- Department of Burn and Plastic Surgery, West China Hospital, Sichuan University, Chengdu, China
The prevalence of Merkel cell polyomavirus(MCPyV) in Merkel cell carcinoma(MCC) and non-MCC skin lesions and its possible role in the etiology of other skin diseases remain controversial. To systematically assess the association between MCPyV infection and MCC, non-MCC skin lesions, and normal skin. For this systematic review and meta-analysis, a comprehensive search for eligible studies was conducted using Medline Ovid, Pubmed, Web of Science, and the Cochrane CENTRAL databases until August 2021; references were searched to identify additional studies. Observational studies that investigated the association between MCPyV infection and MCC, non-MCC skin lesions, and normal skin using polymerase chain reaction(PCR) as a detection method and provided sufficient data to calculate the prevalence of MCPyV positivity. A total of 50 articles were included in the study after exclusion criteria were applied. Two reviewers independently reviewed and assessed the eligibility of the studies, and all disagreements were resolved by consensus. To determine the association between MCPyV and MCC, overall odds ratio (OR) were calculated with 95% CI using a random-effects model. Single-arm meta-analyses were performed to examine the prevalence rate of MCPyV+ in MCC, non-MCC skin lesions, and normal skin. The primary analysis was the prevalence rate of MCPyV+ in MCC. Secondary outcomes included the prevalence rate of MCPyV+ in non-MCC skin lesions and normal skin. A total of 50 studies involving 5428 patients were reviewed based on our inclusion and exclusion criteria. Compared with the control group, MCPyV infection was significantly associated with MCC (OR = 3.51, 95% CI = 2.96 - 4.05). The global prevalence of MCPyV+ in MCC, melanoma, squamous cell carcinoma, basal cell carcinoma, Bowen’s disease, actinic keratosis, keratoacanthoma, seborrheic keratosis, and normal skin was 80%, 4%, 15%, 15%, 21%, 6%, 20%, 10%, and 11%, respectively. The current results suggest that MCPyV infection is significantly associated with an increased risk of MCC. However, the low prevalence rate of MCPyV+ in non-MCC skin lesions does not exclude a pathogenic association of this virus with the development of non-MCC skin lesions.
Introduction
Merkel cell carcinoma(MCC) is a rare, high-grade, aggressive cutaneous neuroendocrine tumor originally discovered in 1972 (1–3). MCC is prone to recurrence, regional metastases that frequently recur in lymph nodes, and distant metastases. Advanced age(> 50 years), demographic characteristics(predominantly European) and sun-exposed skin(ultraviolet radiation) are established risk factors for MCC (4, 5). In recent decades, the incidence of MCC has increased, as has the mortality rate (6).
Polyomaviruses(PyVs) are small, double-stranded DNA-based viruses that are usually non- oncogenic for their hosts but may be oncogenic to some species under certain circumstances (7). PyVs have three major genomic regions: an early region encoding large T antigen (LTA) and small T antigen (STA), both viral oncoproteins with replicative functions; a late region encoding viral structural proteins such as VP1, VP2, and VP3; and a noncoding control region(NCCR) that controls viral replication (8, 9). The identification of Merkel cell polyomavirus(MCPyV) by digital transcriptome analysis was a significant leap in the knowledge of the pathogenesis of MCC (8). According to molecular epidemiological studies, MCPyV has a wide range of prevalences in MCC. The prevalence of MCPyV varies widely worldwide, ranging from approximately 25% in Australian MCC patients to 100% in a French study (10, 11). In addition, MCPyV DNA has also been detected in non-MCC skin lesions and normal skin (12, 13). However, the mechanism of MCPyV infection and the prevalence of MCPyV in non-MCC skin lesions and its potential role in the pathogenesis of other malignant skin diseases are still unknown. To better understand this problem, we performed a systematic review and meta-analysis to examine the relationship between MCPyV and MCC, non-MCC skin lesions, and normal skin.
Methods
Literature Search
This article complies with the Declaration of Helsinki. Preferred Reporting Items for Systematic Reviews and Meta-analyses(PRISMA) guideline was used to conduct the study. Two of us(WAW and LY) comprehensively searched Medline Ovid, Pubmed, Web of Science, and the Cochrane CENTRAL databases from inception to August 1, 2021. Search terms were “merkel cell polyomavirus” and “skin neoplasms,” “skin malignancy,” “skin cancer,” “merkel cell carcinoma,” “squamous cell carcinoma,” “basal cell carcinoma,” “melanoma,” “bowen disease,” “actinic keratosis,” “keratoacanthoma,” “seborrheic keratosis” “non-lesional skin” or “normal skin.” Searches were limited to human participants and English-language publications. We also conducted manual searches of the reference lists of the extracted articles to identify additional relevant publications. Only studies meeting the eligibility criteria outlined below were included in the meta-analysis.
Eligibility Criteria
The extracted data were required to meet the following criteria: (1) designed as a cohort, case-control study, or cross-sectional study; (2) confirmed the presence of MCPyV by polymerase chain reaction(PCR); (3) reported the detection of MCPyV in MCC, squamous cell carcinoma(SCC), basal cell carcinoma(BCC), melanoma, Bowen’s disease, actinic keratosis, keratoacanthoma, seborrheic keratosis or normal skin; (4) full text available.
Studies that met more than one of the following criteria were excluded: (1) insufficient raw data to estimate the outcome; (2) animal study, in vitro study, case report, review, editorial, or commentary; (2) the available data could not be extracted from the article by calculation or by contacting the authors; and (3) multiple studies with overlapping samples. The studies with a more significant number of patients were selected when overlapping study samples were identified. Two reviewers(WAW and LY) independently performed the study selection process, and consensus resolved disagreements.
Data Extraction and Quality Assessment
Data were extracted by the two independent reviewers (WAW and LY) using a structured Excel(Microsoft Corp., Redmond, Washington) data collection spreadsheet as a priori. Discrepancies were discussed and resolved within the research team. The following data were retrieved for the included studies: first author, publication year, country, study design, number of patients in each group (MCC, SCC, BCC, melanoma, Bowen’s disease, actinic keratosis, keratoacanthoma, seborrheic keratosis, and normal skin), number of patients in each group above with MCPyV+, sample types [frozen section(FR) or formalin-fixed paraffin-embedded (FFPE)], PCR primers, and immune status. Eligible studies were further divided into two different analyses: primary and secondary. The primary analysis was the prevalence rate of MCPyV in MCC. Secondary outcomes included the prevalence rate of MCPyV in non-MCC skin lesions(melanoma, SCC, BCC, Bowen’s disease, actinic keratosis, keratoacanthoma, and seborrheic keratosis) and normal skin.
Quality assessment of included studies was performed using the Newcastle-Ottawa scale for cohort and case-control studies (14). The Newcastle-Ottawa scale consists of selection, comparability, and outcome(or exposure for case-control studies). A study can receive one score in each of the domains of selection and outcome and two scores in the domain of comparability. Studies with a low risk of bias had a score of less than 4, those with a score of 4 to 6 had an intermediate risk of bias, and those with a score of 7 or higher had a low risk of bias.
