- 1Department of Hepatopancreatobiliary Surgery, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 2Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Naval Medical University), Shanghai, China
Background: There are few studies comparing the oncological outcomes of laparoscopic pancreaticoduodenectomy (LPD) and open pancreaticoduodenectomy (OPD) for distal cholangiocarcinoma (DCC). Our objective was to assess the short-term efficacy and long-term survival of LPD and OPD in patients with DCC.
Methods: The data of 124 DCC patients who underwent LPD or OPD at the Third Affiliated Hospital of Soochow University from May 2010 to May 2021 were retrospectively analyzed. Propensity score matching was performed to balance the two groups of baseline characteristics. After 1:1 matching, the overall survival (OS) of the two groups was compared by the Kaplan−Meier method. Univariate and multivariate Cox regression analyses were used to identify independent predictors of OS.
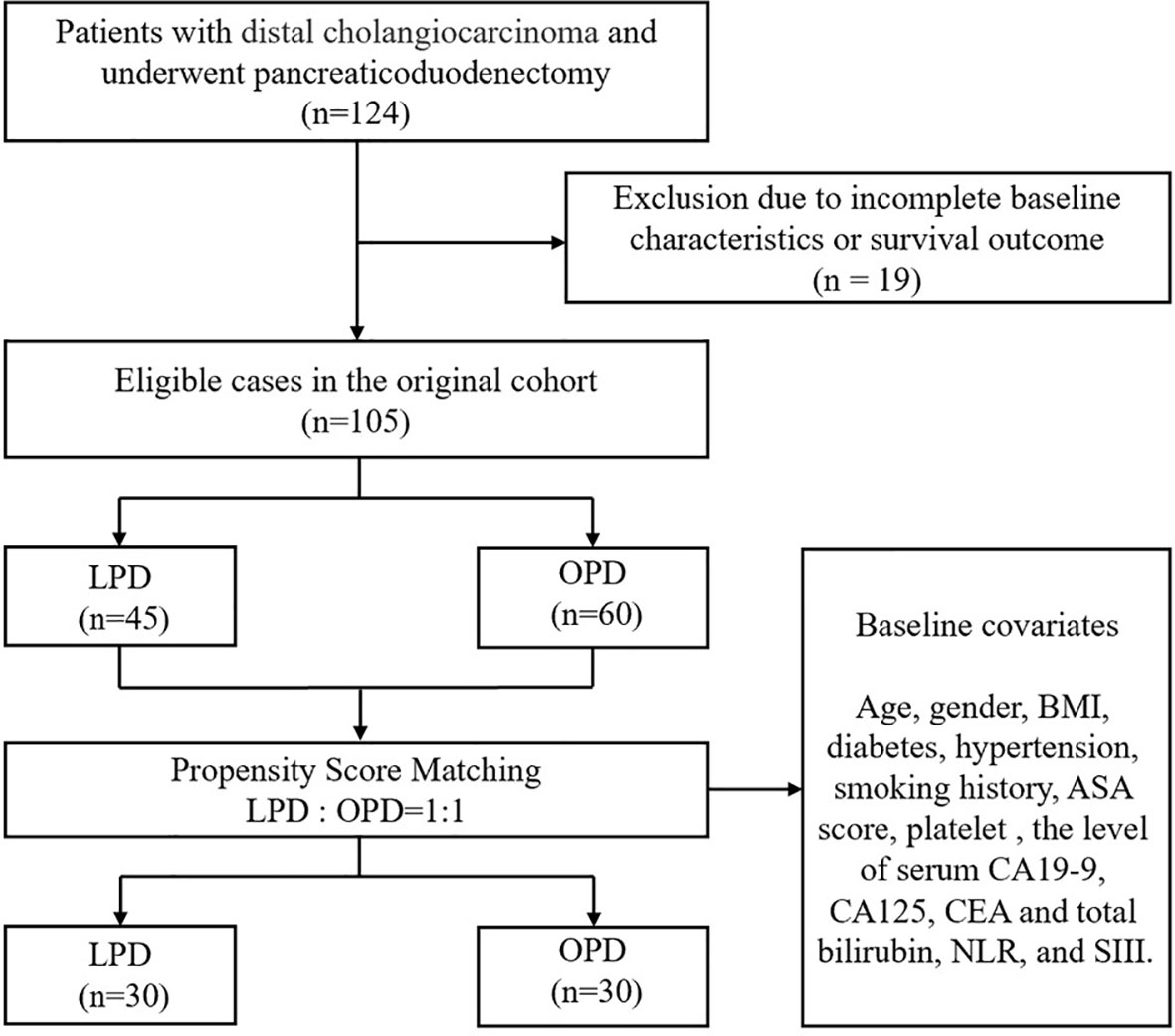
Results: The original cohort consisted of 124 patients. Nineteen patients were excluded because of incomplete baseline or follow-up data, and the remaining 105 patients were divided into two cohorts (45 in the LPD group and 60 in the OPD group). The LPD group showed more favorable results in OS analysis (LPD vs. OPD, 56.4 [46.2-66.5] vs. 48.9 [36.4-61.4], months, P=0. 01). PSM analysis identified 30 pairs of patients, and differences between matching groups were still significant (LPD vs. OPD, 67.9[58.2-77.6] vs. 47.4[31.4-67.5], months, P=0.002). Moreover, the LPD group experienced less intraoperative bleeding (LPD vs. OPD, 292.67 vs. 519.17 mL, P=0.002). Univariate analysis showed that surgical modality (P=0.012), carbohydrate antigen 19-9 (P=0.043), carcinoembryonic antigen (P=0.003), neutrophil-to-lymphocyte ratio (P=0.012), blood transfusion (P=0.031), clinically relevant postoperative pancreatic fistula (P<0.001) and lymphatic metastasis (P=0.004) were predictors of OS. Multivariate Cox analysis demonstrated that carbohydrate antigen 19-9 (P=0.048), carcinoembryonic antigen (P=0.031) and lymphatic metastasis (P=0.023) were independent predictive factors of OS. However, adjuvant therapy had no significant effect on the OS of DCC patients after radical pancreaticoduodenectomy (P>0.05).
