patients with <65 years of age, who underwent elective VS surgery, served as a matched control group (surgCONTROL) to specifically compare surgical treatment in the elderly and the young (Figure 1). Pairing was based on the EOR, surgical approach (semi-sitting or prone position), gender, and tumor size (closest match). All patients were treated by the retrosigmoid approach with intraoperative neurophysiological monitoring. Large VS (e.g., Koos °III–°IV) were generally treated in the semi-sitting position, while small VS (e.g., Koos °I–°II) in the prone position (14). VS associated with neurofibromatosis was systematically excluded from this study. Same was applied to the previously treated VS (by surgery or radiotherapy). All histopathological examinations of surgELDERLY and surgCONTROL were graded as schwannoma by a board-certified neuropathologist. The study population was identified through a prospective registry. The local ethics committee approved data collection and post-hoc analyses.
Data collection
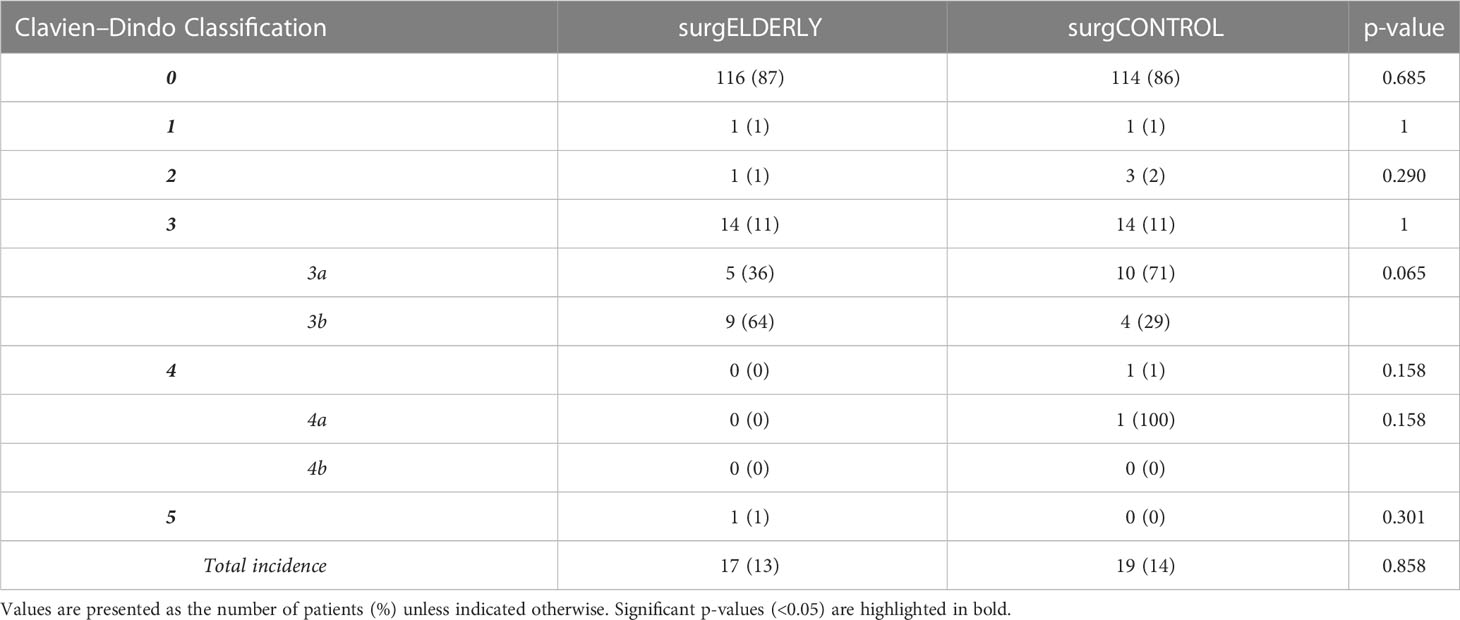
Medical records of each patient were reviewed, and various demographic, tumor, and treatment variables were recorded. Magnetic resonance images (MRIs) were retrospectively analyzed to determine the tumor size according to the Koos grading system in a blinded fashion (14). The EOR was determined by postoperative MRI (3 months postoperatively) and classified by gross total resection (GTR), STR (i.e., residual tumor exclusively in the internal auditory canal), and DS (i.e., residual tumor beyond the internal auditory canal) (Figure 2). MR-graphic tumor progression/recurrence was defined as tumor progress or new tumor recurrence during MR-graphic surveillance with gadolinium contrast. Symptom-affected everyday-life dependency was acquired with the Karnofsky Performance Score (KPS) (15). Pre- and postoperative symptoms were recorded including tinnitus, functional hearing loss in Gardner–Robertson (GR) classes (16), gait uncertainty, vertigo, trigeminal affection (neuralgia or hypesthesia), double vision, swallowing deficit, headache, gustatory deficit, hydrocephalus, and facial palsy. Facial nerve function was reported using the House and Brackmann (H&B) scale pre- and postoperatively, as well as after a follow-up of 3 months and 1 year (17). Recorded patient comorbidities were assessed using the Charlson Comorbidity Index (CCI) (18). Adverse postoperative events were classified according to the Clavien–Dindo Classification (CDC) (19).
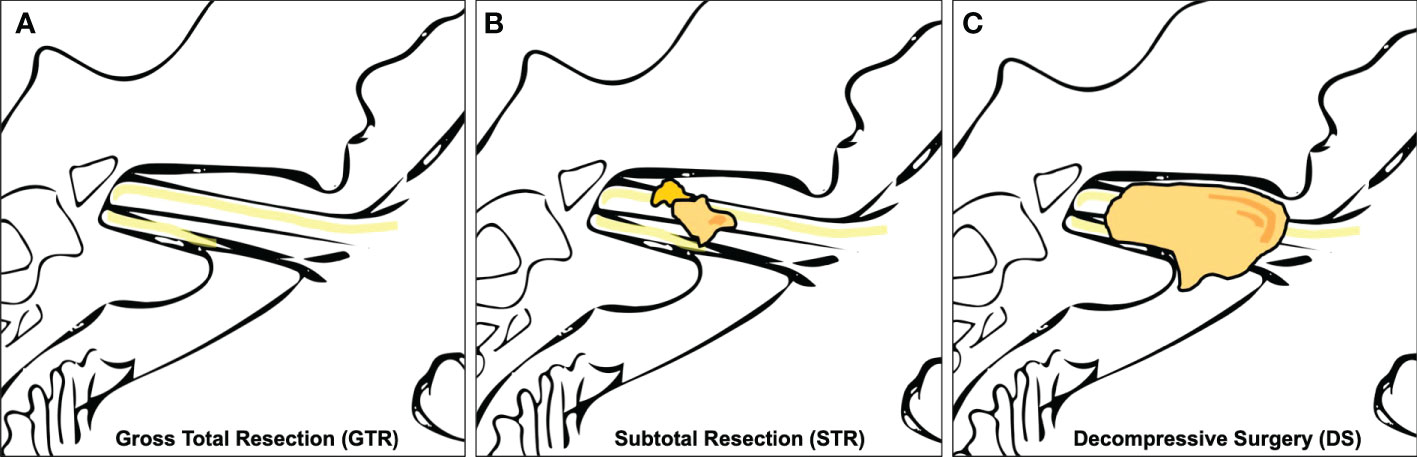
Figure 2 Extent of resection (EOR). (A) shows gross total resection (GTR), (B) subtotal resection (STR), and (C) shows a schematic representation of decompressive surgery (DS) with no decompression of the tumor in the internal auditory canal.