Statistical Analysis
Stata 15.1(StataCorp, College Station, TX USA, 2018) was used to analyze the data after it had been checked for consistency. The “metaprop” command was used to generate pooled effect size(ES) for noncomparative binary outcomes. The 95% confidence interval (CI) was generated using the DerSimonian-Laird random-effects model with FreemanTukey double arcsine transformation and evaluated using the Wilson score technique. The Cochran Q and I2 statistics were used to test for heterogeneity among the chosen studies. Mild, moderate, and severe heterogeneity were defined as I2 statistics of 25% - 50%, 50% - 75%, and >75%, respectively. A random-effects model was used to produce the pooled estimate and 95% CI if heterogeneity was more than 50%. The Mantel-Haenszel method was used to evaluate dichotomous variables, and the results are presented as ORs. Subgroup analysis and meta-regression were employed when heterogeneity was evident based on important variables(country, continent, sample type). Sensitivity analysis was performed to estimate the influence of a single study on the pooled ORs. Statistical significance is defined as a two-tailed P-value of less than 0.05. The visual estimation of a funnel pot, Egger’s test, Begg’s test, and the trim & fill method were used to determine and correct publication bias (P =0.05 was considered significant).
Results
Search Results and Included Trials
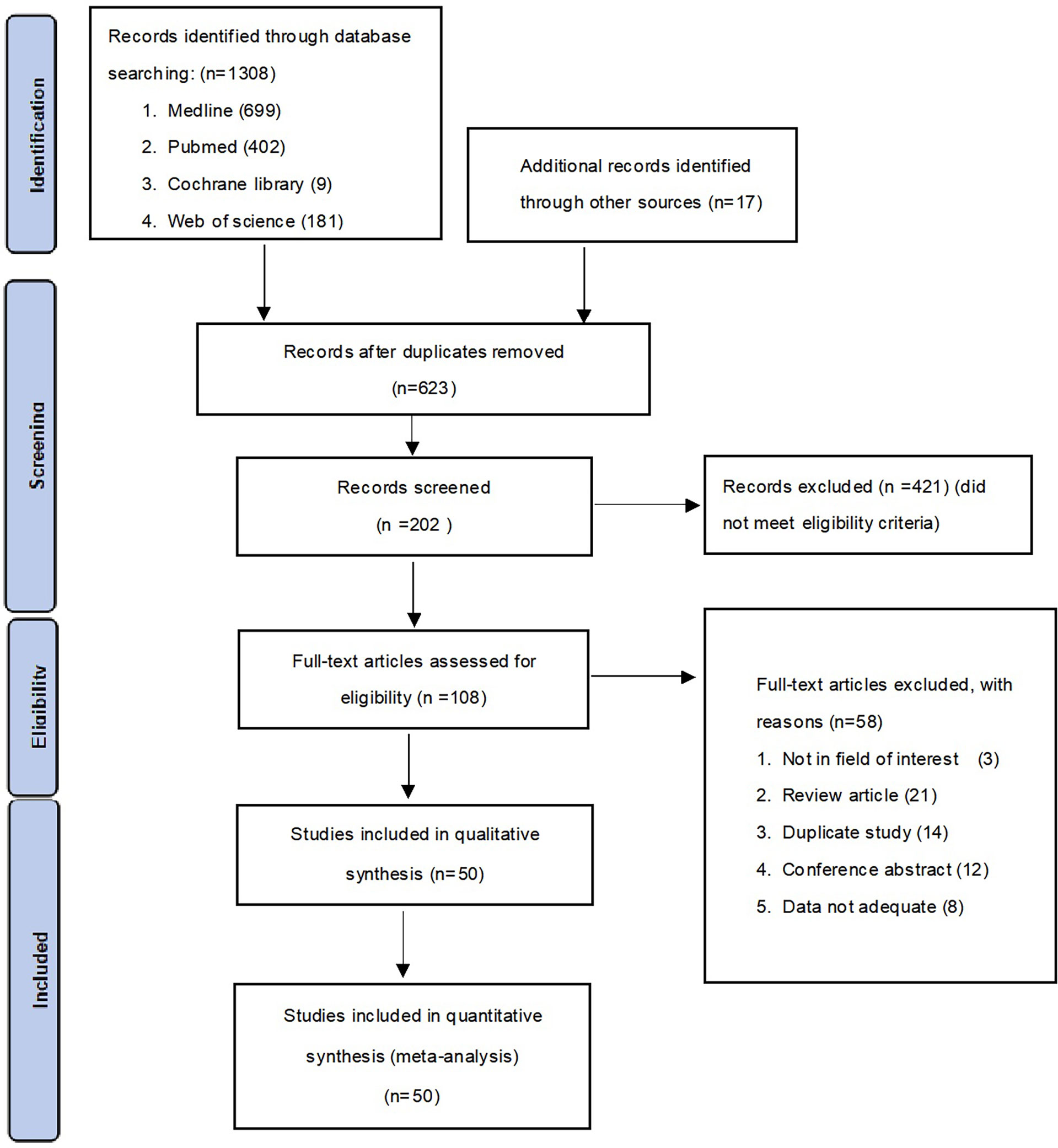
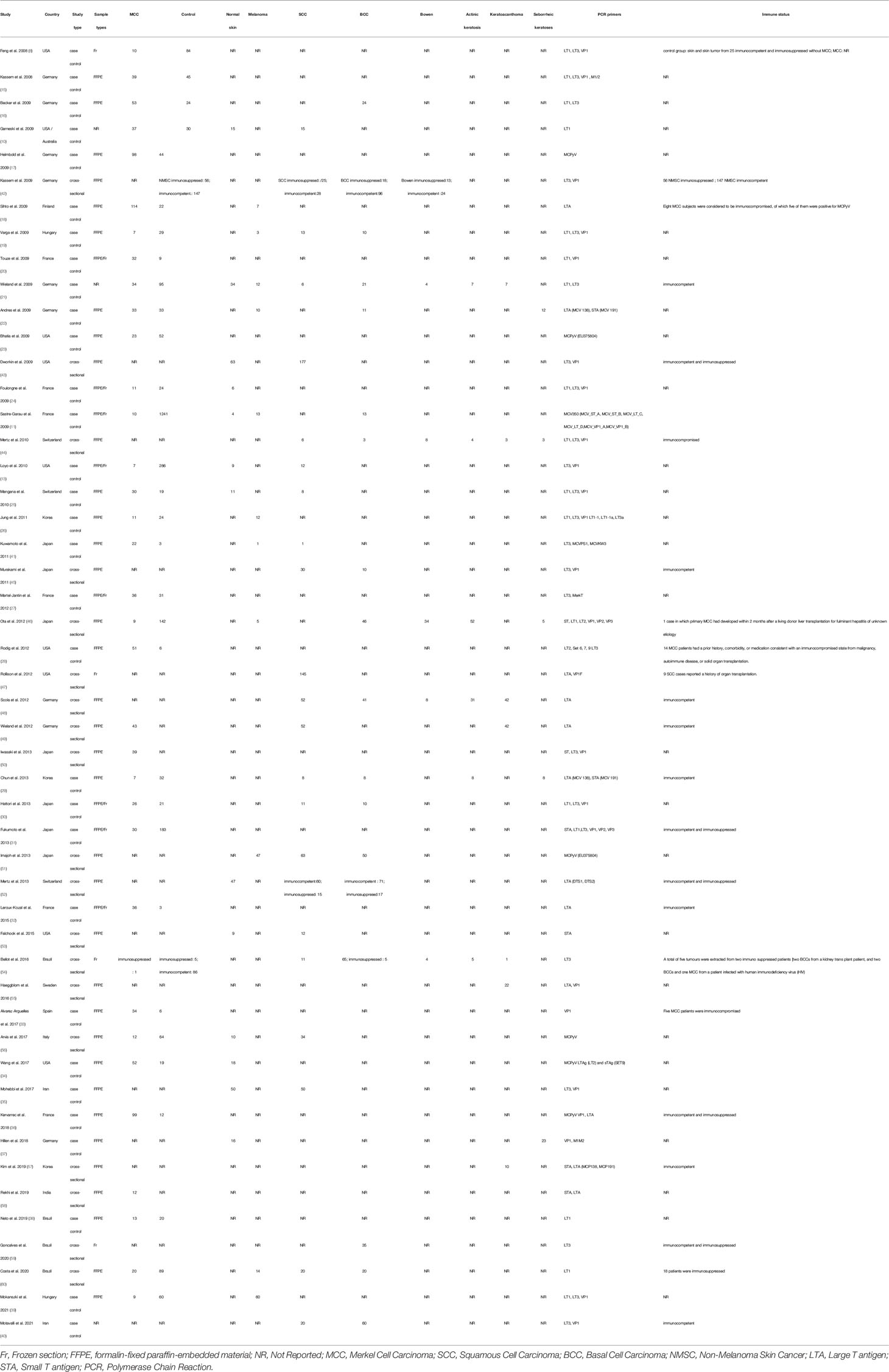
A total of 1308 studies were identified through the literature search. After adjustment for duplicates, 623 articles remained. Of these, 421 articles were removed after reviewing the titles and abstracts. After a full-text review of the remaining 108 articles, 58 articles were further excluded based on the following criteria: 3 studies were not in the field of interest, 21 studies were review articles, 14 studies were duplicates, 12 were conference abstracts, and eight studies had insufficient data. Finally, 50 studies consisting of 31 case-control studies (1812 participants) and 19 cross-sectional studies (3616 participants) were included in the meta-analysis. The flowchart for the selection process and detailed identification is shown in Figure 1. The 50 included studies were published between 2008 and 2021 in 15 different countries. Thirty five studies reported the prevalence of MCPyV+ in MCC patients, 13 studies in normal skin, 11 studies in cutaneous melanoma patients, 23 studies in SCC patients, 17 studies in BCC patients, seven studies in keratoacanthoma patients, six studies in Bowen’s disease and actinic keratosis patients, and five studies in patients with seborrheic keratosis. Thirty studies (8, 10, 11, 13, 15–40) received a score of 7 on the NOS score, while 1 study (41) received a score of 6. All were classified as low risk of bias after quality assessment. However, 19 studies (42–60) had a intermediate risk of bias. Table 1 summarizes the characteristics of the included articles, and the quality of the papers is assessed in Table S1.
