- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
- 2Department of Gastrointestinal Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Trauma & Emergency Surgery, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
Objective: Malnutrition is recognized as a risk factor for poor outcome in patients with gastric cancer (GC). In 2018, the Global Leadership Initiative on Malnutrition (GLIM) published standardized criteria for the diagnosis of malnutrition. Our aim was to investigate whether any of the components of the GLIM diagnostic criteria were related to worse clinical outcomes in patients with GC.
Methods: This study analyzed patients with GC who underwent radical gastrectomy in our hospital between 2014 and 2019. A preoperative nutritional assessment was performed for each patient. Matching was based on the presence of three GLIM components: high weight loss (WL), low body mass index (BMI), and low skeletal muscle index (SMI).
Results: The analysis included 1,188 patients, including 241 (20.3%) with high WL, 156 (13.1%) with low BMI, and 355 (29.9%) with low SMI. Before matching, patients who met the GLIM component criteria were mostly associated with older age, low nutritional reserves, and late tumor progression. After matching, the clinical characteristics of the three cohorts were balanced. In the matched queue, the survival prognosis of the high WL group was worse than that of the non-WL group, and the postoperative complication rate was higher in the low SMI group than in the normal SMI group (P <0.05). In addition, the clinical outcomes in the low and normal BMI groups were similar (P >0.05).
Conclusion: Of the GLIM criteria, high WL and low SMI may be associated with poor clinical outcomes in patients with GC, while a low BMI may not be associated with outcome.
Introduction
In 2020, the estimated number of new global cases of gastric cancer (GC) was more than one million, ranking fifth of all newly diagnosed cancers (1). As a malignant tumor of the upper digestive tract, GC often aggressively impacts the nutritional status of the patient. According to the previous different definitions of malnutrition, the prevalence in GC patients ranges from 20.9% to 80.4% (2–4). GC patients often experience tumor-related symptoms, such as cancer pain, anorexia, and malabsorption, and additional malnutrition may be induced by efforts to treat the patient, e.g., the gastrectomy itself and the side effects of adjuvant chemotherapy. Approximately 20% of cancer patients die from cachexia rather than from the malignant tumor itself (5). Timely detection of malnutrition and effective nutritional interventions have been proven to have considerable clinical and financial benefits (6, 7).
Many methods for nutritional screening and evaluation have been used to determine the nutritional status of patients, but there is no current consensus on the diagnostic criteria for malnutrition. The Global Leadership Initiative on Malnutrition (GLIM) has published a new definition of adult malnutrition to establish a global consensus concerning the diagnostic criteria of malnutrition in clinical diagnosis and treatment (8). After ranking the GLIM criteria, five core diagnostic criteria were screened, including three phenotypic criteria and two etiological criteria. To date, some studies have demonstrated the effectiveness of the GLIM criteria in nutritional assessment and prognosis prediction in adult cancer patients (9–11). However, to the best of our knowledge, only a few studies have explored the correlation between the different components of GLIM and the clinical outcomes of patients in medical institutions (12, 13). Studies have shown that malnutrition defined by GLIM criteria is associated with poor prognosis in GC patients (14, 15), whereas evidence for the correlation of GLIM components with GC prognosis is still insufficient. Therefore, this study aimed to explore the correlation between any GLIM phenotypic criteria, survival time, and postoperative complications in patients with GC.
Materials and Methods
Participants
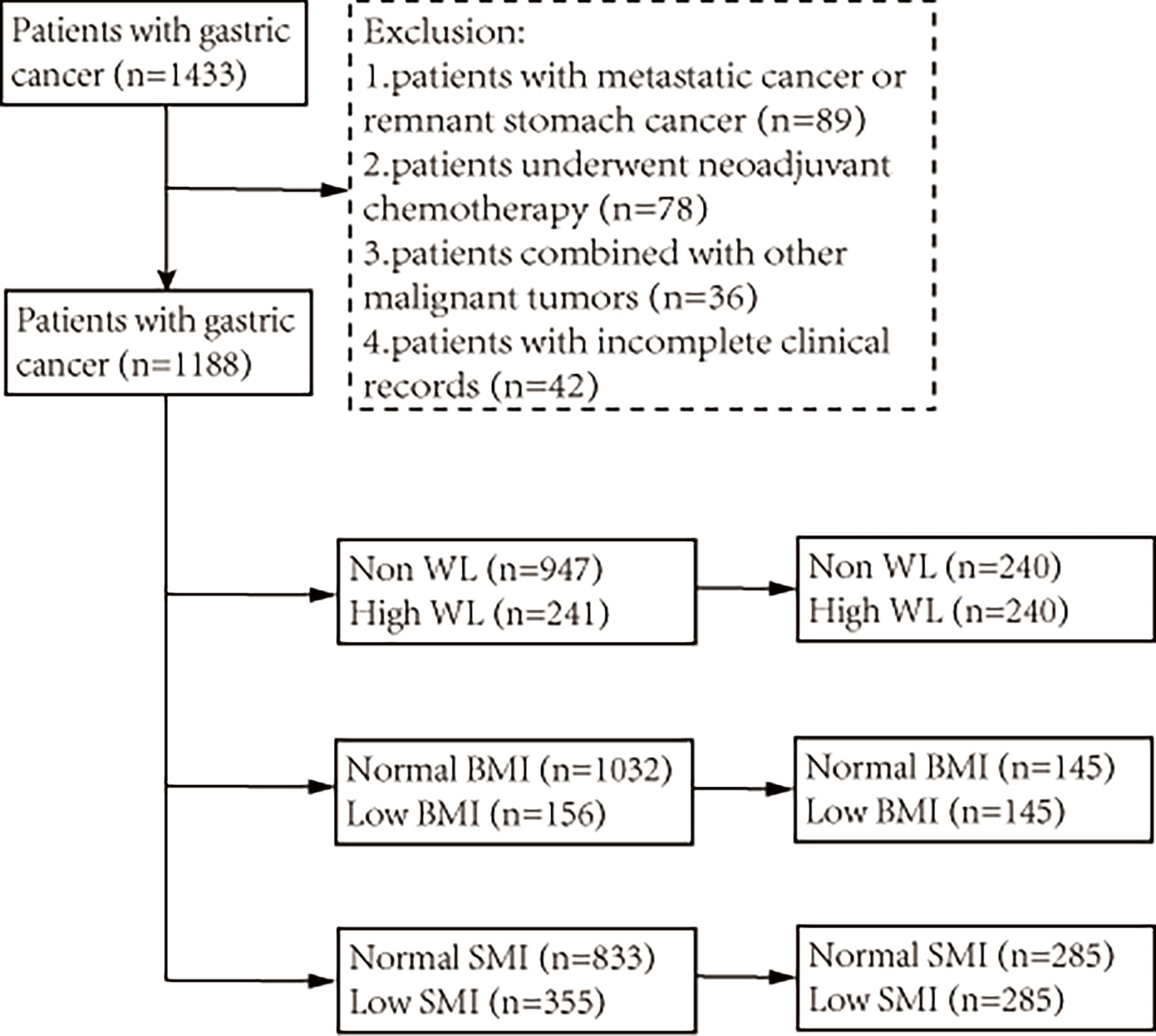
A total of 1,433 patients with GC who underwent radical gastrectomy between July 2014 and February 2019 at the First Affiliated Hospital of Wenzhou Medical University were recruited for this retrospective cohort study. The inclusion criteria were as follows: (a) pathologically diagnosed primary gastric adenocarcinoma before surgery; and (b) planned radical gastrectomy. The exclusion criteria were as follows: (a) patients with metastatic cancer or remnant stomach cancer; (b) patients who underwent neoadjuvant chemotherapy; (c) patients with other malignant tumors; and (d) patients with incomplete clinical records. Ultimately, 1,188 patients were included in the analysis. The management of GC was mainly based on the Japanese Gastric Cancer Treatment Guidelines 2010 (16). Relevant departments were invited to develop individualized treatment plans for patients when necessary. In addition, malnutrition was not an index of avoiding surgical treatment for this study’s cohort. The detailed elimination process and group analysis are shown in Figure 1. All research data was obtained from the database collected by the institution prospectively and the inpatient electronic medical record system. This study was approved by the ethics committee of our hospital and complied with the Declaration of Helsinki. Considering the nature of retrospective research, the requirement for informed consent form was waived.