Conclusions: For DCC patients, LPD may be a more recommended procedure because of its advantages over OPD in terms of intraoperative bleeding and long-term survival.
Introduction
Distal cholangiocarcinoma is an epithelial malignancy originating in the middle and lower segments of the common bile duct and the ampulla of Vater (1), which accounts for approximately 20%-40% of cholangiocarcinoma (2). DCC is highly malignant and has a poor prognosis. The 1-year, 3-year and 5-year OS rates are 46%, 18% and 11%, respectively (3). Old age among males and chronic biliary tract disease are potential risk factors for its occurrence (4). Lymphatic metastasis and nerve infiltration are the main modes of invasion. Pancreaticoduodenectomy is the normative and sole therapeutic method for DCC patients (5, 6). The first case of laparoscopic pancreaticoduodenectomy (LPD) was reported by Gagner et al. in 1994 (7, 8) and has been widely carried out worldwide since then. In the past two decades, LPD has been widely used to treat DCC (3). With the advancement of operative techniques and perioperative care, the postoperative survival rate of DCC patients has been significantly improved (9). However, it is still unclear whether LPD is superior to OPD in terms of short-term outcomes and long-term survival (10). Some studies suggest that LPD takes a long time, has a complicated operation and is high risk, which has high requirements for the surgical team and a high incidence of postoperative complications (11, 12). Several recent multicenter studies have shown that LPD is a secure and practical approach. In high-volume centers with sufficient surgical experience, LPD appears to be an effective alternative, which is associated with a shorter hospital stay and similar short-term morbidity and mortality to OPD. Nevertheless, despite extensive procedural expertise, the clinical benefits of LPD compared to OPD are still insignificant (13–16). However, few studies have focused on LPD and OPD in DCC, so we conducted this study to focus on the differences between LPD and OPD in DCC to help guide the surgical treatment of DCC.
Methods
Study design and patient selection
The data of DCC patients who underwent pancreatoduodenectomy at the Third Affiliated Hospital of Soochow University from May 2010 to May 2021 were retrospectively analyzed. The inclusion criteria were as follows: 1. all patients underwent PD radical surgery; 2. postoperative pathological examination confirmed distal cholangiocarcinoma; 3. preoperative imaging examination showed that the tumor had no distant metastasis; and 4. there was no other malignant tumor resection history.
The exclusion criteria were as follows: 1. incomplete clinical records or loss during follow-up; 2. patients who received neoadjuvant therapy before surgery; 3. patients with severe underlying disease who could not tolerate surgery; and 4. patients only received palliative treatment. The surgeon explained the procedure of pancreaticoduodenectomy clearly.
Data collection
We obtained patient demographics, laboratory data, postoperative pathological results and follow-up data from the medical records database. Preoperative data consisted of age, body mass index (BMI), smoking history, sex, height, history of diabetes, history of hypertension, American Society of Anesthesiologists (ASA) score (17) and tumor markers, including carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 125 (CA125) and carcinoembryonic antigen (CEA). Liver function biochemical data and routine blood indicators, such as platelets, total bilirubin, neutrophil (N) count, lymphocyte (L) count, neutrophil-to-lymphocyte ratio (NLR) and systemic immune inflammation index (SIII=P*N/L), were also calculated (17).
Intraoperative observation indicators included the harvested lymphatic nodes, intraoperative bleeding volume and blood transfusion (obtained through surgical records and anesthesia records). Pathological results included differentiation degree, R0 resection, lymphatic metastasis and tumor stage. The tumor stages were determined according to the 8th edition of the DCC TNM staging definition proposed by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) in 2018 (18). The depth of tumor invasion and lymphatic metastasis were recorded in every patient.
Postoperative observation data included hospitalization time after operation, postoperative complications, postoperative adjuvant therapy and overall survival. Postoperative hospital stay was defined as the number of days from surgery to discharge. Postoperative complications were classified according to the Clavien−Dindo (CD) classification (19), including abdominal infection, clinically relevant postoperative pancreatic fistula (CR-POPF), and delayed gastric emptying (DGE) (17). According to the International Research Group on Pancreatic Surgery (ISGPS) definition, in the current study, only grade B and C POPF were thought to be complications, and the previously defined grade A biochemical pancreatic fistula report was no longer considered a clinical complication (20, 21). OS was defined as the duration from surgical resection to clinical death or last follow-up. To ensure adequate follow-up time for survival analysis, patients who received PD after May 2021 were excluded. Postoperative follow-up was conducted by outpatient visits, inpatient medical record systems and telephone calls. The follow-up period lasted until the end of May 2022 or the patient’s death.