Primary Meta-Analysis: Merkel Cell Polyomavirus Prevalence in MCC
In the pooled analysis, the association between MCPyV and MCC was significant with an adjusted pooled OR of 3.51 (95% CI = 2.96 - 4.05, P<0.05) in the random-effects model due to significant heterogeneity between studies (I2 = 58.02%)(Figure 2). The meta-regression analysis revealed that country (P=0.474), continent (P=0.220) and sample type (P=0.675) did not influence the heterogeneity between studies. The sensitivity analysis showed that no single study influenced the recalculated pooled ORs (Figure S1). Visual inspection of the funnel plot showed evidence of publication bias (Figure S2), which was confirmed by Egger’s test(P= 0.0006) and Begg’s test(P= 0.0037). We then applied the trim and fill method to correct the asymmetry of the funnel plot (Figure S3). Pooled analysis included the imputed studies continued to indicate a statistically significant association between MCPyV and MCC. The result showed that the effect of publication bias was not significant and the conclusion was relatively stable.
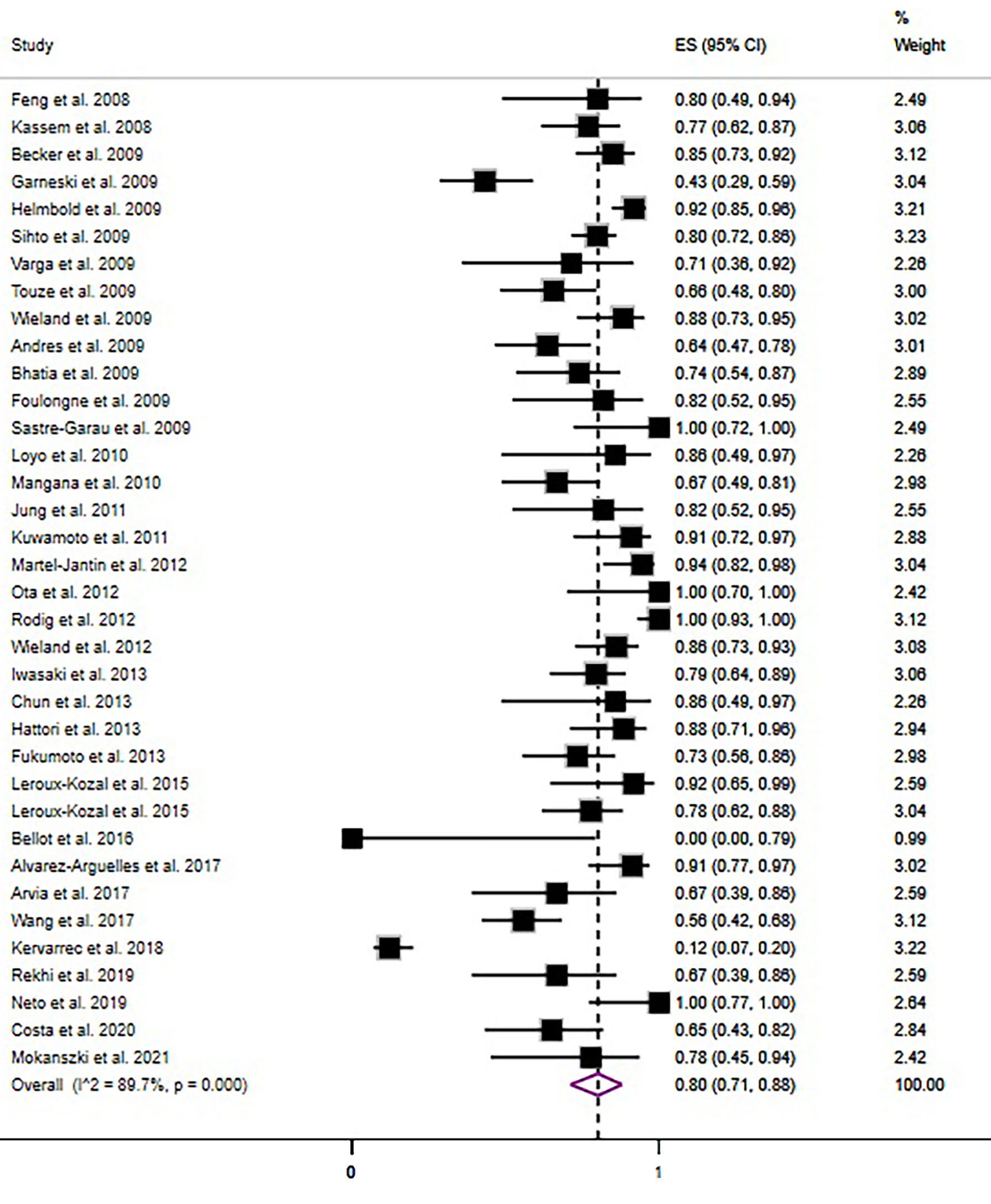
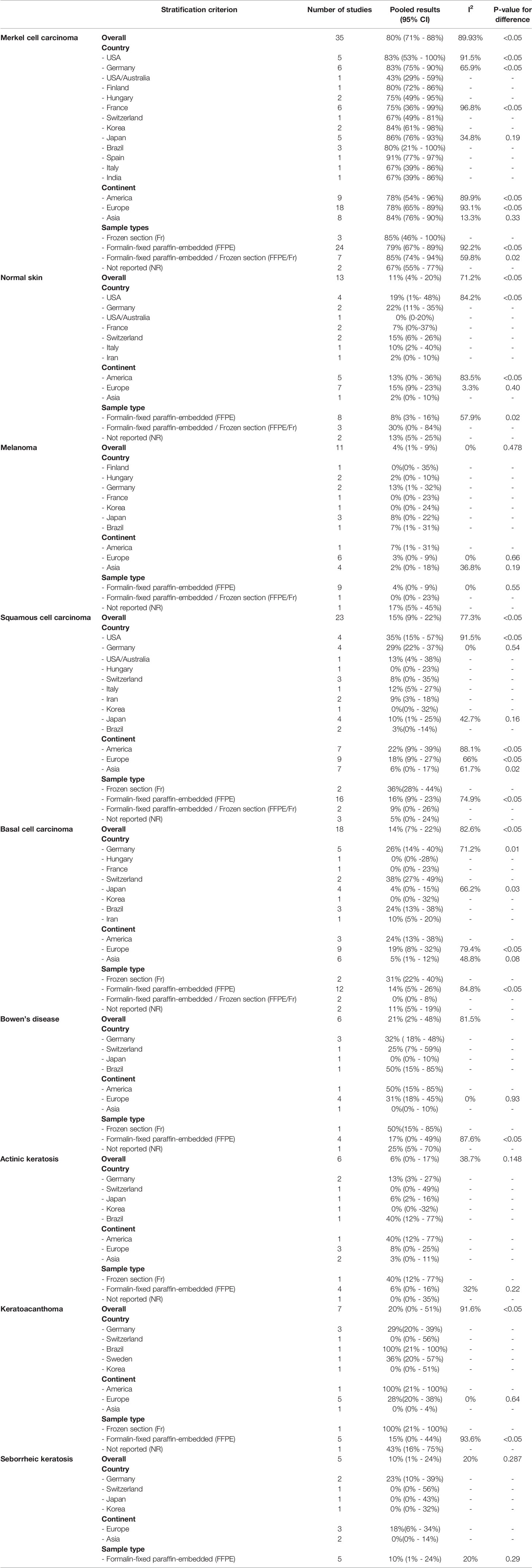
The overall pooled prevalence rate of MCPyV+ in MCC was 80% (95% CI = 71% - 88%, I2 = 89.93%, P<0.05)(Figure 3). We then performed a subgroup analysis based on country, continent, and sample type (frozen section or formalin-fixed paraffin-embedded material). This pooled rate remained consistent in the subgroup analysis, with statistically significant heterogeneity between subgroups (Table 2 and Figures S4–6). There was no obvious source of heterogeneity in the meta-regression analysis(P=0.587). The funnel plot, Egger’s test (P = 0.284) and Begg’s test (P = 0.173) did not indicate publication bias.
Secondary Meta-Analyses: Non-MCC Skin Lesions and Normal Skin
Melanoma
Eleven studies (11, 18, 19, 21, 22, 26, 39, 41, 46, 51, 60)investigated the prevalence rate of MCPyV+ in melanoma, the overall prevalence rate was 4% (95% CI = 1% - 9%, I2 = 0%, P = 0.473)(Figure 4A). In addition, subgroup analysis by country, continent, and sample type still showed significant heterogeneity (Table 2 and Figures S10–12). The funnel plot, Egger’s test (P = 0.150), and Begg’s test (P = 0.080) detected no publication bias.

Figure 4 Forest plot illustrating the pooled prevalence rate of the MCPyV positivity in non-MCC skin lesions and normal skin. (A) melanoma; (B) squamous cell carcinoma; (C) basal cell carcinoma; (D) Bowen’s disease; (E) actinic keratosis; (F) keratoacanthoma; (G) seborrheic keratosis; (H) normal skin.
Squamous Cell Carcinoma