Data Collection
The following data were collected and analyzed for all patients: (1) preoperative basic information, including sex, age, height, weight, hemoglobin concentration, albumin concentration, and comorbidities; (2) nutritional data, including weight loss (WL), body mass index (BMI), and skeletal muscle index (SMI); (3) postoperative pathology information, including tumor size, pathologic tumor–node–metastasis (pTNM) stage, and tumor differentiation degree; and (4) prognostic information, including postoperative complications (PC), postoperative length of stay (LOS), readmission rate within 1 month, and long-term survival information. Complications were classified in accordance with the Clavien–Dindo classification (17). Major PC was defined as a complication of grade II or above, while severe PC was defined as a complication of grade III or above.
Each patient underwent an outpatient follow-up examination in the first month after surgery. Afterwards, additional follow-ups were performed by phone or outpatient examination every 3–6 months. Each follow-up visit included a physical examination, laboratory examination, and necessary imaging examinations, such as computed tomography (CT), ultrasound, and endoscopy. Overall survival (OS) time was defined as the time from the date of surgery to death from any cause or the last follow-up visit. Disease-free survival (DFS) time was defined as the date of surgery to the date of tumor recurrence or death from any cause. The last follow-up was conducted in July 2021.
Components of GLIM Criteria
The GLIM criteria have five components, including three phenotypic criteria (involuntary WL, low BMI, and low muscle mass) and two etiological criteria (reduced food intake or absorption, and disease burden or inflammation) (8). As a tumor of the digestive tract, GC is considered a chronic disease, and studies have shown that GC is closely associated with inflammation (18, 19). Therefore, all patients with GC in this study are considered to meet the etiological criteria of burden of disease/inflammation. Thus, this study aimed to explore the correlation between the three phenotypic criteria and the prognosis of patients with GC. Involuntary WL was calculated as the weight change in GC patients before surgery. According to the GLIM criteria (8), high WL was defined as a WL >5% within 6 months or >10% over 6 months. According to the GLIM recommendations for Asians (8), low BMI was defined as a BMI <18.5, when patients were <70 years old or <20 when patients were ≥70 years old.
Low SMI was defined as ≤40.8 cm2/m2 for males and ≤34.9 cm2/m2 for females according to the prospective study (21, 22).
Statistical Analysis
The Kolmogorov–Smirnov test was used to determine the continuous data distribution state. Continuous data were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) according to the data distribution, and the differences between groups were compared using Student’s t-test or Mann-Whitney U test. Categorical data were expressed as counts and percentages and were compared using the chi-square test.
To reduce the influence of selection bias and potential confounding factors on the prognostic analysis, we performed propensity score matching (PSM) analysis to obtain matched data. Three different matches were used to explore the correlation between the three phenotypic criteria and prognoses of patients with GC. Propensity scores were calculated based on the following patient data: age, Charlson score, hemoglobin, albumin, tumor size, TNM stage, and two phenotypic indicators (BMI and SMI, WL and SMI, WL, and BMI). Three PSMs were conducted at a ratio of 1:1 using a caliper set to 0.20. Then, three sets of matched cohorts were obtained: the high and non-WL groups, the low and normal BMI groups, and the low and normal SMI groups. The Kaplan–Meier method was used to analyze the OS and DFS rates. The log-rank test was used to compare survival differences between the groups. Univariate and multivariate logistic and Cox regression analyses were used to analyze the risk factors of PCs and survival rates in the entire cohort. Factors with P <0.05 in the univariate analysis, were included in the multivariate analysis. Differences were considered statistically significant at P <0.05. All data were analyzed using SPSS Statistics (version 25.0; IBM Corp., Armonk, NY, USA) and R version 4.1.0 (http://www.r-project.org).