Propensity score matching
In recent years, an increasing number of surgical studies have begun to apply PSM analysis to effectively reduce the influence of confounding factors in the study (22). In this study, two groups were matched by propensity score matching. Age, sex, BMI, diabetes, smoking history, ASA score and clinical laboratory test data, including CA125, CEA, platelets, total bilirubin, neutrophil count, lymphocyte count, SIII and other factors related to surgical or postoperative management, were selected as matching factors. Next, PSM was performed at 1:1. After matching, 45 patients were excluded. The LPD group included 30 patients, and the OPD group included 30 patients, for a total of 60 patients. To prevent prognostic factors such as adjuvant therapy and R0 resection from affecting the construction of the propensity score model, only baseline variables were included.
Statistical analyses
For the entire cohort, categorical variables were expressed as frequencies and percentages (%) and compared using the chi-square test (X2) or Fisher’s exact test. Continuous variables conforming to a normal distribution were calculated as the mean ± standard deviation (SD) and compared by Student’s t test. Continuous variables with a nonnormal distribution are represented by the median and interquartile range (IQR), and the differences were compared by the Mann−Whitney U test. The Kaplan−Meier method was adopted to draw survival curves, and the log-rank test was used for univariate analysis of the clinicopathological factors associated with OS. Factors showing statistically significant differences in univariate analysis were included in multivariate Cox regression analysis, to determine the independent risk factors affecting the prognosis of patients. P<0.05 was considered to indicate statistical significance. All statistical analyses were performed by using IBM SPSS Statistics (version 26.0, IBM Corp).
Results
The baseline data
As shown in Figure 1, 124 people with DCC who received PD were enrolled in the study. Nineteen patients were excluded because of incomplete baseline or follow-up data, and the remaining 105 patients were divided into two cohorts (45 in LPD and 60 in OPD). Covariates such as sex, age, BMI, comorbidities, ASA, TNM stage, and preoperative tumor markers were included in the 1:1 PSM analysis. After PSM analysis, 30 LPD patients (66.7%) and 30 OPD patients (50.0%) were matched. Baseline characteristics were equilibrated to reduce the impact of confounding factors between the two groups of patients on the study.
The mean age was 65.3 ± 7.7 years in the LPD group and 63.1 ± 9.9 years in the OPD group (P=0.232). The proportion of male patients was 82.2% in the LPD group and 48.3% in the OPD group (P=0.804). There was no statistical significance before and after PSM (P>0.05). The two teams had similarities in BMI and comorbidities. Furthermore, there was no statistical significance in total bilirubin, platelet, NLR, SIII or preoperative cancer biomarkers including CA19-9, CA125 and CEA, between the two groups, as detailed in Table 1.
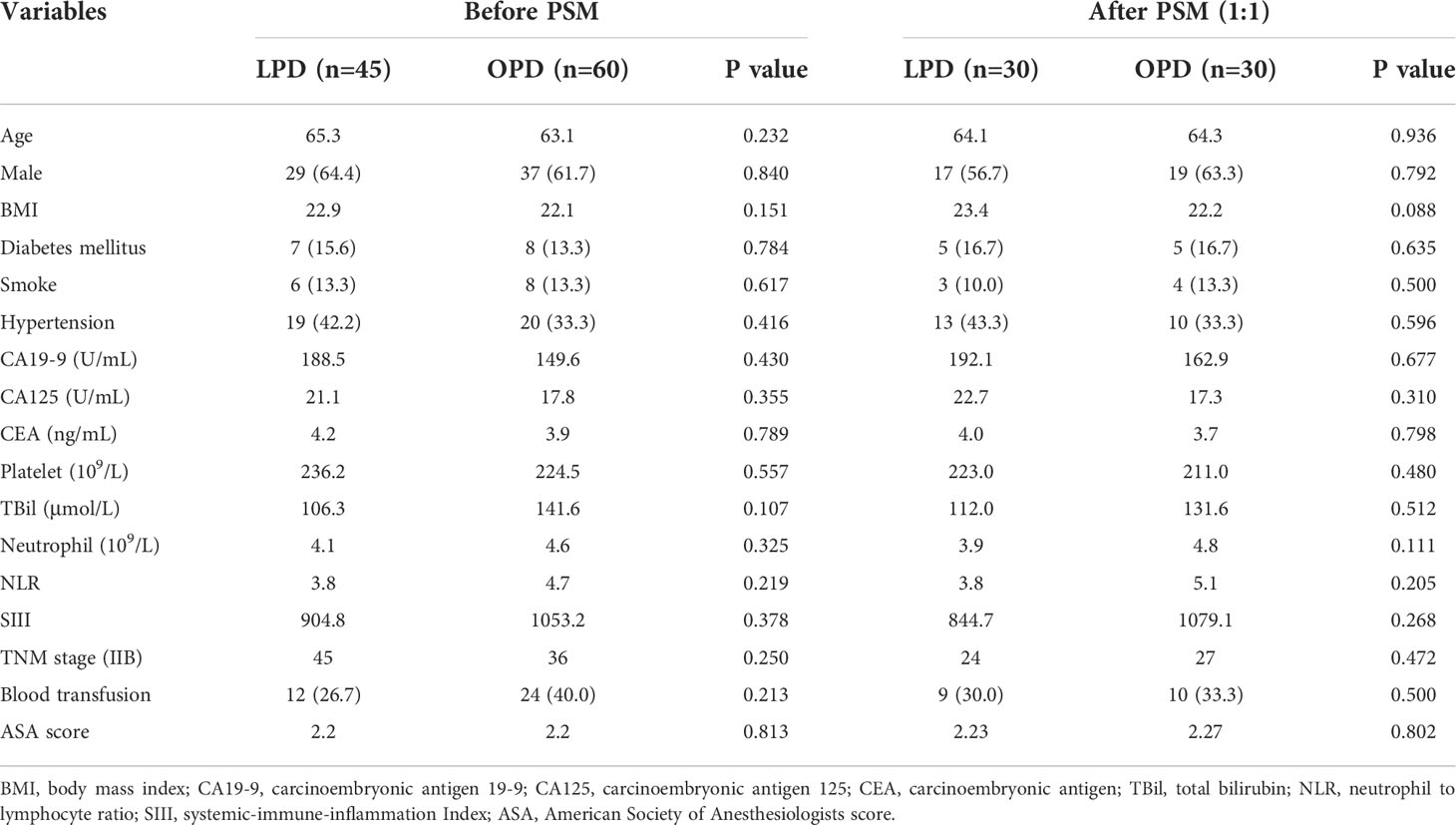
Table 1 Comparisons of patients’ clinicopathologic characteristics between patients with DCC underwent LPD and OPD before and after propensity score matching.