Twenty three studies (10, 13, 19, 21, 25, 29, 30, 35, 40–45, 47–49, 51–54, 56, 60) reported the prevalence rate of MCPyV+ in squamous cell carcinoma samples, with the overall prevalence rate was 15%(95% CI = 9% - 22%, I2 = 77.3%, P<0.05)(Figure 4B). The pooled prevalence rate remained similar in the stratified analysis, with statistically significant heterogeneity across all subgroups(Table 2 and Figures S13-15). We discovered a significant difference in pooled MCPyV+ prevalence in squamous cell carcinoma in American studies 22%(95% CI = 9% - 39%) when compared to Asian studies 6%(95% CI = 0% - 17%), but not when compared to prevalence in Europe 18%(95% CI = 9% - 27%). The point estimates for the prevalence of MCPyV+ in squamous cell carcinoma in frozen section sample 36%(95% CI = 28% - 44%) was twice of the formalin-fixed paraffin-embedded sample. There was no evidence of publication bias as indicated by funnel plot analysis, Egger’s test(P = 0.133), and Begg’s test(P = 0.065).
Basal Cell Carcinoma
The 18 included studies (11, 16, 19, 21, 22, 29, 30, 40, 42, 44–46, 48, 51, 52, 54, 59, 60) reported the prevalence rate of the MCPyV+ in basal cell carcinoma, with the overall prevalence rate was 14%(95% CI = 7% - 22%, I2 = 82.58%, P<0.05)(Figure 4C). Stratification analysis showed increasing trends for American studies 24%(95% CI = 13% - 38%) and stable trends for European 19%(95% CI = 8% - 32%) and Asian studies 5%(95% CI = 1% - 12%). Frozen section samples 31%(95% CI = 22% - 40%) showed a higher prevalence rate than FFPE samples 14%(95% CI = 5% - 26%). While stratification analysis still showed significant heterogeneity(Table 2 and Figures S16-18). According to the funnel plot, Egger’s test(P = 0.059), and Begg’s test(P = 0.075), there was no significant publication bias across the studies for either analysis.
Bowen’s Disease
Several studies (21, 42, 44, 46, 48, 54) investigated the prevalence rate of MCPyV+ in Bowen’s disease, with the pooled prevalence rate was 21%(95% CI = 2% - 48%, I2 = 81.53%, P<0.05)(Figure 4D). All subgroup analysis still showed significant heterogeneity (Table 2 and Figures S19-21). In addition, there was an apparent lower prevalence in Asia than Americas(0% vs 50%). The funnel plot, Egger’s test(P = 0.257), and Begg’s test(P = 0.388) revealed no substantial publication bias.
Actinic Keratosis
The pooled analysis of six studies (21, 29, 44, 46, 48, 54) reporting the prevalence of MCPyV+ in actinic keratosis showed a prevalence rate of 6%(95% CI = 0% - 17%, I2 = 38.69%, P = 0.15)(Figure 4E). Results of the stratification analysis are shown in Table 2 and Figures S22-24. Visual inspection of the funnel plot, Egger’s test(P = 0.899), and Begg’s test(P = 0.274), there was no evidence of significant publication bias.
Keratoacanthoma
According to seven publications (21, 44, 48, 49, 54, 55, 57) that examined the prevalence rate of MCPyV+ in keratoacanthoma, the pooled prevalence rate was 20%(95% CI = 0% - 51%), I2 = 91.58%, P<0.05)(Figure 4F). Stratified analysis showed statistically significant heterogeneity in all subgroups, although the pooled prevalence rate remained identical (Table 2 and Figures S25–27). There was no evidence of substantial publication bias, as determined by visual inspection of the funnel plot, Egger’s test(P = 0.126), and Begg’s test(P = 0.301).