Results
Patient Characteristics
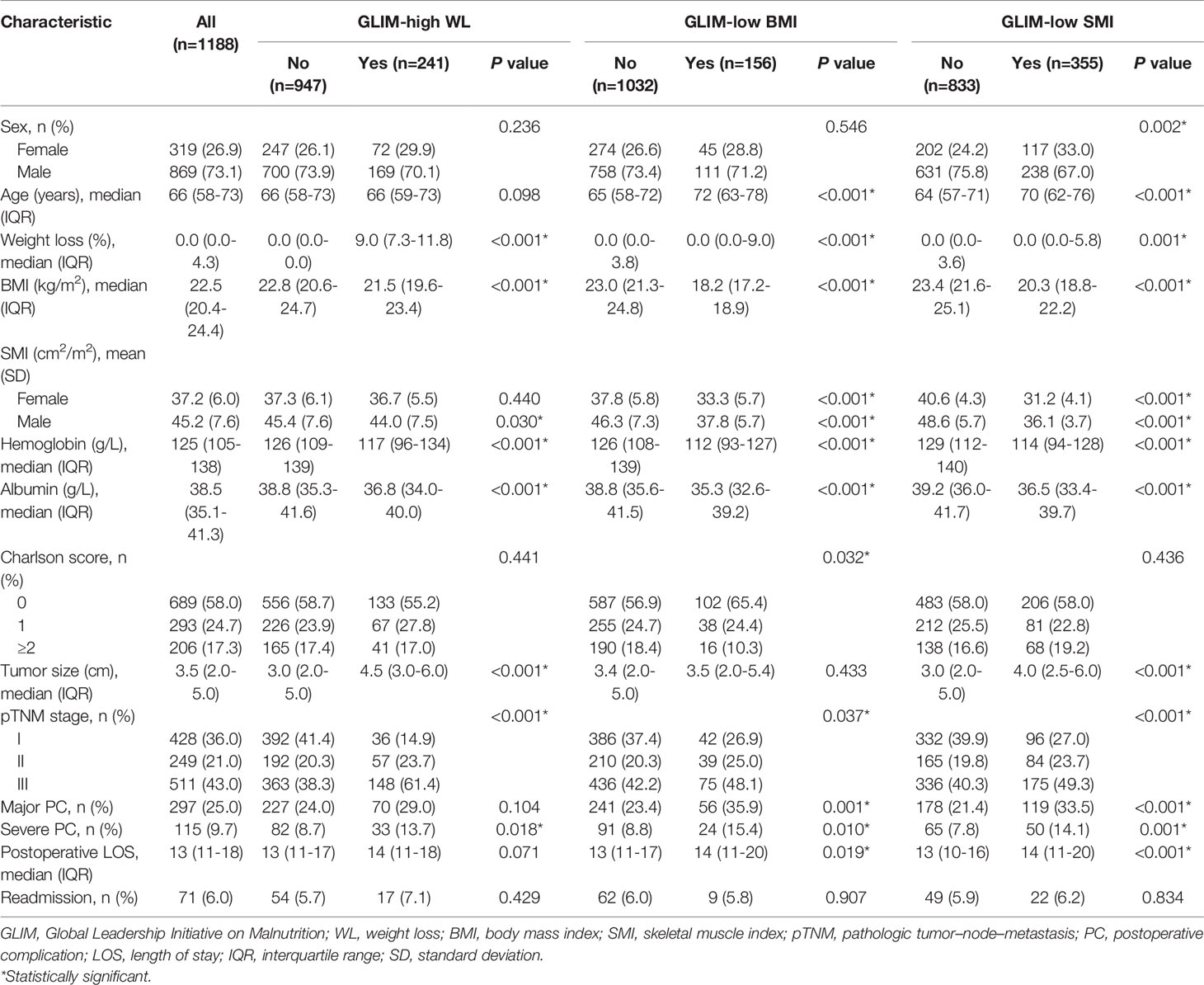
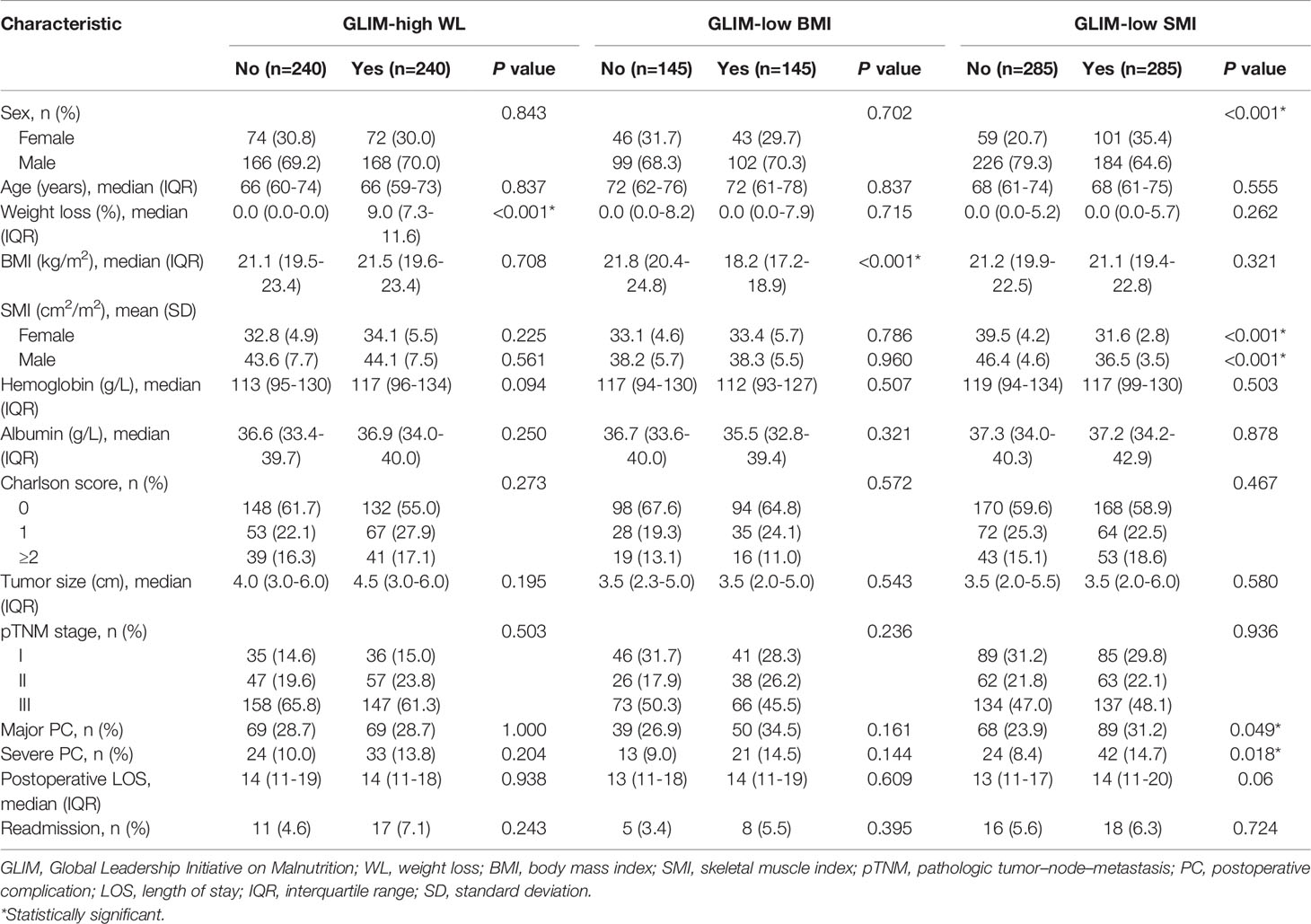
A total of 1,188 patients were included in the analysis, of which 867 (73.1%) were male. Baseline characteristics before matching are shown in Table 1. According to the GLIM criteria, 241 (20.3%) patients had high WL, 156 (13.1%) patients had low BMI, and 355 (29.9%) patients had low SMI. Before matching, patients who met the phenotypic criteria often had older age, anemia, hypoalbuminemia, and a more advanced tumor stage. After PSM analysis, there were 480 patients in the WL cohort (high WL group, n=240; non-WL group, n=240), 290 patients in the BMI cohort (low BMI group, n=145; normal BMI group, n=145), and 570 patients in the SMI cohort (low SMI group, n=285; normal SMI group, n=285). As shown in Table 2, the baseline characteristics of each group reached a balance after matching, except that female were more likely to have a low SMI.