Statistical analysis
Statistical analysis was performed in R Studio (Version 1.2) using descriptive statistics. To compare nonnumeric parameters of both groups, the chi-square test and Fisher’s exact test were applied. For numeric parameters, Welch’s two-sample t-test was used. Recurrence-free and overall survival were estimated using the Kaplan–Meier method and compared between cases and controls using a log-rank test. The length of follow-up for recurrence-free survival was calculated from the date of surgical intervention to the date of either recurrence or the last clinical visit. Significance was defined as the probability of a two-sided type 1 error being <5% (p < 0.05). Data are presented as mean ± standard deviation (SD) if not indicated otherwise. Due to the low incidence of complications and perioperative morbidity, for its analysis and comparison dependent on the EOR, DS and STR were grouped together.
Results
Study cohorts
Among 2,167 patients with VS, 296 patients (14%) were of >65 years of age at date of diagnosis and classified as elderly (Figure 1). Mean age was 71.1 ± 5.0 [range 65–89] years in all elderly patients. Tumor size was equally distributed across Koos grades (°I: 69/296, 23%; °II: 86/296, 29%; °III: 80/296, 27%; °IV: 61/296. 21%). The majority of elderly patients (163/296, 55%) was managed non-surgically (nonsurgELDERLY) by either SRS (45/163, 28%) or watchful observation (118/163, 72%). 133/296 (45%) of elderly patients were treated microsurgically (surgELDERLY) via a retrosigmoid approach. A separate cohort of patients younger than 65 years (N = 133) served as a matched control group (surgCONTROL). The mean age of the surgCONTROL cohort was 46.3 ± 11.9 years (Table 1).
The most common initial symptom in the elderly was functional hearing loss in 172/296 (58%) cases, followed by vertigo in 117/296 (40%) patients. Tinnitus and gait uncertainty were similarly common with 84/296 (28%) cases and 85/296 (29%) cases, respectively. Facial palsy was a leading initial symptom in only 27/296 (9%) cases in the elderly. Trigeminal affection and swallowing deficits were very rare clinical symptoms with 24/296 (8%) and 5/296 (2%) cases, respectively. No patient presented with double vision (Table 1).
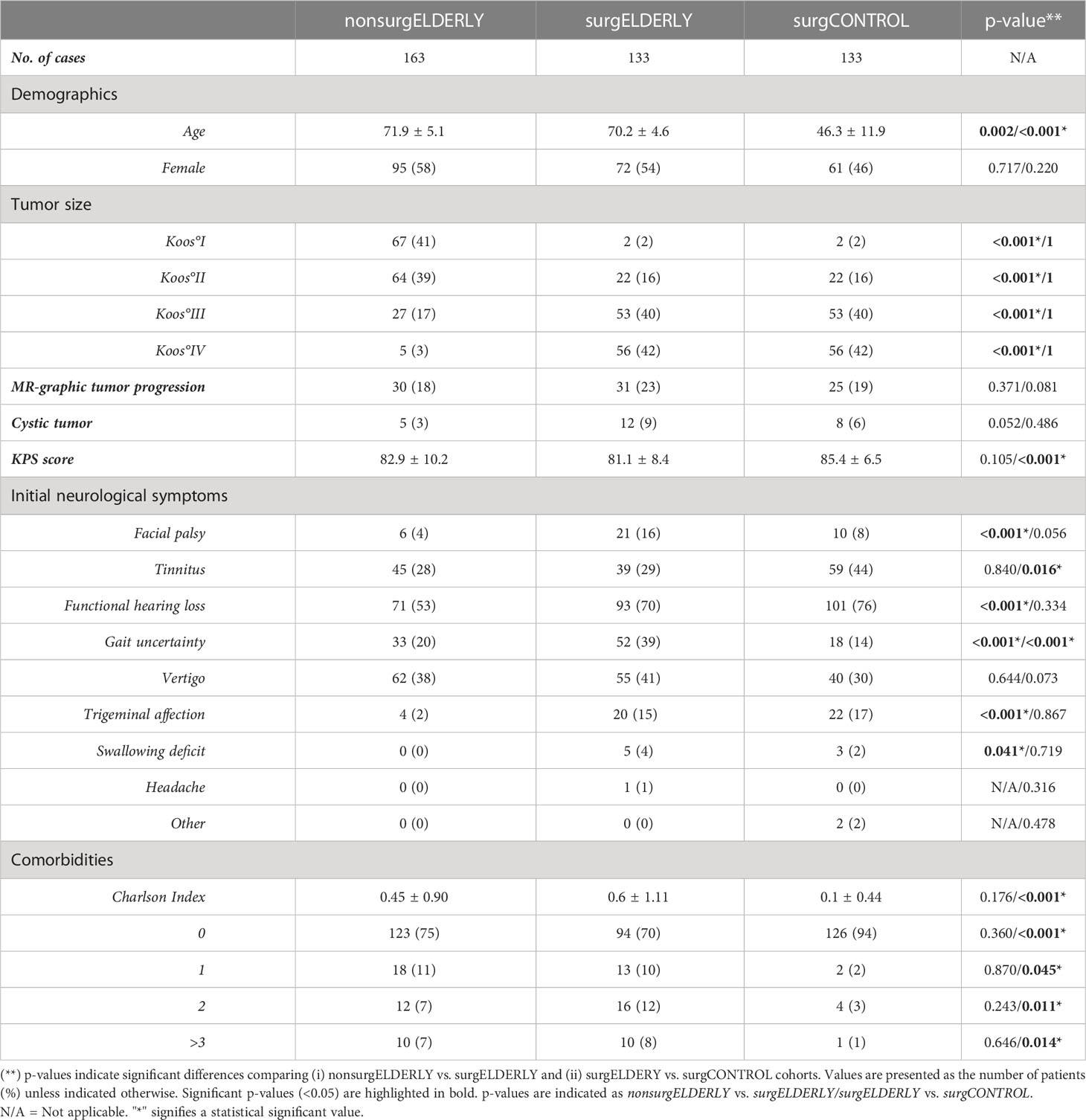
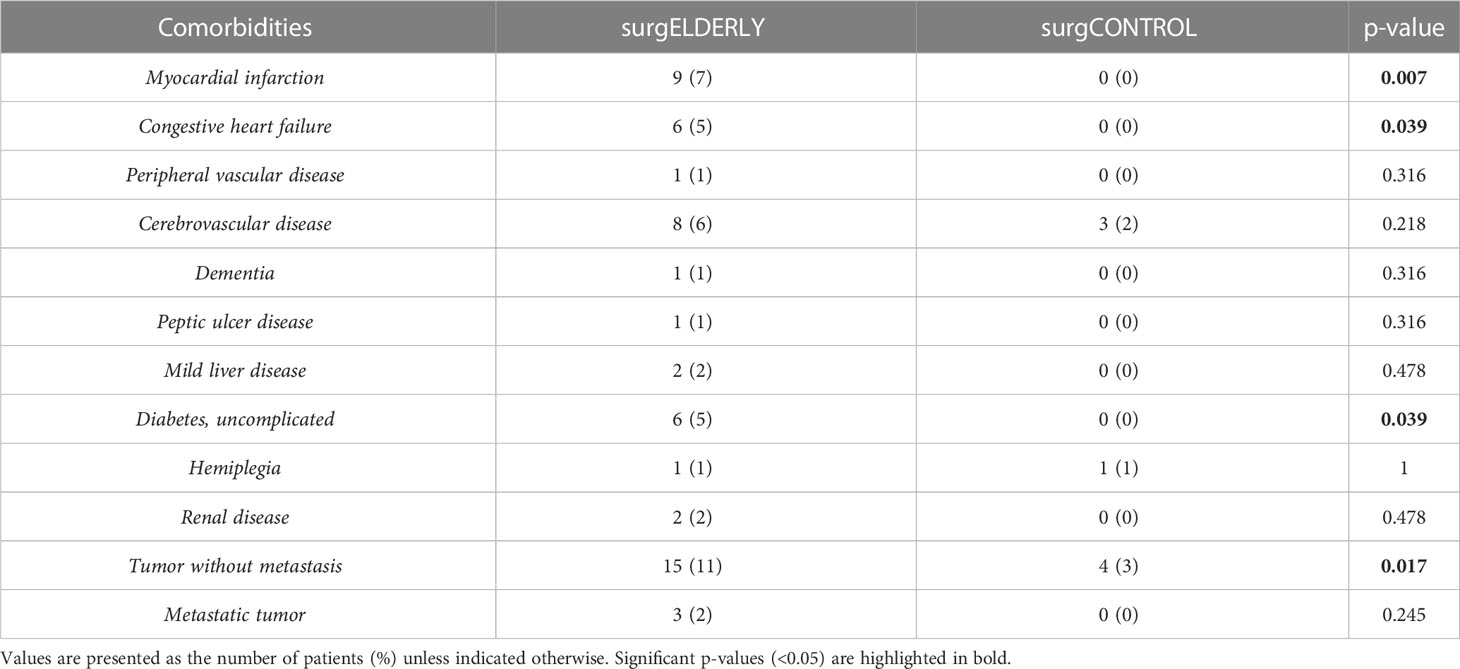