Seborrheic Keratosis
Five studies (22, 29, 37, 44, 46) were included in the analysis of the prevalence rate of MCPyV+ in seborrheic keratosis, with the overall prevalence rate was 10% (95% CI = 1% - 24%, I2 = 19.98%, P = 0.29)(Figure 4G). This pooled rate remained consistent in subgroup analysis, with statistically significant heterogeneity between subgroups (Table 2 and Figures S28-30). According to the funnel plot analysis, Egger’s test(P = 0.105), and Begg’s test(P = 0.072) there was no evidence of publication bias.
Normal Skin
Based on data from 13 publications (10, 11, 13, 21, 24, 25, 34, 35, 37, 43, 52, 53, 56) the overall pooled estimate of the prevalence of MCPyV+ in normal skin was 11% (95% CI = 4% - 20%, I2 = 71.2%, P<0.05)(Figure 4H). Further stratification by country, continent, and sample type are shown in Table 2 and Figures S7-9. In the USA, the American continent, and the FFPE study subgroups, heterogeneity remained significant. No publication bias was detected by funnel plot, Egger’s test (P = 0.967), or Begg’s test (P = 0.802).
Discussion
Numerous factors contribute to the aetiology of non-MCC skin lesions, including UV exposure, immunosuppression, and ageing, which are also risk factors for the development of MCC (45, 53). Feng et al. (8) first discovered MCPyV as a human polyomavirus that reveals clonal integration in MCC. MCPyV showed that the viral genome was integrated into the host genome, disrupting the late region. In addition, a C-terminal truncated LT was expressed. The helicase activity of LT, which is required for viral DNA replication, was removed by this deletion (16). MCPyV infects the majority of people and, according to seroepidemiological studies, causes lifelong harmless chronic infection in healthy people (61–63). MCPyV is also regularly shed from the skin of healthy people, proving that it is a component of the human skin microbiome (64). Dermal fibroblast cells could be the natural host cell for replication of MCPyV in the human body, as the virus could be propagated in human dermal fibroblast cell cultures (65). The role of MCPyV in the development of MCC and the wide distribution of the virus in the body prompted researchers to investigate the prevalence of MCPyV in non-MCC skin lesions. Several studies have shown clonal integration of MCPyV in the non-MCC skin lesions. However, the prevalence of MCPyV in the MCC and non-MCC skin lesions is still controversial. Our study aimed to shed light on this matter.
To the best of our knowledge, this is the first systematic review and meta-analysis to provide comprehensive, up-to-date estimates of the association of MCPyV in MCC and non-MCC skin lesions. We identified a global pooled prevalence of 80% MCPyV+ among 1112 patients with MCC. This finding is consistent with a previous meta-analysis by Santos-Juanes et al. (66) which reported a prevalence of 79%. A geographic and sample type variation of MCPyV+ MCC has well been documented in a previous study. Data from the Americas and Europe show that nearly 80% of MCC cases are MCPyV+ (10, 67), while studies from Australia found that only 24% of cases are MCPyV+ (67). The lower prevalence of MCPyV+ in Australian studies compared to other continents may be due to the increased sun exposure in Australia, making a possible viral contribution less common and the possibility that a different and unknown strain of MCPyV is undetectable (10). In Asia, MCPyV+ is found in 76.9% to 88.5% of Japanese (29, 41, 45, 46, 48), 81.2% to 85.71% of Korean (29, 57), and 25% of Indian MCC patients (58). Several studies have shown that the MCPyV detection rate of DNA was greater in frozen samples than in FFPE tissue samples (12, 27). On the contrary, through subgroup analyses, we found no significant differences in the prevalence rate of MCPyV+ MCC among countries, continents, and different sample types (Table 2).
The discovery of MCPyV DNA in non-melanoma skin cancers(NMSCs) from immunocompromised people was the first observation linking MCPyV to non-MCC (15). MCPyV was later found in various non-MCC skin lesions and normal skin (Table 1). Recent studies showed that non-MCC skin lesions significantly have lower MCPyV DNA viral loads than in MCC. MCPyV DNA was significantly positive in non-melanoma skin cancer in immunosuppressed patients compared with non-immunosuppressed patients (38, 48, 68). Our meta-analytic study showed that the pooled prevalence rate of MCPyV+ in melanoma, SCC, BCC, Bowen’s disease, actinic keratosis, keratoacanthoma, seborrheic keratosis, and normal skin was 4%, 15%, 14%, 21%, 6%, 20%, 10%, and 11%, respectively (Table 2). The low prevalence rate of MCPyV in non-MCC skin lesions, which is similar or even lower to that in normal skin, suggests that MCPyV probably plays a minor role in the development of non-MCC skin lesions. Subgroup analysis by continent showed that trends were higher in the Americas for SCC, BCC, Bowen’s disease, actinic keratosis, and keratoacanthomas, with the corresponding rates being lower or relatively similar to the overall pooled prevalence in the Asian and European continents, respectively. In addition, we found that the detection rate for DNA extracted from frozen section samples was higher than for DNA extracted from FFPE samples, suggesting that degradation of DNA in FFPE tissues caused by formalin fixation makes PCR less sensitive (12, 20, 24, 27). The presence of MCPyV DNA in the skin and non-MCC skin lesions might not be a surprising phenomenon, as one would expect, because it is due to the ability of HPyVs to infect the skin and remain in a latent form that can be reactivated in states of profound immunosuppression (69, 70). MCPyV is a cutaneous microbe that is generally acquired in early childhood when it has the opportunity to integrate into the host genome of dermal fibroblast cells (65, 71). Regardless of these findings, it is apparent that the presence of MCPyV DNA alone is not sufficient to cause malignancy (38). Therefore, the oncogenic significance of MCPyV in non-MCC skin lesions is still debatable.
The limitations of our article also warrant considerations. First, because randomized trials are neither currently available nor likely to be conducted in the future, this meta-analysis relies on observational data. As a result, unmeasured biases in individual studies must be taken into consideration. Second, further assessment revealed that there were several sources of heterogeneity among the included studies: (1) heterogeneity of study population(age, gender, immune status, smoking and drinking habits, geographic differences, sun exposure, etc.), (2) the relatively small number of specimens examined may give a wrong view of the prevalence of MCPyV in specific samples, (3) methods performed to detect MCPyV viral load(i.e., primers selection, viral DNA copy number, etc.), and (4) PCR screening method (i.e., the quality of the samples, viral gene target selection, DNA extraction method, primer selection, PCR technique, false-positivity due to PCR contamination, etc.). To overcome these problems and convincingly determine MCPyV positivity, several multimodal approaches have recently been proposed, such as immunohistochemistry and PCR assay (IHC + PCR), fluorescence in situ hybridization(FISH) coupled with DNA hybridization chain reaction(HCR-DNA FISH), etc., which have been shown to be a highly sensitive approach to detect the viral genome in tissue samples (72, 73). Third, MCPyV may contribute to cancer onset through a “hit-and-run” mechanism (74, 75). Therefore, tumor samples from different stages should be examined because the virus has only transient effects in cellular transformation, as it can be silenced or its genome lost during cancer progression (76).
Conclusion
Our results suggest a ubiquitous distribution of MCPyV in the skin with higher MCPyV positivity in MCC tumors, closely linking MCPyV as a putative etiologic agent to the carcinogenesis of MCC. However, the significantly lower prevalence rate of MCPyV+ in non-MCC skin lesions does not exclude a pathogenic association of this virus with the development of non-MCC skin lesions. Further large-scale studies using uniform viral genome detection methods are needed to determine the precise role of MCPyV in MCC pathogenesis and to define the significance of detecting viral DNA in non-MCC skin lesions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
WW, conceptualization, methodology, visualization, and writing—original draft preparation, formal analysis, investigation, writing— review and editing, and supervision. ZL, YQ, supervision and funding acquisition. WW, YL methodology and visualization. YL data curationand sample contribution. All authors contributed to the article and approved the submitted version.