Short-Term Clinical Outcomes
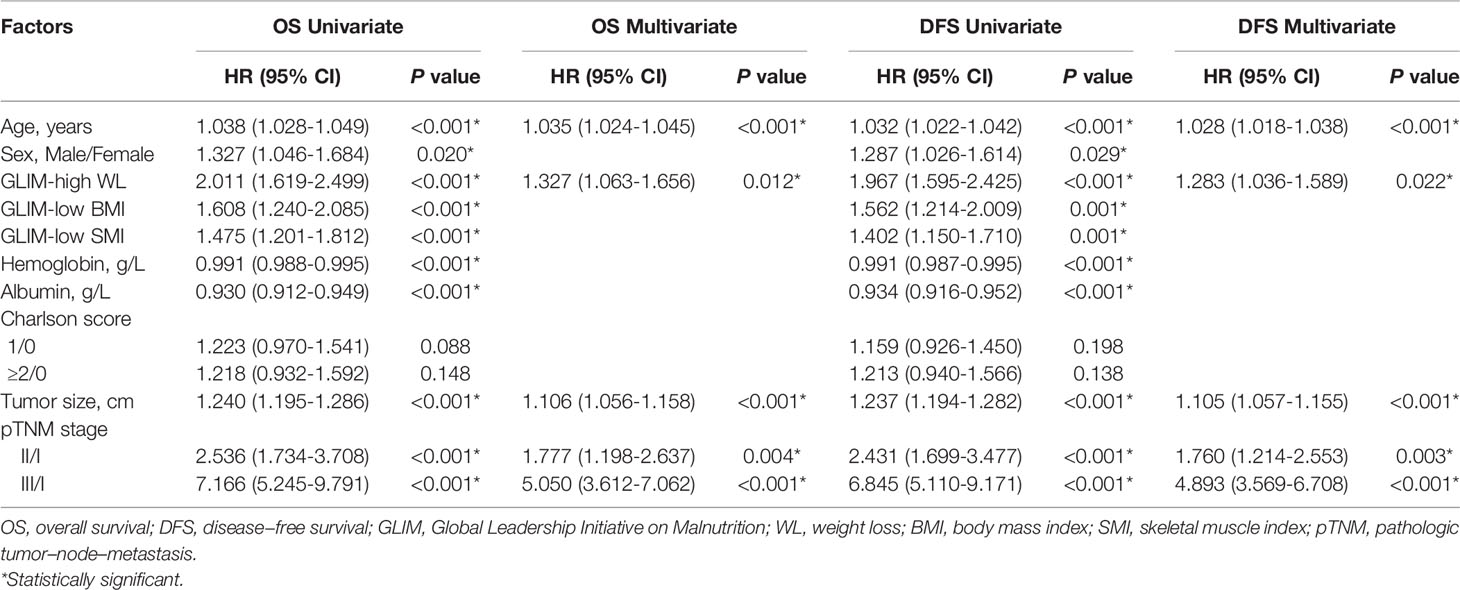
The incidence of major PC in the entire cohort was 25% (297/1,188), of which 9.7% (115/1,188) had severe PC. Univariate and multivariate logistic regression analyses for the risk factors of major and severe PC were conducted in the entire cohort. The results showed that age, low SMI, albumin level, and Charlson score ≥2 were independent risk factors for major and severe PC (all P <0.05, Table 3).
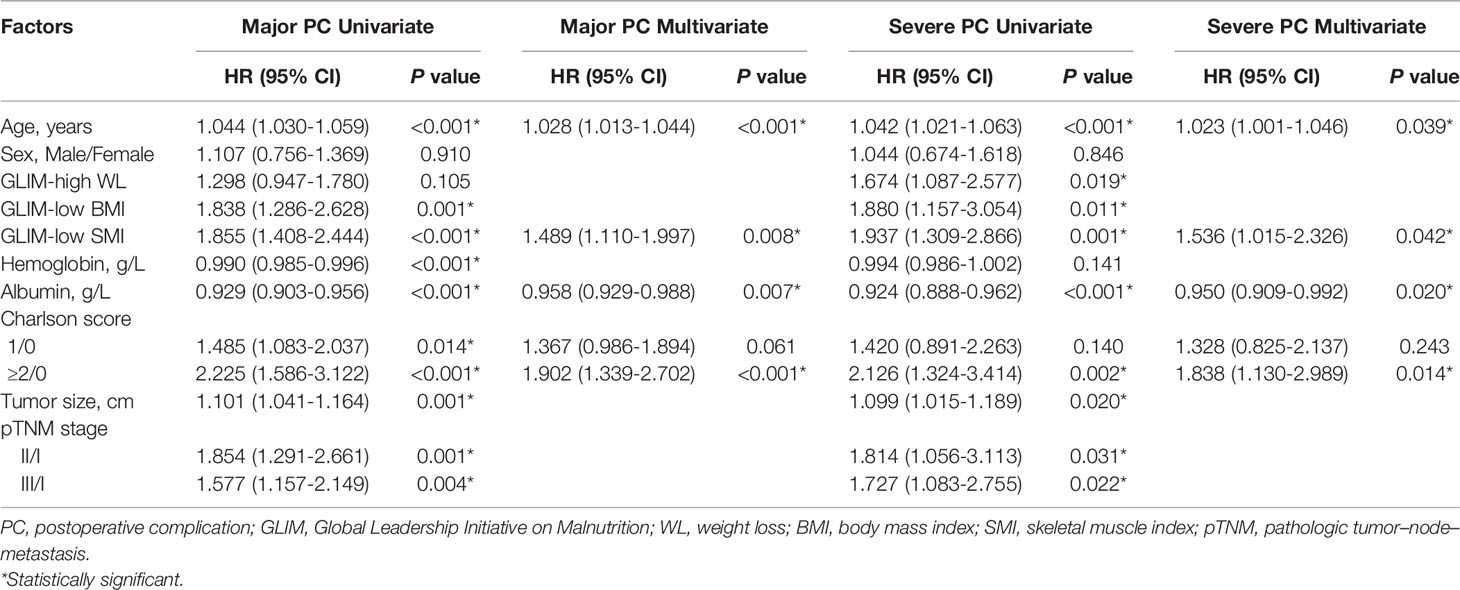
Table 3 Univariate and multivariate logistic regression analysis for postoperative complications in the entire cohort.