When comparing surgELDERLY with the younger surgCONTROL cohort, they presented with a worse initial KPS (81.1 ± 8.4 and 85.4 ± 6.5, respectively; p < 0.001) poorer preoperative comorbidity status (e.g., the incidence of myocardial infarction, congestive heart failure, diabetes, and malignant tumors), yielding in a significantly higher CCI in the surgELDERLY compared to the surgCONTROL group (0.6 ± 1.1 and 0.1 ± 0.4, respectively; p < 0.001) (Tables 1, 2). However, there was no significant difference in the following VS-associated morbidities: initial incidence of facial palsy, functional hearing loss, vertigo, trigeminal affection, swallowing deficit, headache, gustatory deficit, and hydrocephalus. surgELDERLY had a higher incidence of pre-operative gait uncertainty (p < 0.001), but complained of less tinnitus than their controlled matches (p = 0.016).
Non-surgical vestibular schwannoma management in the elderly
The surgELDERLY and nonsurgELDERLY cohort did not differ in age, gender, the clinical status in KPS. The incidence of cystic morphology and MR-graphic progression was higher in the surgically treated but did not reach statistical significance in this cohort. However, nonsurgELDERLY were characterized by a significant smaller tumor size (p < 0.001). The nonsurgELDERLY cohort had less initial severe CN deficits (facial palsy, functional hearing loss, gait uncertainty, trigeminal affection) than surgELDERLY. The CCI was not significantly different in both cohorts (Table 1).
Surgical vestibular schwannoma management in the elderly
The surgCONTROL was younger than the surgELDERLY cohort but did not differ in other patient demographics or tumor characteristics (Table 1). surgELDERLY presented with a worse initial KPS then their younger surgCONTROL cohort (81.1 ± 8.4 and 85.4 ± 6.5, respectively; p < 0.001). There were no significant group difference in the initial incidence of facial palsy, functional hearing loss, vertigo, trigeminal affection, swallowing deficit, headache, gustatory deficit and hydrocephalus in a matched control comparison (“surgELDERLY” vs. “surgCONTROL”). Remarkably, surgELDERLY had a higher incidence of gait uncertainty but complained of less tinnitus than their controlled matches. Additionally, surgELDERLY had a poorer preoperative comorbidity status (e.g., the incidence of myocardial infarction, congestive heart failure, diabetes, and malignant tumors), yielding in a significantly higher CCI in the surgELDERLY compared to the surgCONTROL group (0.6 ± 1.1 and 0.1 ± 0.4, respectively; p < 0.001) (Tables 1, 2).
Surgical data and surgical complications
Surgery was performed by a retrosigmoid craniotomy in all cases using either a semi-sitting (221/266, 83%) or prone position (45/266, 17%) in both surgical study groups. Mean operating time (skin to skin) was noted similarly at 248.0 ± 75.2 minutes in the surgELDERLY and 240.0 ± 82.1 minutes in the surgCONTROL (p = 0.398).