Funding
This research article was funded by the Science and Technology Support Program of Science and Technology Department of Sichuan Province (2020YFS0267), the Key Project Research and Invention Program of Science and Technology Department of Sichuan Province(2021YFS0245), the National Natural Science Foundation of China (81871574).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.868781/full#supplementary-material
References
1. Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of Prognostic Factors From 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol (2016) 23:3564–71. doi: 10.1245/s10434-016-5266-4
2. Harms PW. Update on Merkel Cell Carcinoma. Clin Lab Med (2017) 37:485–501. doi: 10.1016/j.cll.2017.05.004
3. Toker C. Trabecular Carcinoma of the Skin. Arch Dermatol (1972) 105:107–10. doi: 10.1001/archderm.105.1.107
4. Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, et al. Merkel Cell Carcinoma: Critical Review With Guidelines for Multidisciplinary Management. Cancer (2007) 110:1–12. doi: 10.1002/cncr.22765
5. Rockville Merkel Cell Carcinoma Group. Merkel Cell Carcinoma: Recent Progress and Current Priorities on Etiology, Pathogenesis, and Clinical Management. J Clin Oncol (2009) 27:4021–6. doi: 10.1200/JCO.2009.22.6605
6. Hodgson NC. Merkel Cell Carcinoma: Changing Incidence Trends. J Surg Oncol (2005) 89:1–4. doi: 10.1002/jso.20167
7. zur Hausen H. A Specific Signature of Merkel Cell Polyomavirus Persistence in Human Cancer Cells. Proc Natl Acad Sci USA (2008) 105:16063. doi: 10.1073/pnas.0808973105
8. Feng H, Shuda M, Chang Y, Moore PS. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science (2008) 319(5866):1096–100. doi: 10.1126/science.1152586
9. Lim ES, Reyes A, Antonio M, Saha D, Ikumapayi UN, Adeyemi M, et al. Discovery of STL Polyomavirus, a Polyomavirus of Ancestral Recombinant Origin That Encodes a Unique T Antigen by Alternative Splicing. Virology (2013) 436(2):295–303. doi: 10.1016/j.virol.2012.12.005
10. Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel Cell Polyomavirus is More Frequently Present in North American Than Australian Merkel Cell Carcinoma Tumors. J Invest Dermatol (2009) 129(1):246–8. doi: 10.1038/jid.2008.229
11. Sastre-Garau X, Peter M, Avril MF, Laude H, Couturier J, Rozenberg F, et al. Merkel Cell Carcinoma of the Skin: Pathological and Molecular Evidence for a Causative Role of MCV in Oncogenesis. J Pathol (2009) 218(1):48–56. doi: 10.1002/path.2532
12. Laude HC, Jonchere B, Maubec E, Carlotti A, Marinho E, Couturaud B, et al. Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and non Tumour Specimens From Patients With Merkel Cell Carcinoma. PloS Pathog (2010) 6(8):e1001076. doi: 10.1371/journal.ppat.1001076
13. Loyo M, Guerrero-Preston R, Brait M, Hoque MO, Chuang A, Kim MS, et al. Quantitative Detection of Merkel Cell Virus in Human Tissues and Possible Mode of Transmission. Int J Cancer (2010) 126(12):2991–6. doi: 10.1002/ijc.24737
14. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
15. Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent Detection of Merkel Cell Polyomavirus in Human Merkel Cell Carcinomas and Identification of a Unique Deletion in the VP1 Gene. Cancer Res (2008) 68(13):5009–13. doi: 10.1158/0008-5472.CAN-08-0949
16. Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC Polyomavirus is Frequently Present in Merkel Cell Carcinoma of European Patients. J Invest Dermatol (2009) 129(1):248–50. doi: 10.1038/jid.2008.198
17. Helmbold P, Lahtz C, Enk A, Herrmann-Trost P, Marsch WCH, Kutzner H, et al. Frequent Occurrence of RASSF1A Promoter Hypermethylation and Merkel Cell Polyomavirus in Merkel Cell Carcinoma. Research Support, non-U.S. Gov’t. Mol Carcinog (2009) 48(10):903–9. doi: 10.1002/mc.20540
18. Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical Factors Associated With Merkel Cell Polyomavirus Infection in Merkel Cell Carcinoma. J Natl Cancer Instit (2009) 101(13):938–45. doi: 10.1093/jnci/djp139
19. Varga E, Kiss M, Szabo K, Kemeny L. Detection of the Merkel Cell Polyomavirus (MCV) DNA in Tumor Samples of Merkel Cell Carcinoma Patients. Conference Abstract. J Invest Dermatol (2009) 129(2):S74. doi: 10.1111/j.1365-2133.2009.09221
20. Touze A, Gaitan J, Maruani A, Le Bidre E, Doussinaud A, Clavel C, et al. Merkel Cell Polyomavirus Strains in Patients With Merkel Cell Carcinoma. Emerg Infect Dis (2009) 15(6):960–2. doi: 10.3201/eid1506.081463
21. Wieland U, Mauch C, Kreuter A, Krieg T, Pfister H. Merkel Cell Polyomavirus Is Prevalent in Normal and Lesional Skin and Mucosa of Individuals Without Merkel Cell Carcinoma. Conference Abstract. J Invest Dermatol (2009) 129(2):S101. doi: 10.3201/eid1509.081575
22. Andres C, Belloni B, Puchta U, Sander CA, Flaig MJ. Prevalence of Mcpyv in Merkel Cell Carcinoma and non-MCC Tumors. J Cutaneous Pathol (2010) 37(1):28–34. doi: 10.1111/j.1600-0560.2009.01352.x
23. Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Merkel Cell Carcinoma Subgroups by Merkel Cell Polyomavirus DNA Relative Abundance and Oncogene Expression. Int J Cancer (2010) 126(9):2240–6. doi: 10.1002/ijc.24676
24. Foulongne V, Dereure O, Kluger N, Molès J, Guillot B, Segondy M. Merkel Cell Polyomavirus DNA Detection in Lesional and Nonlesional Skin From Patients With Merkel Cell Carcinoma or Other Skin Diseases. Br J Dermatol (2010) 162(1):59–63. doi: 10.1111/j.1365-2133.2009.09381.x
25. Mangana J, Dziunycz P, Kerl K, Dummer R, Cozzio A. Prevalence of Merkel Cell Polyomavirus Among Swiss Merkel Cell Carcinoma Patients. Dermatology (2010) 221(2):184–8. doi: 10.1159/000315067
26. Jung HS, Choi YL, Choi JS, Roh JH, Pyon JK, Woo KJ, et al. Detection of Merkel Cell Polyomavirus in Merkel Cell Carcinomas and Small Cell Carcinomas by PCR and Immunohistochemistry. Histol Histopathol (2011) 26(10):1231–41. doi: 10.14670/HH-26.1231