Clinicopathological and postoperative characteristics
As demonstrated in Table 2, the intraoperative bleeding in the LPD group was less than that in the OPD group (mean, 292.67 vs. 519.17 mL, P=0.002), and less intraoperative blood transfusion was required (mean, 591.67 vs. 880.00 mL, P=0.033). Although the LPD group had more harvested lymph nodes in the original cohort (mean, 14.58 vs. 10.20, P=0.01), there was no statistical significance after PSM analysis (mean, 14.3 vs. 9.93, P=0.076). Other surgical results and pathological features, such as lymphatic metastasis, R0 resection and AJCC TNM stage, were not significantly different between the two matched groups.
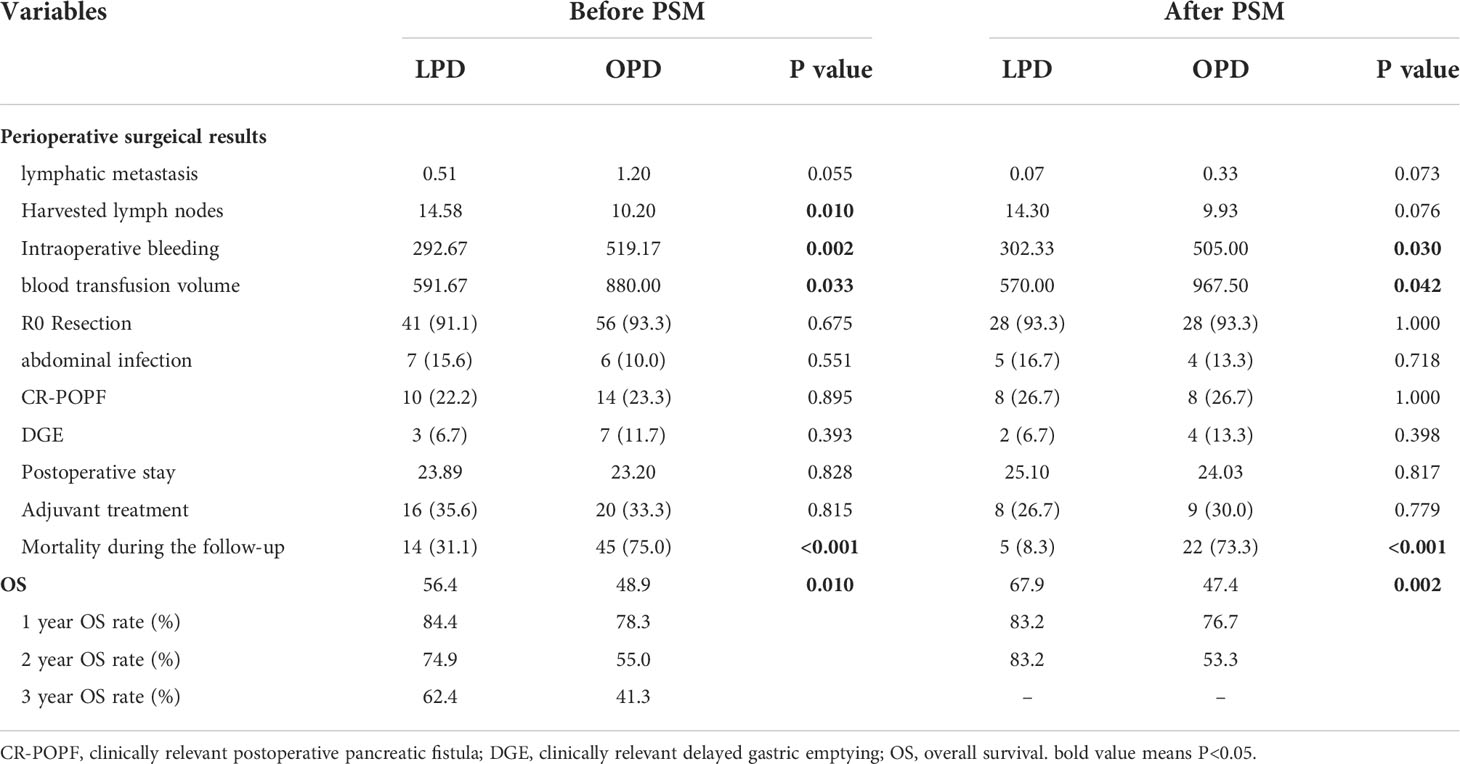
Table 2 Comparisons of clinical outcomes between patients with LPD and OPD before and after propensity score matching.