After PSM analysis, the postoperative LOS and readmission rates among the groups in the three matched cohorts were not statistically significant (all P >0.05, Table 2). In addition, the PC rates in the matched low SMI group were significantly higher than those in the normal SMI group (major: 31.2% vs. 23.9%, P =0.049; severe: 14.7% vs. 8.4%, P =0.018), while there was no statistical difference in the matched WL and BMI cohorts (all P >0.05, Table 2). However, in terms of specific complications, no statistical difference was observed between the low and normal SMI groups (all P >0.05, Table S1).
Long-Term Survival Outcomes
The median follow-up period was 49.2 (range: 0.5–81.5) months. The three-year OS and DFS rates in the entire cohort were 67.5% and 64.2%, respectively. Univariate and multivariate Cox regression analyses for the risk factors of OS and DFS rates were performed in the entire cohort. The analysis results showed that age, high WL, tumor size, and TNM stage were independent risk factors for OS and DFS rates (all P <0.05, Table 4).
The Kaplan–Meier curves in the entire cohort showed that high WL, low BMI, and low SMI were associated with poor OS and DFS rates (all P <0.001, Figure S1). After matching, high WL was still associated with poor OS and DFS rates (P =0.041; P =0.033, Figures 2A–D), while low BMI and low SMI were not (P =0.575; P =0.910, Figures 2E, F). In addition, the median follow-up for the WL-matched cohort was 49.0 (range: 0.5–81.5) months. The three-year OS and DFS rates in the WL-matched cohort were 55.0% and 53.0%, respectively.
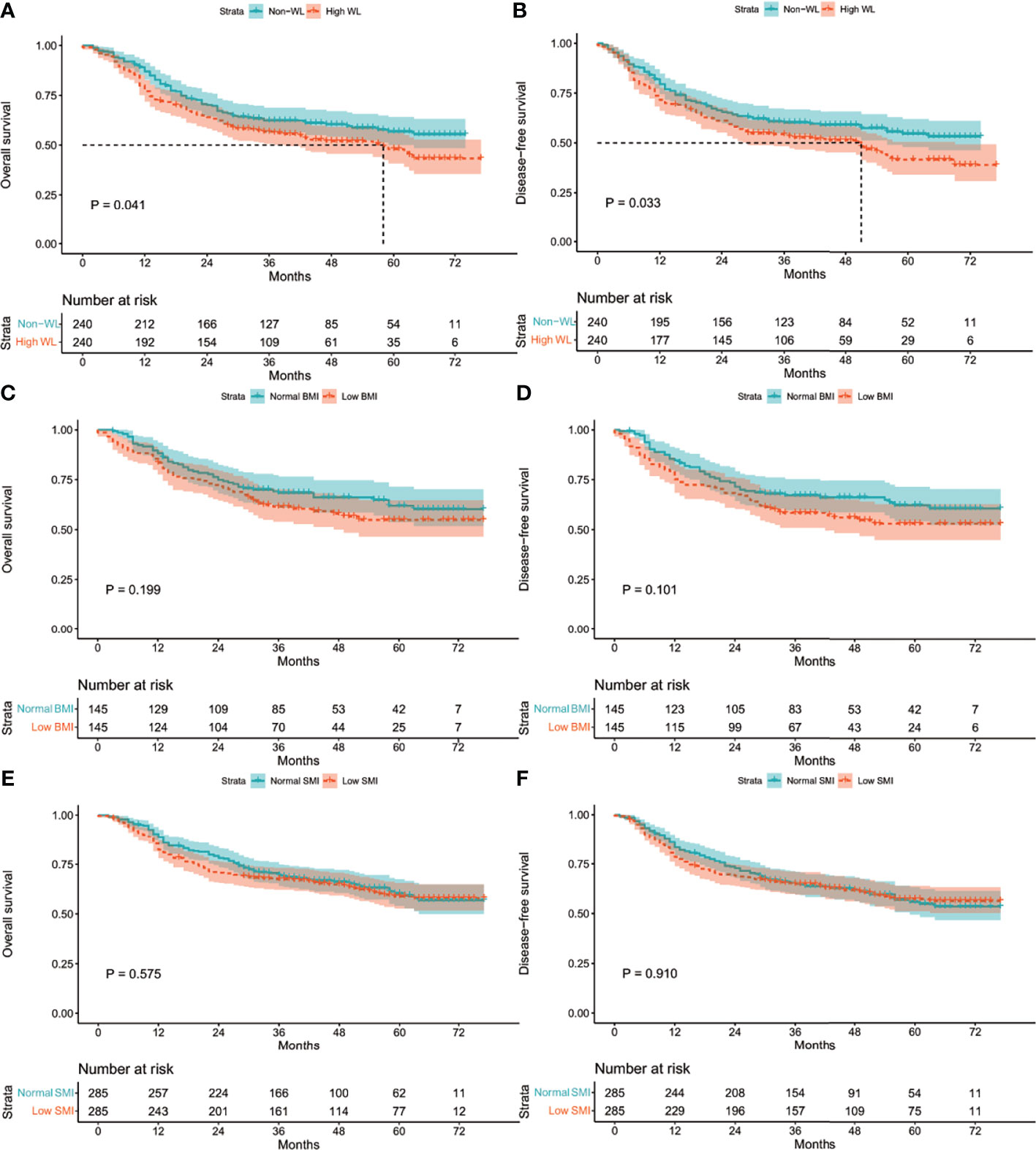
Figure 2 Kaplan-Meier curves: (A) in the matched WL cohort for OS rate; (B) in the matched WL cohort for DFS rate; (C) in the matched BMI cohort for OS rate; (D) in the matched BMI cohort for DFS rate; (E) in the matched SMI cohort for OS rate; (F) in the matched SMI cohort for DFS rate. WL, weight loss; BMI, body mass index; SMI, skeletal muscle index; OS, overall survival; DFS, disease-free survival.