27. Martel-Jantin C, Filippone C, Cassar O, Peter M, Tomasic G, Vielh P, et al. Genetic Variability and Integration of Merkel Cell Polyomavirus in Merkel Cell Carcinoma. Virology (2012) 426(2):134–42. doi: 10.1016/j.virol.2012.01.018
28. Rodig SJ, Cheng JW, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved Detection Suggests All Merkel Cell Carcinomas Harbor Merkel Polyomavirus. J Clin Invest (2012) 122(12):4645–53. doi: 10.1172/JCI64116
29. Chun SM, Yun SJ, Lee SC, Won YH, Lee JB. Merkel Cell Polyomavirus is Frequently Detected in Korean Patients With Merkel Cell Carcinoma. Ann Dermatol (2013) 25(2):203–7. doi: 10.5021/ad.2013.25.2.203
30. Hattori T, Takeuchi Y, Takenouchi T, Hirofuji A, Tsuchida T, Kabumoto T, et al. The Prevalence of Merkel Cell Polyomavirus in Japanese Patients With Merkel Cell Carcinoma. Multicenter Study. J Dermatol Sci (2013) 70(2):99–107. doi: 10.1016/j.jdermsci.2013.02.010
31. Fukumoto H, Sato Y, Hasegawa H, Katano H. Frequent Detection of Merkel Cell Polyomavirus DNA in Sera of HIV-1-Positive Patients. Virol J (2013) 10:84. doi: 10.1186/1743-422X-10-84
32. Leroux-Kozal V, Leveque N, Brodard V, Lesage C, Dudez O, Makeieff M, et al. Merkel Cell Carcinoma: Histopathologic and Prognostic Features According to the Immunohistochemical Expression of Merkel Cell Polyomavirus Large T Antigen Correlated With Viral Load. Hum Pathol (2015) 46(3):443–53. doi: 10.1016/j.humpath.2014.12.001
33. Alvarez-Arguelles ME, Melon S, Rojo S, Fernandez-Blázquez A, Boga JA, Palacio A, et al. Detection and Quantification of Merkel Cell Polyomavirus. Analysis of Merkel Cell Carcinoma Cases From 1977 to 2015. J Med Virol (2017) 89(12):2224–9. doi: 10.1002/jmv.24896
34. Wang L, Harms PW, Palanisamy N, Carskadon S, Cao X, Siddiqui J, et al. Age and Gender Associations of Virus Positivity in Merkel Cell Carcinoma Characterized Using a Novel RNA in Situ Hybridization Assay. Clin Cancer Res (2017) 23(18):5622–30. doi: 10.1158/1078-0432.CCR-17-0299
35. Mohebbi E, Noormohamadi Z, Sadeghi-Rad H, Sadeghi F, Yahyapour Y, Vaziri F, et al. Low Viral Load of Merkel Cell Polyomavirus in Iranian Patients With Head and Neck Squamous Cell Carcinoma: Is it Clinically Important? J Med Virol (2018) 90(2):344–50. doi: 10.1002/jmv.24953
36. Kervarrec T, Samimi M, Gaboriaud P, Gheit T, Beby-Defaux A, Houben R, et al. Detection of the Merkel Cell Polyomavirus in the Neuroendocrine Component of Combined Merkel Cell Carcinoma. Virchows Archiv (2018) 472(5):825–37. doi: 10.1007/s00428-018-2342-0
37. Hillen LM, Rennspiess D, Speel EJ, Haugg AM, Winnepenninckx V, Hausen AZ. Detection of Merkel Cell Polyomavirus in Seborrheic Keratosis. Front Microbiol (2018) 8:2648. doi: 10.3389/fmicb.2017.02648
38. Neto CF, Oliveira WRP, Costa PVA, Cardoso MK, Barreto PG, Romano CM, et al. The First Observation of the Association of Merkel Cell Polyomavirus and Merkel Cell Carcinoma in Brazil. Int J Dermatol (2019) 58(6):703–6. doi: 10.1111/ijd.14325
39. Mokánszki A, Méhes G, Csoma SL, Kollár S, Chang Chien YC. Molecular Profiling of Merkel Cell Polyomavirus-Associated Merkel Cell Carcinoma and Cutaneous Melanoma. Diagn (Basel) (2021) 11(2):212. doi: 10.3390/diagnostics11020212
40. Motavalli Khiavi F, Nasimi M, Rahimi H. Merkel Cell Polyomavirus Gene Expression and Mutational Analysis of Large Tumor Antigen in non-Merkel Cell Carcinoma Tumors of Iranian Patients. Public Health Genomics (2021) 23(5-6):210–7. doi: 10.1159/000510254
41. Kuwamoto S, Higaki H, Kanai K, Iwasaki T, Sano H, Nagata K, et al. Association of Merkel Cell Polyomavirus Infection With Morphologic Differences in Merkel Cell Carcinoma. Hum Pathol (2011) 42(5):632–40. doi: 10.1016/j.humpath.2010.09.011
42. Kassem A, Technau K, Kurz AK, Pantulu D, Löning M, Kayser G, et al. Merkel Cell Polyomavirus Sequences Are Frequently Detected in Nonmelanoma Skin Cancer of Immunosuppressed Patients. Int J Cancer (2009) 125(2):356–61. doi: 10.1002/ijc.24323
43. Dworkin AM. Merkel Cell Polyomavirus in Cutaneous Squamous Cell Carcinoma of Immunocompetent Individuals. J Invest Dermatol (2011) 131(6):1388–8. doi: 10.1038/jid.2011.97
44. Mertz K, Pfaltz M, Junt T, Schmid M, Fernandez Figueras MT, Pfaltz K, et al. Merkel Cell Polyomavirus Is Present in Common Warts and Carcinoma in Situ of the Skin. Hum Pathol (2010) 41(10):1369–79. doi: 10.1016/j.humpath.2010.01.023
45. Murakami M, Imajoh M, Ikawa T, Nakajima H, Kamioka M, Nemoto Y, et al. Presence of Merkel Cell Polyomavirus in Japanese Cutaneous Squamous Cell Carcinoma. J Clin Virol (2011) 50(1):37–41. doi: 10.1016/j.jcv.2010.09.013
46. Ota S, Ishikawa S, Takazawa Y, Goto A, Fujii T, Ohashi K, et al. Quantitative Analysis of Viral Load Per Haploid Genome Revealed the Different Biological Features of Merkel Cell Polyomavirus Infection in Skin Tumor. PloS One (2012) 7(6):e39954. doi: 10.1371/journal.pone.0039954
47. Rollison DE, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, et al. Case-Control Study of Merkel Cell Polyomavirus Infection and Cutaneous Squamous Cell Carcinoma. Cancer Epidemiol Biomark Prev (2012) 21(1):74–81. doi: 10.1158/1055-9965.EPI-11-0764
48. Scola N, Wieland U, Silling S, Altmeyer P, Stucker M, Kreuter A. Prevalence of Human Polyomaviruses in Common and Rare Types of non-Merkel Cell Carcinoma Skin Cancer. Br J Dermatol (2012) 167(6):1315–20. doi: 10.1111/j.1365-2133.2012.11141.x
49. Wieland U, Scola N, Stolte B, Stucker M, Silling S, Kreuter A. No Evidence for a Causal Role of Merkel Cell Polyomavirus in Keratoacanthoma. J Am Acad Dermatol (2012) 67(1):41–6. doi: 10.1016/j.jaad.2011.07.026
50. Iwasaki T, Matsushita M, Kuwamoto S, Kato M, Murakami I, Higaki-Mori H, et al. Usefulness of Significant Morphologic Characteristics in Distinguishing Between Merkel Cell Polyomavirus-Positive and Merkel Cell Polyomavirus-Negative Merkel Cell Carcinomas. Hum Pathol (2013) 44(9):1912–7. doi: 10.1016/j.humpath.2013.01.026