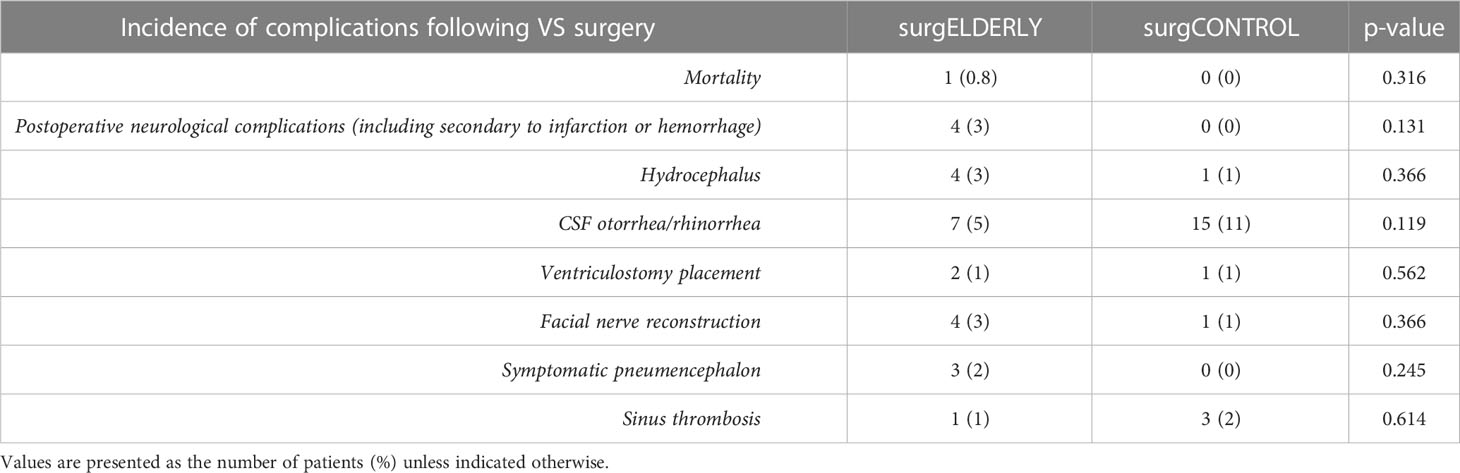
The incidence of perioperative complication was comparable in the surgELDERLY (17/133, 13%) and surgCONTROL group (19/133, 14%) (p=0.858). Larger tumors (Koos °III and °IV) more often yielded in postoperative complications (surgELDERLY: °II: N = 3; °III: N = 2; °IV: N = 12 and surgCONTROL: °I: N = 1; °II: N = 2; °III: N = 8; °IV: N = 8). Postoperative hemorrhage, venous thrombosis and symptomatic pneumoncephalon occurred more frequently in the surgELDERLY, but this did not reach any statistical significance. In contrast, younger patients were more prone to postoperative CSF leakage. Overall, the complication rate in the surgELDERLY cohort was not significantly raised. One patient suffered a postoperative hemorrhage and treatment was terminated after the patient’s presumed will, yielding in a mortality rate of N = 1 (Table 3). The CDC Index of complication severity is summarized in Table 4. Discharge modality (home, rehab, and other hospital) was significantly indifferent in both groups.
Functional outcome
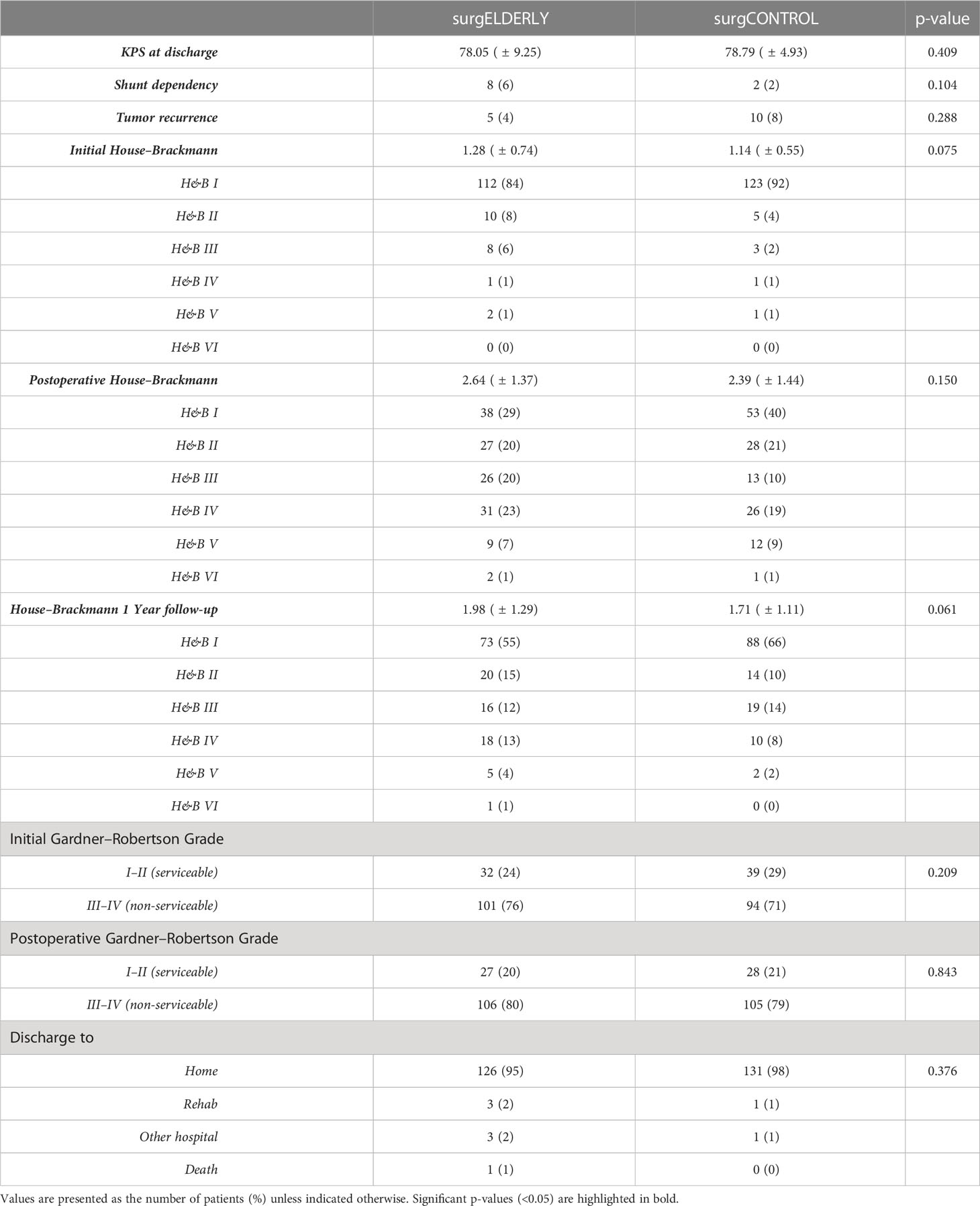
Postoperative KPS did not differ between surgELDERLY and surgCONTROL patients (78.1 ± 9.3 and 78.8 ± 4.9; p = 0.409). There was no significant difference in discharge modality (p = 0.377). Impeccable facial function (H&B = 1) was completely unaffected by the surgery in 38/112 (34%) and 53/123 (43%) in surgELDERLY and surgCONTROL, respectively (p = 0.096). After surgery-related deterioration, facial function recovered to a favorable outcome (i.e., H&B°I and H&B°II) in 45/75 (60%) and 46/70 (65%) surgELDERLY and surgCONTROL patients, respectively (Table 5). Therefore, overall favorable facial function outcome after 1 year was 70% (93/133) in the elderly and 76% (102/133) in the young. Hearing preservation was insignificantly better in surgELDERLY at 84% (27 out of 32 with functional hearing) than in surgCONTROL at 71% (28 out of 39 with functional hearing) with p = 0.164. New trigeminal deficit was observed in 2%. Of the 20 patients suffering from preoperative trigeminal affection (hypesthesia or neuralgia), 17 patients recovered clinically in the surgELDERLY group (85%); the same was observed in 20/22 patients (90%) in the surgCONTROL group (p = 0.277). New postoperative gait uncertainty was observed in N = 4 in the surgELDERLY but none in the younger surgCONTROL group. Postoperative recovery