As two sets of postoperative data showed, there was no statistical significance in hospitalization time after operation, DGE, CR-POPF or intra-abdominal infection between the two teams (P>0.05).
Survival analysis
Patients who underwent PD from 2010 to 2021 (n=105) were included in the survival analysis. Comparing the tumor prognosis of the two groups, the LPD group had a lower mortality during the follow-up (31.1 vs. 75.0%, P<0.001) and showed preferable results in OS analysis (LPD vs. OPD, 56.4[46.2-66.5] vs. 48.9[36.4-61.4], months, P=0. 01) (Figure 2A). After PSM analysis, the difference in mortality during the follow-up period was still marked (8.3 vs. 73.3%, P<0.001), and the OS benefit of LPD was still superior to that of OPD (LPD vs. OPD, 67.9[58.2-77.6] vs. 47.4[31.4-67.5], months, P=0. 002) (Figure 2B).
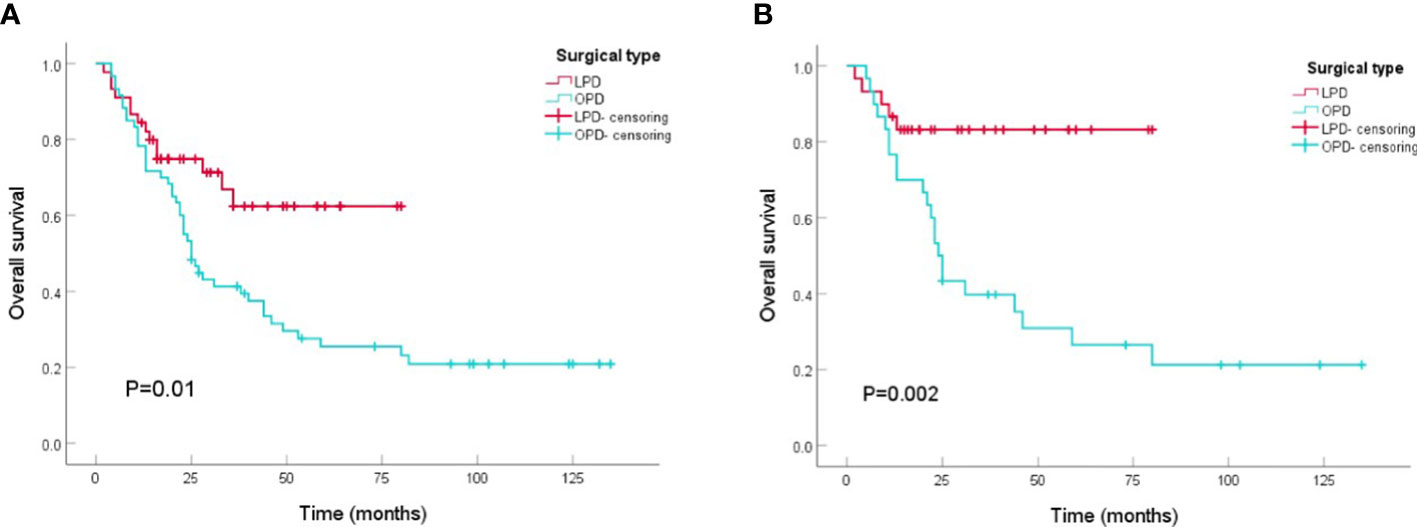
Figure 2 Kaplan-Meier curves of overall survival between patients with OPD and LPD. (A) Kaplan-Meier curves of overall survival between patients with OPD and LPD in the original cohort. (B) Kaplan-Meier curves of overall survival between patients with OPD and LPD in the PSM cohort.
Univariate analysis of prognosis
Univariate Cox regression analysis indicated that the surgical approach was a predictor of overall survival (HR=0.463[0.253-0.847], P=0.012). Other OS-related factors included CA19-9 (HR=2.561[1.030-6.369], P=0.043), CEA (HR=4.095[1.639-10.232], P=0.003), NLR (HR=2.885[1.258-6.620], P=0.012), blood transfusion (HR=1.768[1.053-2.967], P=0.031), CR-POPF (HR=0.249[0.124-0.500], P<0.001), and lymphatic metastasis (HR=1.170[1.052-1.302], P=0.004). Sex, BMI, complications and abdominal infection were not important prognostic factors (Table 3).