Discussion
Malnutrition is a public health problem often associated with a poor prognosis in patients with gastric malignancies. Early recognition of malnutrition and active nutritional support measures can improve the nutritional status and clinical outcomes of patients with GC (6, 23). Malnutrition has recently attracted the attention of clinicians as a risk factor that may be corrected. The GLIM recently published new standard guidelines for diagnosing malnutrition and have encouraged independent verification and research on these standard guidelines in specific populations (8). For the first time, the current study used the PSM analysis method was used to explore the correlation between the three phenotype-based criteria of GLIM and the short- and long-term prognosis of patients with GC.
It is well known that malnourished patients often present with worse nutritional reserve indicators, such as hemoglobin and serum albumin levels (2, 9). In addition, elderly patients are more prone to suffer from malnutrition, sarcopenia, and weakness due to weakened body functions, organ decline, and reduced immunity (24). GC is a highly aggressive form of cancer, especially in the advanced or metastatic stage, and the symptoms of reduced nutritional intake and absorption and gastrointestinal obstruction were more obvious than in other cancer patients (23, 25). Considering that the clinical baselines of malnourished patients were worse than those of well-nourished patients, and the associated adverse outcomes were unsurprising and obvious, consistent with the findings in this study. However, it was hard to implement a randomized controlled trial to explore the correlation between the components of the GLIM criteria and the prognosis of GC patients. Therefore, this study adopted the PSM analysis method to control the bias to the greatest extent possible.
A history of WL is an essential part of nutritional screening and evaluation (4, 6) and has a long history of use in the clinical assessment of malnutrition. It has been reported that high WL in GLIM criteria was significantly related to severe PC and mortality following major abdominal surgery (12). In the matched cohort of this study, high WL was also associated with poor survival prognosis, but not with major and severe PCs. Therefore, we inferred that cut-off value of the high WL in GLIM criteria has a better predictive ability on survival prognosis of GC, and that the poor clinical baselines of patients with high WL may be the reason for the higher rate of complications. In addition, the weight of patients after gastrectomy is typically reduced by 6–17% (26) and is closely related to poor compliance with postoperative adjuvant chemotherapy. Thus, WL is also an important risk factor for quality of life (QOL) and survival (27, 28).
BMI is usually used to assess nutritional status and is currently the most widely studied body composition parameter. However, preoperative low BMI as an indicator of poor prognosis in GC patients is still controversial (29, 30). BMI was not sufficient to identify all patients facing nutritional challenges, especially in those with increased BMI. It was reported that cancer associated malnutrition was still prevalent among overweight and obese people as defined by BMI (31). Loss of muscle mass may be masked by a high fat mass, making it difficult for BMI to accurately assess actual nutritional and functional status (20, 31). The median BMI of the patients in this study was relatively low, which may be more representative of the Asian population. Therefore, we used the recommended BMI values for Asians reported in the GLIM guidelines that define low BMI as <18.5 kg/m2 for patients <70 years old or <20 kg/m2 in patients ≥70 years old (8). Before the matching analysis, patients with low BMI were closely related to advanced age, high WL, low SMI, anemia, hypoalbuminemia, high Charlson score, and late tumor stage. Therefore, patients in the low BMI group had more frequent complications and worse long-term prognoses. However, the results of the matching analysis showed that low BMI was not associated with the long- and short-term prognoses of patients with GC. Therefore, further prospective studies are needed to analyze the effectiveness of these criteria in predicting the prognoses of patients with GC.
Abdominal CT imaging has the advantages of accuracy, non-invasiveness, and convenience. It is often used in patients with GC to evaluate tumor staging and monitor treatment during follow-up. Importantly, the accuracy of CT scans in quantifying whole-body skeletal muscle mass can reach 99% (32). Decreased skeletal muscle mass is a key clinical manifestation of cancer-related malnutrition that can lead to the development of sarcopenia (33, 34). A recent meta-analysis reported that low SMI in the L3 cross section is closely related to poor prognosis in GC patients (35). However, there is no consensus on the cut-off value of SMI in the L3 cross section; thus, the current study adopted a recommended cut-off value from our institution’s previous research (22). We found that low SMI was an independent risk factor for complications, rather than long-term survival prognosis, which was consistent with the results of the PSM analysis. Lee et al. found that, compared with preoperative low SMI, postoperative low SMI may be a more important risk factor for long-term survival (34). Although sex had no significant effect on complications and survival, female patients in this study seemed to be more likely to experience low SMI. This may be explained by the sexual dimorphism of skeletal muscle mass that is attributed to differences in hormone levels and gene expression between males and females (36, 37) and can also lead to differences in muscle loss associated with aging.
Exercise and nutritional interventions are critical for maintaining muscle mass and preventing involuntary body weight loss (BWL). Resistance exercise contributes to the synthesis of muscle proteins (38), and the anabolic effect of exogenous amino acids on muscle proteins is enhanced by previous exercise (39). A prospective study by Yamamoto et al. found that some GC patients with sarcopenia can reverse their sarcopenic state by participating in preoperative exercise and nutritional interventions that may improve the postoperative clinical outcome (40). A randomized controlled trial conducted in Japan proposed that daily oral nutritional supplements (ONS) >200 mL after gastrectomy can significantly improve BWL after 1 year (41). In addition, Meng et al. reported that, for patients with nutritional risk after gastrectomy, ONS after discharge can improve nutritional outcomes, skeletal muscle maintenance, chemotherapy tolerance, and some QOL variables (42). These results indicate that preoperative and postoperative supportive measures may improve the clinical outcomes of patients with GC.