of symptomatic gait uncertainty was observed in 71% (37/52) and 76% (12/16) of the surgELDERLY and surgCONTROL, respectively (p = 0.382). The recovery of vertigo symptoms was observed in N = 40/55 (71%) of all surgELDERLY presenting with preoperative vertigo; this rate was higher in the surgCONTROL cohort with N = 37/40 (90%) (p = 0.013). The incidence of new postoperative vertigo was N = 0 (0%) in surgELDERLY but N = 8/91 (9%) in its control cohort. Considering new postoperative tinnitus, the incidence was higher in the young (N = 1 in surgELDERLY and N = 9 in surgCONTROL), whereas the postoperative improvement of known tinnitus was similar at 14% and 16%, respectively (surgELDERLY and surgCONTROL) (p = 0.585).
Extent of resection and recurrence-free survival
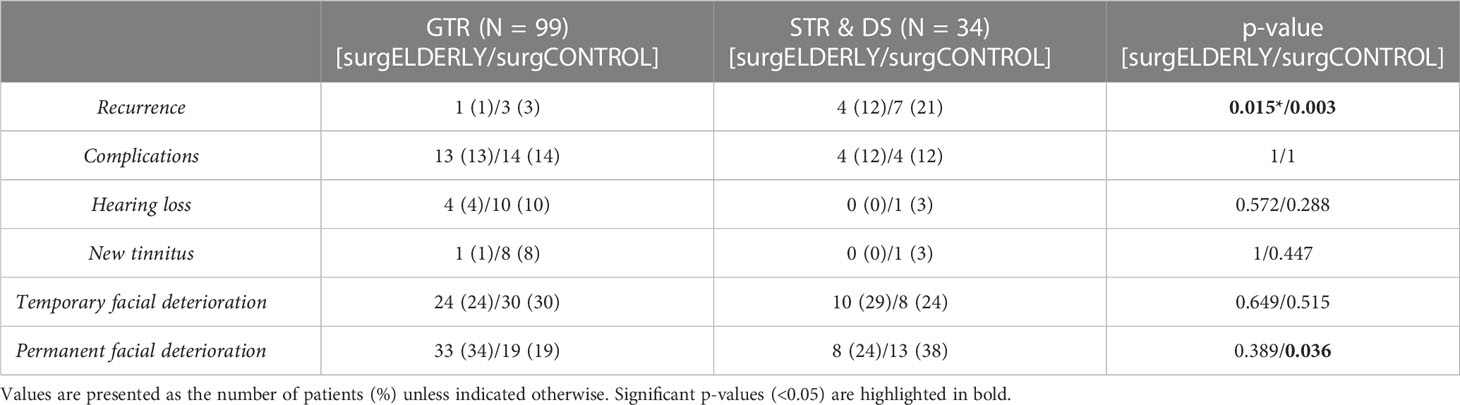
GTR was achieved in the majority of surgELDERLY cases (99/133, 74%). When taking the EOR into account, there was no significantly higher postoperative CN affection (facial, trigeminal, and vestibulocochlear) in surgELDERLY associated with GTR and STR/DS (Table 6). However, the rate of permanent facial deterioration within patients treated with GTR remained significantly higher in the surgELDERLY compared to the surgCONTROL (p = 0.035). Additionally, within the surgCONTROL group, STR/DS was associated with a significantly higher incidence of permanent facial deterioration (p = 0.036) compared to GTR.
STR/DS was significantly associated with the incidence of recurrence compared to GTR in both subgroups (surgELDERLY: p = 0.015; surgCONTROL: p = 0.003). The incidence of recurrence was statistically insignificant in surgELDERLY compared to surgCONTROL, when treated with GTR (p = 0.621). Mean time for surveillance was 38 ± 36 months in surgCONTROL (median: 25 months) and 31 ± 37 months (median: 34 months) in surgELDERLY. The overall incidence for recurrence was 5/133 (4%) and 10/133 (8%) after neurosurgical tumor resection in the surgELDERLY and surgCONTROL, respectively, with no statistical significance (p = 0.143). Mean time to recurrence was 67.33 ± 42.02 months in surgELDERLY and 63.2 ± 70.98 months in surgCONTROL. Kaplan–Meier analysis on RFS depending on the EOR is shown in Figure 3. The EOR was significantly associated with RFS in both surgELDERLY and surgCONTROL cohorts (p < 0.001). Mean time to recurrence in surgELDERLY was 67 ± 53 months and 79 ± 71 months in surgCONTROL.