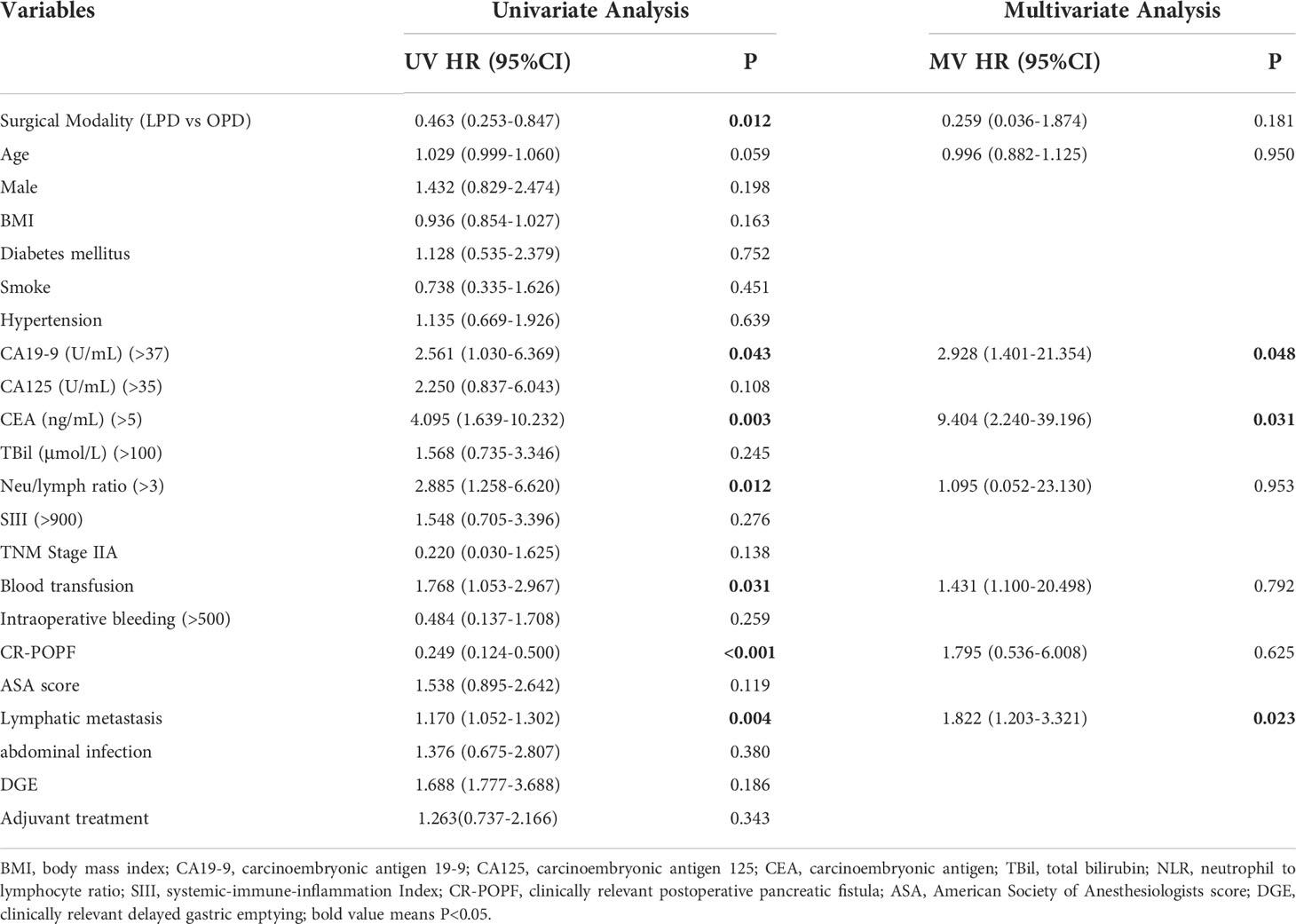
Table 3 Independent prognostic factors of OS by Cox-regression analysis of the whole cohort before and after the PSM.
Multivariate analysis of predictors
The OS-related factors with a P value less than 0.1 in the univariate analysis were included in the multivariate analysis, which indicated that age, surgical method, blood transfusion, CR-POPF and NLR were no longer prognostic factors. CA19-9 (HR=2.928[1.401-21.354], P=0.048), CEA (HR=9.404[2.240-39.196], P=0.031) and lymphatic metastasis (HR=1.822[1.203-3.321], P=0.023) were still markedly related to OS, while postoperative adjuvant chemotherapy had no significant influence on OS (P=0.343) (Table 3).
Discussion
DCC is highly malignant and prone to lymphatic metastasis or nerve infiltration, and the prognosis is poor (4). At present, PD is the sole feasible and effective method for the treatment of resectable DCC. To reach the purpose of radical resection, lymph nodes need to be carefully cleaned to ensure R0 resection in PD (23). With the continuous progress of surgical technology, LPD has become a routine procedure in some pancreatic centers in recent years (24). Some studies have reported that LPD has a shorter hospital stay and rapid postoperative recovery and is not inferior to OPD in short-term oncology results (25, 26). However, there are still few studies on the treatment of DCC with LPD and OPD. This study found that the LPD group achieved similar clinical outcomes in terms of surgical safety and radical effects, and the LPD group had better long-term survival than OPD.
The incidence of total complications following LPD and OPD was reported to be comparable. In the LPD group and the OPD group, the incidence of CR-POPF was 18.02% and 18.73%, respectively, while the incidence of intra-abdominal infection was 10% and 11% (15, 27). Our study indicated that the average intraoperative bleeding volume in the LPD group was less than that in the OPD group (mean, 292.67 vs. 519. 17 mL, P=0.002), and fewer intraoperative blood transfusions were required (mean, 591.67 vs. 880. 00 mL, P=0.033). This may be due to laparoscopic amplification and clearer vision; thus, the intraoperative vascular exposure could be conducted, and intraoperative bleeding could be managed more clearly and precisely. The data in this study showed that the rate of CR-POPF in the LPD group and the OPD group was 22.2% and 23.3%, respectively, and the incidence of intra-abdominal infection was 15.6% and 10.0%, respectively. There was no statistical significance (P>0.05) and similar to the results reported in the literature, indicating that LPD can achieve short-term efficacy similar to OPD for DCC.