51. Imajoh M, Hashida Y, Nakajima H, Sano S, Daibata M. Prevalence and Viral DNA Loads of Three Novel Human Polyomaviruses in Skin Cancers From Japanese Patients. J Dermatol (2013) 40(8):657–60. doi: 10.1111/1346-8138.12180
52. Mertz KD, Paasinen A, Arnold A, Baumann M, Offner F, Willi N, et al. Merkel Cell Polyomavirus Large T Antigen Is Detected in Rare Cases of Nonmelanoma Skin Cancer. J Cutaneous Pathol (2013) 40(6):543–9. doi: 10.1111/cup.12129
53. Falchook GS, Rady P, Konopinski JC, Busaidy N, Hess K, Hymes S, et al. Merkel Cell Polyomavirus and Human Papilloma Virus in Proliferative Skin Lesions Arising in Patients Treated With BRAF Inhibitors. Arch Dermatol Res (2016) 308(5):357–65. doi: 10.1007/s00403-016-1650-y
54. Bellott TR, Baez CF, Almeida SG, Venceslau MT, Zalis MG, Guimarães MA, et al. Molecular Prevalence of Merkel Cell Polyomavirus in Nonmelanoma Skin Cancer in a Brazilian Population. Clin Exp Dermatol (2017) 42(4):390–394. doi: 10.1111/ced.13069
55. Haeggblom L, Franzen J, Nasman A. Human Polyomavirus DNA Detection in Keratoacanthoma and Spitz Naevus: No Evidence for a Causal Role. J Clin Pathol (2017) 70(5):451–3. doi: 10.1136/jclinpath-2016-204197
56. Arvia R, Sollai M, Pierucci F, Urso C, Massi D, Zakrzewska K. Droplet Digital PCR (Ddpcr) vs Quantitative Real-Time PCR (Qpcr) Approach for Detection and Quantification of Merkel Cell Polyomavirus (Mcpyv) DNA in Formalin Fixed Paraffin Embedded (FFPE) Cutaneous Biopsies. J Virol Methods (2017) 246:15–20. doi: 10.1016/j.jviromet.2017.04.003
57. Kim DK. No Association Between Merkel Cell Polymavirus Infection and Keratoacanthoma in Korean Patients. Asian Pacific J Cancer Prevention: APJCP (2019) 20(5):1299–301. doi: 10.31557/APJCP.2019.20.5.1299
58. Rekhi B, Arora R, Chandrani P, Krishna S, Dutt A. Merkel Cell Polyomavirus is Implicated in a Subset of Cases of Merkel Cell Carcinomas From the Indian Subcontinent. Conf Abstract Modern Pathol (2020) 33(3):491–2. doi: 10.1016/j.micpath.2019.103778
59. Goncalves MTV, Varella RB, Almeida NKO, Guimaraes M, Luz FB. Molecular Detection of Merkel Cell Polyomavirus in Basal Cell Carcinoma and Perilesional Tissue: A Cross-Sectional Study. Anais Brasileiros Dermatol (2020) 95(4):527–8. doi: 10.1016/j.abd.2019.10.007
60. Costa PVA, Ishiy PS, Urbano PRP, Romano CM, Tyring SK, Oliveira WRP, et al. Identification of Polyomaviruses in Skin Cancers. Intervirology (2021) 64(3):119–25. doi: 10.1159/000513544
61. Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of Human Polyomaviruses. PloS Pathog (2009) 5:e1000363. doi: 10.1371/journal.ppat.1000363
62. Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. Quantitation of Human Seroresponsiveness to Merkel Cell Polyomavirus. PloS Pathog (2009) 5(9):e1000578. doi: 10.1371/journal.ppat.1000578
63. Kamminga S, van der Meijden E, Feltkamp MCW, Zaaijer HL. Seroprevalence of Fourteen Human Polyomaviruses Determined in Blood Donors. PloS One (2018) 13(10):e0206273. doi: 10.1371/journal.pone.0206273
64. Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel Cell Polyomavirus and Two Previously Unknown Polyomaviruses are Chronically Shed From Human Skin. Cell Host Microbe (2010) 7:509–15. doi: 10.1016/j.chom.2010.05.006
65. Liu W, Yang R, Payne AS, Schowalter RM, Spurgeon ME, Lambert PF, et al. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe (2016) 19:775–87. doi: 10.1016/j.chom.2016.04.024
66. Santos-Juanes J, Fernandez-Vega I, Fuentes N, Galache C, Coto-Segura P, Vivanco B, et al. Merkel Cell Carcinoma and Merkel Cell Polyomavirus: A Systematic Review and Meta-Analysis. Rev Br J Dermatol (2015) 173(1):42–9. doi: 10.1111/bjd.13870
67. Colunga A, Pulliam T, Nghiem P. Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes. 26 Clin Cancer Res (2018) 24:2035–43. doi: 10.1158/1078-0432.CCR-17-0439
68. Dalianis T, Hirsch HH. Human Polyomaviruses in Disease and Cancer. Virology (2013) 437(2):63–72. doi: 10.1016/j.virol.2012.12.015
69. Moens U, Ludvigsen M, Van Ghelue M. Human Polyomaviruses in Skin Diseases. Patholog Res Int (2011) 2011:123491–12. doi: 10.4061/2011/123491
70. Sheu JC, Tran J, Rady PL, Dao H, Tyring SK, Nguyen HP. Polyomaviruses of the Skin: Integrating Molecular and Clinical Advances in an Emerging Class of Viruses. Br J Dermatol (2019) 180(6):1–10. doi: 10.1111/bjd.17947
71. Amber K, McLeod MP, Nouri K. The Merkel Cell Polyomavirus and its Involvement in Merkel Cell Carcinoma. Dermatol Surg (2013) 39:232–8. doi: 10.1111/dsu.12079
72. Liu W, Krump NA, Buck CB, You J. Merkel Cell Polyomavirus Infection and Detection. J Vis Exp (2019) 144:10.3791/58950. doi: 10.3791/58950
73. Moshiri AS, Doumani R, Yelistratova L, Blom A, Lachance K, Shinohara MM, et al. Polyomavirus-Negative 31 Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor 32 Virus Detection. J Invest Dermatol (2017) 137:819–27. doi: 10.1016/j.jid.2016.10.028
74. Sadeghi F, Salehi-Vaziri M, Alizadeh A, Ghodsi SM, Bokharaei-Salim F, Fateh A, et al. Detection of Merkel Cell Polyomavirus Large T-Antigen Sequences in Human Central Nervous System Tumors. J Med Virol (2015) 87(7):1241– 1247. doi: 10.1002/jmv.24178
75. Behdarvand A, Zamani MS, Sadeghi F, Yahyapour Y, Vaziri F, Jamnani FR, et al. Evaluation of Merkel Cell Polyomavirus in Non-Small Cell Lung Cancer and Adjacent Normal Cells. Microb Pathog (2017) 108:21–6. doi: 10.1016/j.micpath.2017.04.033
Keywords: merkel cell carcinoma, merkel cell polyomavirus, prevalence, infectivity, pathogenesis, skin cancer
Citation: Wijaya WA, Liu Y, Qing Y and Li Z (2022) Prevalence of Merkel Cell Polyomavirus in Normal and Lesional Skin: A Systematic Review and Meta-Analysis. Front. Oncol. 12:868781. doi: 10.3389/fonc.2022.868781
Received: 03 February 2022; Accepted: 28 February 2022;
Published: 22 March 2022.
Edited by:
Motoki Nakamura, Nagoya City University, JapanCopyright © 2022 Wijaya, Liu, Qing and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyong Li, 302992694@qq.com; Yong Qing, hxqingyong@163.com
 Wilson A. Wijaya
Wilson A. Wijaya Yu Liu
Yu Liu