The current study has some limitations. First, this study was limited by the retrospective single-center design despite its large sample size, and further multi-regional validation studies are necessary. Second, despite the rigorous PSM analysis, our conclusions were limited by other variables that could not be measured. For example, we did not include preoperative and postoperative nutritional support data in the analysis due to incomplete data collection. Third, this study only analyzed the data of GC patients who underwent radical resection, so the conclusions drawn from this study cannot be generalized to GC patients receiving neoadjuvant chemotherapy or metastatic patients.
In conclusion, high WL was associated with poor OS and DFS rates, and low SMI was associated with high PC rates. However, a low BMI may not be associated with poor clinical outcomes. Of the GLIM criteria, high WL and low SMI may be more valuable in predicting prognosis than low BMI in order to optimize clinical intervention.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Y-SH and X-DC contributed to conception and design of the study. L-BX, Y-QC, and W-JC acquired the data. L-BX, S-XZ, and LW analyzed and interpreted data and carried out the statistical analysis. L-BX and T-TM wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.851091/full#supplementary-material
References
1. Sung H, Ferlay J, RL S, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Zheng H-L, Lu J, Li P, Xie J-W, Wang J-B, Lin J-X, et al. Effects of Preoperative Malnutrition on Short- and Long-Term Outcomes of Patients With Gastric Cancer: Can We do Better? Ann Surg Oncol (2017) 24(11):3376–85. doi: 10.1245/s10434-017-5998-9
3. Lidoriki I, Schizas D, Mpaili E, Vailas M, Sotiropoulou M, Papalampros A, et al. Associations Between Skeletal Muscle Mass Index, Nutritional and Functional Status of Patients With Oesophago-Gastric Cancer. Clin Nutr ESPEN (2019) 34:61–7. doi: 10.1016/j.clnesp.2019.08.012
4. Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, YY S, et al. Survey and Analysis of the Nutritional Status in Hospitalized Patients With Malignant Gastric Tumors and its Influence on the Quality of Life. Support Care Cancer (2020) 28(1):373–80. doi: 10.1007/s00520-019-04803-3
5. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer Cachexia: Understanding the Molecular Basis. Nat Rev Cancer (2014) 14(11):754–62. doi: 10.1038/nrc3829
6. Jie B, Jiang Z-M, MT N, Zhu S-N, Yu K, Kondrup J. Impact of Preoperative Nutritional Support on Clinical Outcome in Abdominal Surgical Patients at Nutritional Risk. Nutrition (2012) 28(10):1022–7. doi: 10.1016/j.nut.2012.01.017
7. Muscaritoli M, Krznarić Z, Singer P, Barazzoni R, Cederholm T, Golay A, et al. Effectiveness and Efficacy of Nutritional Therapy: A Systematic Review Following Cochrane Methodology. Clin Nutr (2017) 36(4):939–57. doi: 10.1016/j.clnu.2016.06.022
8. Cederholm T, GL J, Correia MITD, MC G, Fukushima R, Higashiguchi T, et al. GLIM Criteria for the Diagnosis of Malnutrition - a Consensus Report From the Global Clinical Nutrition Community. Clin Nutr (2019) 38(1):1–9. doi: 10.1016/j.clnu.2018.08.002
9. Qin L, Tian Q, Zhu W, Wu B. The Validity of the GLIM Criteria for Malnutrition in Hospitalized Patients With Gastric Cancer. Nutr Cancer (2020) 73(11–12):2732–9. doi: 10.1080/01635581.2020.1856894
10. Zhang K-P, Tang M, Fu Z-M, Zhang Q, Zhang X, Guo Z-Q, et al. Global Leadership Initiative on Malnutrition Criteria as a Nutrition Assessment Tool for Patients With Cancer. Nutrition (2021), 91–2:111379. doi: 10.1016/j.nut.2021.111379
11. Zhang Z, Wan Z, Zhu Y, Zhang L, Zhang L, Wan H. Prevalence of Malnutrition Comparing NRS2002, MUST, and PG-SGA With the GLIM Criteria in Adults With Cancer: A Multi-Center Study. Nutrition (2021) 83:111072. doi: 10.1016/j.nut.2020.111072
12. Skeie E, Tangvik RJ, Nymo LS, Harthug S, Lassen K, Viste A. Weight Loss and BMI Criteria in GLIM’s Definition of Malnutrition is Associated With Postoperative Complications Following Abdominal Resections - Results From a National Quality Registry. Clin Nutr (2020) 39(5):1593–9. doi: 10.1016/j.clnu.2019.07.003
13. Yeung SSY, Chan JHY, Chan RSM, Sham A, Ho SC, Woo J. Predictive Value of the GLIM Criteria in Chinese Community-Dwelling and Institutionalized Older Adults Aged 70 Years and Over. J Nutr Health Aging (2021) 25(5):645–52. doi: 10.1007/s12603-021-1610-x
14. Huang D-D, Yu D-Y, Song H-N, Wang W-B, Luo X, Wu G-F, et al. The Relationship Between the GLIM-Defined Malnutrition, Body Composition and Functional Parameters, and Clinical Outcomes in Elderly Patients Undergoing Radical Gastrectomy for Gastric Cancer. Eur J Surg Oncol (2021) 47(9):2323–31. doi: 10.1016/j.ejso.2021.02.032
15. Xu L-B, Shi M-M, Huang Z-X, Zhang W-T, Zhang H-H, Shen X, et al. Impact of Malnutrition Diagnosed Using Global Leadership Initiative on Malnutrition Criteria on Clinical Outcomes of Patients With Gastric Cancer. JPEN J Parenter Enteral Nutr (2021). doi: 10.1002/jpen.2127
16. Japanese Gastric Cancer A. Japanese Gastric Cancer Treatment Guidelines 2010 (Ver. 3). Gastric Cancer (2011) 14(2):113–23. doi: 10.1007/s10120-011-0042-4
17. Clavien PA, Barkun J, deO ML, JN V, Dindo D, RD S, et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann Surg (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
18. Montinaro A, Walczak H. Sterile Inflammation Fuels Gastric Cancer. Immunity (2018) 48(3):481–3. doi: 10.1016/j.immuni.2018.03.011
19. Fichtner-Feigl S, Kesselring R, Strober W. Chronic Inflammation and the Development of Malignancy in the GI Tract. Trends Immunol (2015) 36(8):451–9. doi: 10.1016/j.it.2015.06.007
20. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients With Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol (2008) 9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0
21. Lou N, CH C, Chen XD, Zhou CJ, Wang SL, Zhuang CL, et al. Sarcopenia in Overweight and Obese Patients is a Predictive Factor for Postoperative Complication in Gastric Cancer: A Prospective Study. Eur J Surg Oncol (2017) 43(1):188–95. doi: 10.1016/j.ejso.2016.09.006
22. Zhuang C-L, Huang D-D, Pang W-Y, Zhou C-J, Wang S-L, Lou N, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis From a Large-Scale Cohort. Med (Baltimore) (2016) 95(13):e3164. doi: 10.1097/MD.0000000000003164
23. Xu R, Chen X-D, Ding Z. Perioperative Nutrition Management for Gastric Cancer. Nutrition (2021) 93:111492. doi: 10.1016/j.nut.2021.111492
24. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
25. Mizukami T, Piao Y. Role of Nutritional Care and General Guidance for Patients With Advanced or Metastatic Gastric Cancer. Future Oncol (2021) 17(23):3101–9. doi: 10.2217/fon-2021-0186
26. Davis JL, LV S, JF C, Schattner M, DH I, Capanu M, et al. Patterns and Predictors of Weight Loss After Gastrectomy for Cancer. Ann Surg Oncol (2016) 23(5):1639–45. doi: 10.1245/s10434-015-5065-3
27. Kong H, Kwon OK, Yu W. Changes of Quality of Life After Gastric Cancer Surgery. J Gastric Cancer (2012) 12(3):194–200. doi: 10.5230/jgc.2012.12.3.194
28. Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative Weight Loss Leads to Poor Survival Through Poor s-1 Efficacy in Patients With Stage II/III Gastric Cancer. Int J Clin Oncol (2017) 22(3):476–83. doi: 10.1007/s10147-017-1089-y
29. Lee HH, Park JM, Song KY, Choi M-G, Park CH. Survival Impact of Postoperative Body Mass Index in Gastric Cancer Patients Undergoing Gastrectomy. Eur J Cancer (2016) 52:129–37. doi: 10.1016/j.ejca.2015.10.061
30. Chen H-N, Chen X-Z, Zhang W-H, Yang K, Chen X-L, Zhang B, et al. The Impact of Body Mass Index on the Surgical Outcomes of Patients With Gastric Cancer: A 10-Year, Single-Institution Cohort Study. Med (Baltimore) (2015) 94(42):e1769. doi: 10.1097/MD.0000000000001769
31. Martin L, Gioulbasanis I, Senesse P, Baracos VE. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis are Endemic in Overweight and Obese Patients. JPEN J Parenter Enteral Nutr (2020) 44(2):227–38. doi: 10.1002/jpen.1597
32. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver Validation of Skeletal Muscle Measurement by Magnetic Resonance Imaging and Computerized Tomography. J Appl Physiol (1998) 85(1):115–22. doi: 10.1152/jappl.1998.85.1.115
33. Fearon K, Strasser F, SD A, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol (2011) 12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7
34. Lee JK, Park YS, Lee K, Youn SI, Won Y, Min S-H, et al. Prognostic Significance of Surgery-Induced Sarcopenia in the Survival of Gastric Cancer Patients: A Sex-Specific Analysis. J Cachexia Sarcopenia Muscle (2021) 12(6):1897–907. doi: 10.1002/jcsm.12793
35. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Muscle Mass, Assessed at Diagnosis by L3-CT Scan as a Prognostic Marker of Clinical Outcomes in Patients With Gastric Cancer: A Systematic Review and Meta-Analysis. Clin Nutr (2020) 39(7):2045–54. doi: 10.1016/j.clnu.2019.10.021
36. Haizlip KM, Harrison BC, Leinwand LA. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiol (Bethesda) (2015) 30(1):30–9. doi: 10.1152/physiol.00024.2014
37. Welle S, Tawil R, Thornton CA. Sex-Related Differences in Gene Expression in Human Skeletal Muscle. PloS One (2008) 3(1):e1385. doi: 10.1371/journal.pone.0001385
38. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased Rates of Muscle Protein Turnover and Amino Acid Transport After Resistance Exercise in Humans. Am J Physiol (1995) 268(3 Pt 1):E514–20. doi: 10.1152/ajpendo.1995.268.3.E514
39. Biolo G, Tipton KD, Klein S, Wolfe RR. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am J Physiol (1997) 273(1 Pt 1):E122–9. doi: 10.1152/ajpendo.1997.273.1.E122
40. Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao M, Nishikawa K, Miyamoto A, et al. Effectiveness of a Preoperative Exercise and Nutritional Support Program for Elderly Sarcopenic Patients With Gastric Cancer. Gastric Cancer (2017) 20(5):913–8. doi: 10.1007/s10120-016-0683-4
41. Miyazaki Y, Omori T, Fujitani K, Fujita J, Kawabata R, Imamura H, et al. Oral Nutritional Supplements Versus a Regular Diet Alone for Body Weight Loss After Gastrectomy: A Phase 3, Multicenter, Open-Label Randomized Controlled Trial. Gastric Cancer (2021) 24(5):1150–9. doi: 10.1007/s10120-021-01188-3
Keywords: malnutrition, gastric cancer, clinical outcomes, propensity score matching, global leadership initiative on malnutrition
Citation: Xu L-B, Mei T-T, Cai Y-Q, Chen W-J, Zheng S-X, Wang L, Chen X-D and Huang Y-S (2022) Correlation Between Components of Malnutrition Diagnosed by Global Leadership Initiative on Malnutrition Criteria and the Clinical Outcomes in Gastric Cancer Patients: A Propensity Score Matching Analysis. Front. Oncol. 12:851091. doi: 10.3389/fonc.2022.851091
Received: 09 January 2022; Accepted: 07 February 2022;
Published: 03 March 2022.
Edited by:
Dimitrios Schizas, National and Kapodistrian University of Athens, GreeceReviewed by:
Dionysios Dellaportas, National and Kapodistrian University of Athens, GreeceIrene Lidoriki, National and Kapodistrian University of Athens, Greece
Maximos Frountzas, National and Kapodistrian University of Athens, Greece
Copyright © 2022 Xu, Mei, Cai, Chen, Zheng, Wang, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Chen, 352161451@qq.com; Yun-Shi Huang, huang412280246@163.com
 Li-Bin Xu
Li-Bin Xu Ting-Ting Mei2
Ting-Ting Mei2 Yi-Qi Cai
Yi-Qi Cai Yun-Shi Huang
Yun-Shi Huang