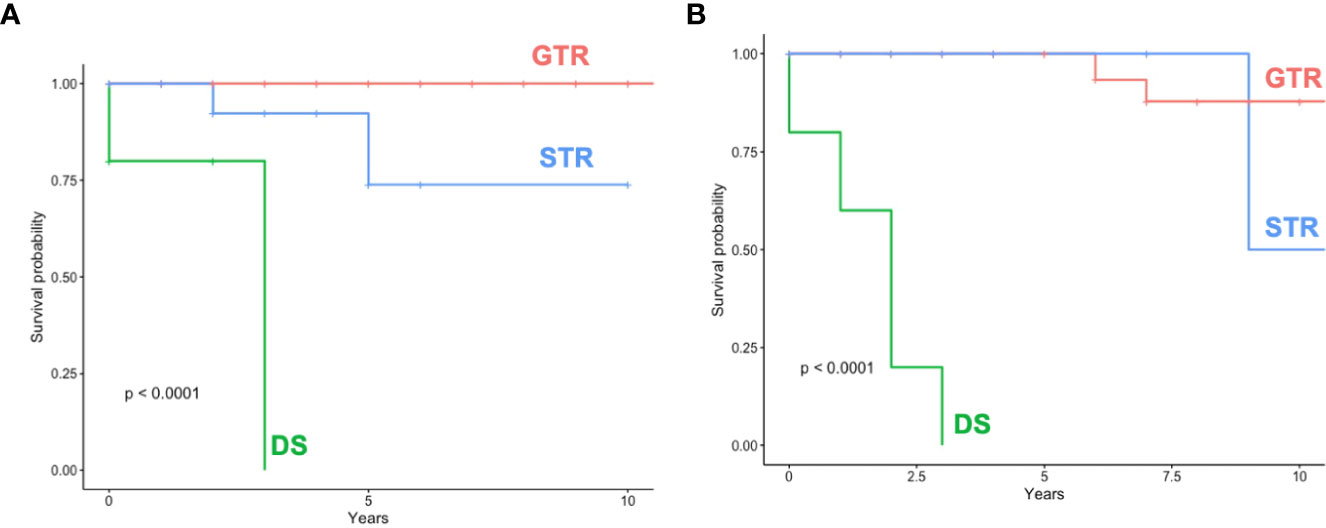
Figure 3 Recurrence/Progression-free-survival (RFS) (A) shows RFS dependent on the EOR in surgELDERLY and (B) surgCONTROL. GTR, gross total resection; STR, subtotal resection; DS, decompressive surgery.
Discussion
The present study aimed to evaluate the management of VS in the elderly (>65 years) in a tertiary neurosurgical center and to assess the oncological and functional outcome of this particular cohort in comparison to matched young controls and in regard to the EOR. The elderly represented approx. 14% of all VS patients in our cohort. The majority was eligible for non-surgical treatment. Patients were allocated for surgical treatment due to large tumor size affecting the brainstem and worse preoperative clinical symptomatology (e.g., the presence of vertigo and imbalance). In comparison to a matched-control cohort, elderly selected for surgery were characterized by a worse preoperative clinical condition (KPS and CCI). Despite this, postoperative mortality, morbidity and functional outcome did not differ between both surgical groups. These data suggest that the retrosigmoid approach for VS resection is safe in the elderly >65 years of age. Patients significantly benefit in regard to preoperative imbalance. GTR was accomplishable in the majority of patients. Notably, the EOR determined RFS.
Patterns of care and clinical characteristics
In the present population of surgically treated elderly (surgELDERLY), preoperative CN deficits (facial function, hearing, gait, and trigeminal affection) were distinctively more severe than in the conservatively managed (nonsurgELDERLY). Same was described in a nationwide registry study in Sweden with 58 elderly patients and by a series of surgically managed elderly patients by Samii et al. (12, 20). When comparing the nuances of clinical deficits, surgELDERLY rarely presented with tinnitus but suffered from gait uncertainty more often than their matched and younger cohort. Interestingly, preoperative functional hearing was similar in both groups and not decreased in the elderly, even though presbyacusis is a common phenomenon in the general elderly population (21). This observation suggests that symptoms leading to the diagnosis of VS most likely are not hearing function but other vestibulocochlear (gait and vertigo), facial, or trigeminal symptoms.
Microsurgical care
Microsurgical tumor resection of VS by a retrosigmoid craniotomy was safe and did not yield in a higher risk for postoperative CN deficits in the elderly in comparison to the matched surgCONTROL cohort. Even with a highly significant incidence of relevant comorbidity (i.e., higher CCI and worse KPS), perioperative complication rates and morbidity are statistically comparable. Perioperative complications in the elderly have been described by current literature from 20% to 57% (1, 3, 10). In contrast, the present population showed an incidence of perioperative complication of 13% and 14% in the elderly and the young, respectively. Due to a higher case load of elderly VS patients in the past 15 years (i.e., nine cases per year) in comparison to previous studies (approx. two to three per year) (3, 10), our department was able to acquire and develop a surgical routine and protocols for VS surgery in this patient cohort. The operative experience of the surgical team has been described as an important factor affecting onco-functional outcome in VS management, and therefore, treatment in high-volume centers has been recommended (9). The distribution of complications is different in both cohorts with a higher prevalence of CSF leakage and venous thrombosis in the younger population, while the elderly more often suffered from postoperative hemorrhage and symptomatic pneumocephalus, which has been described in the literature before (1, 10, 12, 22). Previous studies have mixed surgical approaches (translabyrinthine, middle fossa, and retrosigmoid) (23). This is the first comparative series to describe a large elderly cohort treated exclusively by a microsurgical, retrosigmoid approach. Thus, lower perioperative morbidity of the present study might be partially attributed by differences in the surgical approach.
Postoperative functional outcome
There were no differences in general functional outcome or the independency score (KPS) comparing the surgELDERLY to the surgCONTROL. In contrast, the surgELDERLY cohort showed a significant improvement of gait/vertigo after surgery. Tinnitus was improved in 13%–14% of all surgically treated. Postoperative hearing loss was not worse but significantly better in the surgELDERLY. In approx. 60% of the patients, facial function normalized within 3 months, making postoperative functional deterioration temporary. There was no difference in facial nerve outcome between the surgELDERLY and the surgCONTROL group. While observing a remarkable transient functional deterioration right after surgery, of which the majority recovers, these numbers should not be compared to direct results after other non-surgical treatment options (e.g., radiotherapy or radiosurgery). The rate of permanent facial deterioration was significantly higher in surgELDERLY compared to surgCONTROL in the GTR group suggesting decreased postoperative rehabilitation potential in facial function with advanced age (24).
The explanation for the inversely proportional relationship of permanent facial deterioration and GTR vs. STR/DS in the elderly (more facial deterioration in GTR) and the young (more facial deterioration in STR/DS) could be attributed to the actual intention-to-treat. In the young, we usually intend GTR unless there is a deterioration of the intraoperative neuromonitoring (e.g., facial motor–evoked potentials). This approach explains the GTR rate of 74% in the present study. However, in a larger cohort of the same group, GTR was even higher with approx. 93% (25). This fact shows that we are more aggressive in the young than in the elderly to achieve GTR explaining the higher rate of unfavorable rates in the Young STR/DS subcohort. In turn, the present analysis has shown that surgical management strategy in the elderly is more careful compared to the young.
Long-term results should be chosen to compare functional outcome between such different treatment modalities as radiosurgery and microsurgery, e.g., in hearing outcome. Watchful observation (“WaS”) abandons CN function (hearing and vestibular, more prominently then facial and trigeminal function) to the natural history of VS and tumor dynamic. Thus, it has been shown that 12% lose functional hearing in the course of the VS natural history (26). In line with this, SRS has been shown to result in a long-term hearing preservation of only 35%–51% (27) and therefore similarly to surgical treatment. Additionally, it is well known that hearing function dramatically decreases within the first decade after SRS (8, 13, 27). Additionally, tinnitus and imbalance are shown to increase and facial nerve dysfunction (e.g., hemispasm) might appear after SRS (28). Finally, little is known about radiation-associated tumor malignization (11, 29). The present study design, however, does not allow a direct comparison of functional outcome between radiotherapy and surgery.
It is not to be forgotten that functional outcome, as physicians and/or treating surgeons may define it, does not necessarily transfer to quality-of-life in the patients’ eyes in a proportional way. Leaving residual tumor behind or treating the tumor by non-invasive treatments such as SRS can impact mental health or illness perception (30).
Extent of resection and recurrence-free survival
Due to the study design (matched by tumor size and the EOR), this analysis does not allow any general statement considering the frequency of the achieved EOR in the elderly compared to the young alone. However, when put into context with another series published and treated by the same group and institution with N = 572 primary VS patients, the rate of GTR was significantly higher among a general VS patient cohort of any age. Therefore, we can assume that the management of elderly patients with VS is carried out more conservatively—even in regard to the surgical EOR (25).
Mean time to recurrence was >5 years in both groups, proposing that long-term follow-up in VS patients should be carried out regardless of age. Still, the overall incidence of and mean time to recurrence were statistically insignificant in the elderly compared to the young concordant to previous matched cohort studies with distinctly smaller patient numbers (3, 10). In large VS, where surgery is inevitable, tumor mass reduction with SRS or observation could be suggested to avoid functional deterioration (9). In our cohort, microsurgical care followed by observation was the only treatment (no adjuvant SRS) carried out. Even though the number of STR- and DS-resected patients was low, the risk for tumor recurrence within 5 years was significantly increased in STR and DS. Thus, a deliberate DS as a standard treatment strategy in VS must be critically analyzed, especially in a setting of DS with observation alone (9). Therefore, if DS is inevitable, adjuvant therapy, e.g. SRS, should be evaluated for long-term tumor control. However, our study design does not allow any direct comparison between surgical GTR and DS plus SRS. This issue has yet to be investigated. Still, our results imply that if surgery is indicated in VS—even in patients with advanced age—GTR (or near-total tumor resection) should be the intention of surgery to ensure maximal tumor control. Also, our data imply that subtotal VS resection without adjuvant therapy is not recommended.
The EOR is significantly associated with RFS in both groups independent of age with noted early tumor recurrence in DS compared to STR and GTR. Pre-existing data show that the volume of residual tumor correlates with the incidence of recurrence (31, 32). GTR—whenever safely feasible—should be the primary intention-to-treat, and this study confirms the beneficial aspect in the tumor control of GTR compared to STR/DS even in an elderly cohort. We reckon the fact that STR is defined more strictly (minimal residual only in the IAC and complete removal of the tumor in the CPA) in this presented study than in previously published studies by other groups. Therefore, we suggest that the EOR must be defined homogenously to truly convey this observed relationship of the EOR, recurrence, and functional outcome to clinical day-to-day care in the form of intention-to-resect to patients’ benefit.
A definite statement on surgical treatment of VS is generally very difficult to acquire due to several reasons: (1) the level of evidence: there are no published randomized controlled clinical trials or even prospective studies on surgical resection in the current literature (11), (2) heterogenicity in surgical modality (e.g., approaches) by a heterogenous groups of specialists (neurosurgery and ENT), and 3) the lack of agreement of an EOR classification. To address these issues, an interdisciplinary network should be encouraged and a clinically relevant EOR classification should be enforced (including GTR, NTR, STR, and DS) to homogenously evaluate RFS and perioperative morbidity in larger multicenter settings. Such detailed distinction between the EOR might appear overelaborate, however, as tumor recurrence has shown to be dependent on the residual tumor, the idea of exact EOR classification has shown promise in other brain tumor entities already (33, 34).
Limitations of this study
It is apparent that the retrospective nature of this study bears its limitations and biases. Firstly, this is a single-center study. Therefore, the generalizability and reproducibility of the results may be limited to specialized centers with a comparative caseload in VS. Furthermore, although the number of patients classified as elderly may be regarded as the largest cohort compared to previously published studies (3, 10), still, the patient number and its value have to be put into its statistical context. Moreover, detailed subgroup analysis cannot be carried out (e.g., tumor size and cystic morphology) due to this cohort size.
Conclusion
The evidence level of VS management is remarkably low, and the debate on the risk–benefit ratio of surgical treatment is still ongoing, especially in the elderly. The present matched-cohort study shows that the microsurgical tumor resection of VS is safe and does not bear additional perioperative morbidity or worse functional outcome in the elderly as compared to a young control group. The overall incidence and of recurrence was statistically insignificant in the elderly compared to the young. When treating with surgery alone, the EOR determines RFS and the incidence of recurrence/progression in both study cohorts. For maximal tumor control, GTR should be intended. As incidence of recurrence is not significantly lower compared to the young. Postoperative follow-up should be carried out as mean time to recurrence was > 5 years in both groups. If leaving relevant tumor residual is inevitable, adjuvant therapy, e.g. SRS, should be evaluated in the Elderly.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SW contributed to the acquisition, analysis, interpretation of data, conception and design, and writing the first draft. KM and FE contributed to interpretation of the data and critical review of the final manuscript. GN contributed to data analysis, interpretation of the data, and reviewing the final manuscript. MT contributed to the interpretation of the data and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCI, Charlson Comorbidity Index; CDC, Clavien–Dindo Classification; DS, decompression surgery; ENT, ear, nose, and throat; EOR, extent of resection; G&R, Gardner and Robertson; H&B, House and Brackmann; IAC, internal auditory canal; GTR, gross total resection; KPS, Karnofsky Performance Score; MRI, magnetic resonance imaging; N/A, not applicable; SRS, stereotactic radiosurgery; STR, subtotal resection; VS, vestibular schwannoma.
References
1. McClelland S 3rd, Guo H, Okuyemi KS. Morbidity and mortality following acoustic neuroma excision in the united states: analysis of racial disparities during a decade in the radiosurgery era. Neuro Oncol (2011) 13(11):1252–9. doi: 10.1093/neuonc/nor118
2. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2015-2019. Neuro Oncol (2022) 24(Suppl 5):v1–v95. doi: 10.1093/neuonc/noac202
3. Jiang N, Wang Z, Chen W, Xie Y, Peng Z, Yuan J, et al. Microsurgical outcomes after gross total resection on vestibular schwannoma in elderly patients: a matched cohort study. World Neurosurg (2017) 101:457–65. doi: 10.1016/j.wneu.2017.01.120
4. Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the united states, 1985-1999. Neuro Oncol (2006) 8(1):27–37. doi: 10.1215/S1522851705000323
5. Nakatomi H, Jacob JT, Carlson ML, Tanaka S, Tanaka M, Saito N, et al. Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma. J Neurosurg (2017) 133(4):1–7. doi: 10.3171/2016.11.JNS16498
6. Carlson ML, Link MJ. Vestibular schwannomas. N Engl J Med (2021) 384(14):1335–48. doi: 10.1056/NEJMra2020394
7. Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurg (1991) 28(5):646–50; discussion 50-1. doi: 10.1097/00006123-199105000-00002
8. Kondziolka D, Mousavi SH, Kano H, Flickinger JC, Lunsford LD. The newly diagnosed vestibular schwannoma: radiosurgery, resection, or observation? Neurosurg Focus (2012) 33(3):E8. doi: 10.3171/2012.6.FOCUS12192
9. Goldbrunner R, Weller M, Regis J, Lund-Johansen M, Stavrinou P, Reuss D, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol (2020) 22(1):31–45. doi: 10.1093/neuonc/noz153
10. Van Abel KM, Carlson ML, Driscoll CL, Neff BA, Link MJ. Vestibular schwannoma surgery in the elderly: a matched cohort study. J Neurosurg (2014) 120(1):207–17. doi: 10.3171/2013.6.JNS122433
11. Wolbers JG, Dallenga AH, Mendez Romero A, van Linge A. What intervention is best practice for vestibular schwannomas? a systematic review of controlled studies. BMJ Open (2013) 3(2). doi: 10.1136/bmjopen-2012-001345
12. Samii M, Tatagiba M, Matthies C. Acoustic neurinoma in the elderly: factors predictive of postoperative outcome. Neurosurg (1992) 31(4):615–9; discussion 9-20. doi: 10.1227/00006123-199210000-00001
13. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med (1998) 339(20):1426–33. doi: 10.1056/NEJM199811123392003
14. Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg (1998) 88(3):506–12. doi: 10.3171/jns.1998.88.3.0506
15. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol (1984) 2(3):187–93. doi: 10.1200/JCO.1984.2.3.187
16. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol (1988) 97(1):55–66. doi: 10.1177/000348948809700110
17. Yen TL, Driscoll CL, Lalwani AK. Significance of house-brackmann facial nerve grading global score in the setting of differential facial nerve function. Otol Neurotol (2003) 24(1):118–22. doi: 10.1097/00129492-200301000-00023
18. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol (1994) 47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5
19. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
20. Bartek J Jr., Forander P, Thurin E, Wangerid T, Henriksson R, Hesselager G, et al. Short-term surgical outcome for vestibular schwannoma in Sweden: a nation-wide registry study. Front Neurol (2019) 10:43. doi: 10.3389/fneur.2019.00043
21. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet (2016) 388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6
22. Machetanz K, Leuze F, Mounts K, Trakolis L, Gugel I, Grimm F, et al. Occurrence and management of postoperative pneumocephalus using the semi-sitting position in vestibular schwannoma surgery. Acta Neurochirurgica (2020) 162(11):2629–36. doi: 10.1007/s00701-020-04504-5
23. Ansari SF, Terry C, Cohen-Gadol AA. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus (2012) 33(3):E14. doi: 10.3171/2012.6.FOCUS12163
24. Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst (2000) 5(4):191–208. doi: 10.1111/j.1529-8027.2000.00026.x
25. Tatagiba M, Ebner FH, Nakamura T, Naros G. Evolution in surgical treatment of vestibular schwannomas. Curr Otorhinolaryngol Rep (2021) 9(4):467–76. doi: 10.1007/s40136-021-00366-2
26. Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurg (2011) 68(1):68–77. doi: 10.1227/NEU.0b013e3181fc60cb
27. Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg (2016) 125(Suppl 1):64–72. doi: 10.3171/2016.7.GKS161494
28. Tolisano AM, Hunter JB. Hearing preservation in stereotactic radiosurgery for vestibular schwannoma. J Neurol Surg B Skull Base (2019) 80(2):156–64. doi: 10.1055/s-0039-1677680
29. Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after gamma knife radiosurgery. Lancet (2002) 360(9329):309–10. doi: 10.1016/S0140-6736(02)09521-1
30. Carlson ML, Barnes JH, Nassiri A, Patel NS, Tombers NM, Lohse CM, et al. Prospective study of disease-specific quality-of-Life in sporadic vestibular schwannoma comparing observation, radiosurgery, and microsurgery. Otol Neurotol (2021) 42(2):e199–208. doi: 10.1097/MAO.0000000000002863
31. Vakilian S, Souhami L, Melancon D, Zeitouni A. Volumetric measurement of vestibular schwannoma tumour growth following partial resection: predictors for recurrence. J Neurol Surg B Skull Base (2012) 73(2):117–20. doi: 10.1055/s-0032-1301395
32. Seol HJ, Kim CH, Park CK, Kim CH, Kim DG, Chung YS, et al. Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo) (2006) 46(4):176–80; discussion 80-1. doi: 10.2176/nmc.46.176
33. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry (1957) 20(1):22–39. doi: 10.1136/jnnp.20.1.22
Keywords: acoustic neuroma, neuro-oncology, skull base, geriatric, elderly, vestibular schwannoma
Citation: Wang SS-Y, Machetanz K, Ebner F, Naros G and Tatagiba M (2023) Association of extent of resection on recurrence-free survival and functional outcome in vestibular schwannoma of the elderly. Front. Oncol. 13:1153698. doi: 10.3389/fonc.2023.1153698
Received: 30 January 2023; Accepted: 02 May 2023;
Published: 05 June 2023.
Edited by:
Arianna Rustici, University of Bologna, ItalyReviewed by:
Konstantinos Gousias, University of Münster, GermanyJulius Höhne, University Medical Center Regensburg, Germany
Copyright © 2023 Wang, Machetanz, Ebner, Naros and Tatagiba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Shih-Yüng Wang, sophie.wang@med.uni-tuebingen.de
†These authors have contributed equally to this work
 Sophie Shih-Yüng Wang
Sophie Shih-Yüng Wang Kathrin Machetanz
Kathrin Machetanz Florian Ebner2
Florian Ebner2 Georgios Naros
Georgios Naros Marcos Tatagiba
Marcos Tatagiba