In addition, to achieve the consequence of radical resection, R0 resection is required (23). There was no statistical significance in R0 resection between LPD and OPD (91.1% vs. 93.3%, P=0.675). It is shown that LPD and OPD can achieve similar results in R0 resection, and it is consistent with Boggi et al. ‘‘s report that the R0 resection rate of LPD for tumors can reach 73%~100%. As distal cholangiocarcinoma is prone to lymphatic metastasis, it is widely accepted that lymphatic metastasis is an independent risk factor affecting the prognosis of DCC patients (28). In this research, we also found that lymphatic metastasis was a prognostic factor for the OS of DCC patients (P<0.05). Although research has pointed out that lymphatic metastasis and harvested lymph nodes are prognostic factors for DCC (29), there are still few studies on the relationship between harvested lymph node number and the long-term survival of DCC. In our study, although the LPD group had more harvested lymph nodes in the original cohort (mean, 14.58 vs. 10.20, P=0.01), there was no statistical significance after PSM analysis (mean, 14.3 vs. 9.93, P=0.076). This reveals that the two teams are similar in technical feasibility.
Several studies have shown that LPD and OPD have similar long-term survival rates in the treatment of pancreatic and periampullary cancers (15, 27). A recently published paper showed that there was no significant difference in long-term survival between LPD and OPD in DCC (30). In contrast, our research revealed that the prognosis for LPD was improved. Comparing the potential causes of the different outcomes, although there was no statistically significant difference after PSM, LPD harvested more lymph nodes in the initial cohort, which may be worthy of further study. Second, research has demonstrated that blood transfusions have an impact on PD patients’ long-term survival after surgery (31). Our results supported this finding and showed that LPD patients receive fewer blood transfusions, which may help improve the prognosis for LPD patients.
Many studies have focused upon the adjuvant treatment of DCC (32, 33) because the tumor heterogeneity of DCC leads to poor targeted therapy for this disease. At present, the use of gemcitabine combined with platinum drugs is generally accepted internationally, and the existence of positive lymph is an indication of adjuvant therapy (34, 35). We found that adjuvant therapy was not a predictor of OS, and the proportion of postoperative adjuvant therapy in DCC patients was 34.3%, which was lower than the international average (36). The negative results might be due to the limited numbers of samples who received postoperative adjuvant therapy in the research, and multicenter studies on DCC adjuvant therapy after PD are expected.
The study still has some limitations. First, this is a single-center, small-sample retrospective study and may inevitably involve residual confounding factors. Second, because patients do not receive unified treatment, there may be disunity factors that affect the survival of patients. To provide a clearer conclusion on the LPD of DCC, a massive prospective randomized controlled trial is needed. Hopefully soon, we can conduct a multicenter randomized trial.
In summary, compared with OPD, LPD significantly reduced intraoperative bleeding volume and blood transfusion in DCC treatment and showed a similar postoperative complication rate. With better long-term survival outcomes than OPD, LPD can be a preferred surgical option for DCC patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Third Affiliated Hospital of Soochow University. The patients provided their written informed consent to participate in this study.
Author contributions
WC and XC designed the research and revised the manuscript. XC, WC, YueZ performed the surgery with the help of their colleagues. YuwZ, GZ, DW gathered results of each patient. YueZ and HW analyzed the data. YuwZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Jiangsu “333” high-level talent Project (2022, 3-4-086), Changzhou Health High-level Top-notch Talent Project (2022), Changzhou Science and technology support plan for social development (No. CE20225043). Young Talent Development Plan of Changzhou Health Commission (No. CZQM2020005, No. CZQM2021002), Major Science and Technology Project of Changzhou Health Commission (ZD201906), Applied Basic Research of Changzhou Technology Bureau (CJ20190093), and Young Talent Science and Technology Project of Changzhou Health Commission (No. QN202101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bahra M. Surgical treatment of distal cholangiocarcinoma. Chirurg (2021) 92(9):788–95. doi: 10.1007/s00104-021-01453-2
2. Lee RM, Maithel SK. Approaches and outcomes to distal cholangiocarcinoma. Surg Oncol Clin N Am (2019) 28(4):631–43. doi: 10.1016/j.soc.2019.06.014
3. Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol (2019) 58(7):1048–55. doi: 10.1080/0284186X.2019.1590634
4. Courtin-Tanguy L, Rayar M, Bergeat D, Merdrignac A, Harnoy Y, Boudjema K, et al. The true prognosis of resected distal cholangiocarcinoma. J Surg Oncol (2016) 113(5):575–80. doi: 10.1002/jso.24165
5. Istanbouli A, Patel S, Almerey T, Li Z, Stauffer JA. Surgical treatment for intrahepatic, peri-hilar, and distal cholangiocarcinoma: 20-single institutional year experience. Am Surg (2021). doi: 10.1177/00031348211034751
6. Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepato-Biliary-Pancreatic Surg (2008) 15(1):41–54. doi: 10.1007/s00534-007-1279-5
7. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc (1994) 8(5):408–10. doi: 10.1007/BF00642443
8. Feng Q, Liao W, Xin Z, Jin H, Du J, Cai Y, et al. Laparoscopic pancreaticoduodenectomy versus conventional open approach for patients with pancreatic duct adenocarcinoma: An up-to-Date systematic review and meta-analysis. Front Oncol (2021) 11:749140. doi: 10.3389/fonc.2021.749140
9. Chong EH, Choi SH. Hybrid laparoscopic and robotic hepatopancreaticoduodenectomy for cholangiocarcinoma. J Gastrointest Surg (2019) 23(9):1947–8. doi: 10.1007/s11605-019-04242-9
10. Qin R, Kendrick ML, Wolfgang CL, Edil BH, Palanivelu C, Parks RW, et al. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr (2020) 9(4):464–83. doi: 10.21037/hbsn-20-446
11. El Nakeeb A, Askar W. Laparoscopic pancreaticoduodenectomy for resection of periampullary tumors should be routine? HPB (2018) 20:S649–50. doi: 10.1016/j.hpb.2018.06.2265
12. Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg (2015) 220(5):831–8. doi: 10.1016/j.jamcollsurg.2014.12.052
13. Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg (2012) 255(6):1048–59. doi: 10.1097/SLA.0b013e318251ee09
14. Mazzola M, Giani A, Crippa J, Morini L, Zironda A, Bertoglio CL, et al. Totally laparoscopic versus open pancreaticoduodenectomy: A propensity score matching analysis of short-term outcomes. Eur J Surg Oncol (2021) 47(3 Pt B):674–80. doi: 10.1016/j.ejso.2020.10.036
15. Wang M, Li D, Chen R, Huang X, Li J, Liu Y, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol (2021) 6(6):438–47. doi: 10.1016/S2468-1253(21)00054-6
16. Yin T, Qin T, Wei K, Shen M, Zhang Z, Wen J, et al. Comparison of safety and effectiveness between laparoscopic and open pancreatoduodenectomy: A systematic review and meta-analysis. Int J Surg (2022) 105:106799. doi: 10.1016/j.ijsu.2022.106799
17. Zhang Z, Yin T, Qin T, Pan S, Wang M, Zhang H, et al. Comparison of laparoscopic versus open pancreaticoduodenectomy in patients with resectable pancreatic ductal adenocarcinoma: A propensity score-matching analysis of long-term survival. Pancreatology (2022) 22(2):317–24. doi: 10.1016/j.pan.2021.12.005
18. Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional validation study of the American joint commission on cancer (8th edition) changes for T and n staging in patients with pancreatic adenocarcinoma. Ann Surg (2017) 265(1):185–91. doi: 10.1097/SLA.0000000000001763
19. Bolliger M, Kroehnert JA, Molineus F, Kandioler D, Schindl M, Riss P, et al. Experiences with the standardized classification of surgical complications (Clavien-dindo) in general surgery patients. Eur Surg (2018) 50(6):256–61. doi: 10.1007/s10353-018-0551-z
20. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery (2017) 161(3):584–91. doi: 10.1016/j.surg.2016.11.014
21. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery (2005) 138(1):8–13. doi: 10.1016/j.surg.2005.05.001
22. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg (2018) 53(6):1112–7. doi: 10.1093/ejcts/ezy167
23. Wellner UF, Shen Y, Keck T, Jin W, Xu Z. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: a meta-analysis for the 5-year survival. Surg Today (2017) 47(3):271–9. doi: 10.1007/s00595-016-1362-0
24. Chen K, Pan Y, Liu XL, Jiang GY, Wu D, Maher H, et al. Minimally invasive pancreaticoduodenectomy for periampullary disease: a comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol (2017) 17(1):120. doi: 10.1186/s12876-017-0691-9
25. Dembinski J, Yoh T, Aussilhou B, Ftériche FS, Hounkonnou CPA, Hentic O, et al. The long-term outcomes of laparoscopic versus open pancreatoduodenectomy for ampullary carcinoma showed similar survival: a case-matched comparative study. Surg Endosc (2022) 36(7):4732–40. doi: 10.1007/s00464-021-08813-3
26. Pan S, Qin T, Yin T, Yu X, Li J, Liu J, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: study protocol for a multicentre randomised controlled trial. BMJ Open (2022) 12(4):e057128. doi: 10.1136/bmjopen-2021-057128
27. Dang C, Wang M, Zhu F, Qin T, Qin R. Comparison of laparoscopic and open pancreaticoduodenectomy for the treatment of nonpancreatic periampullary adenocarcinomas: a propensity score matching analysis. Am J Surg (2021) 222(2):377–82. doi: 10.1016/j.amjsurg.2020.12.023
28. Suzuki S, Shimoda M, Shimazaki J, Maruyama T, Oshiro Y, Nishida K, et al. Number of positive lymph nodes and lymphatic invasion are significant prognostic factors after pancreaticoduodenectomy for distal cholangiocarcinoma. Clin Exp Gastroenterol (2019) 12:255–62. doi: 10.2147/CEG.S207333
29. Guilbaud T, Girard E, Lemoine C, Schlienger G, Alao O, Risse O, et al. Intra-pancreatic distal cholangiocarcinoma and pancreatic ductal adenocarcinoma: a common short and long-term prognosis? Updates Surg (2021) 73(2):439–50. doi: 10.1007/s13304-021-00981-0
30. Kim SH, Lee B, Hwang HK, Lee JS, Han HS, Lee WJ, et al. Comparison of postoperative complications and long-term oncological outcomes in minimally invasive versus open pancreatoduodenectomy for distal cholangiocarcinoma: A propensity score-matched analysis. J Hepatobiliary Pancreat Sci (2022) 29(3):329–37. doi: 10.1002/jhbp.1067
31. Lopez-Aguiar AG, Ethun CG, Pawlik TM, Tran T, Poultsides GA, Isom CA, et al. Association of perioperative transfusion with recurrence and survival after resection of distal cholangiocarcinoma: A 10-institution study from the US extrahepatic biliary malignancy consortium. Ann Surg Oncol (2019) 26(6):1814–23. doi: 10.1245/s10434-019-07306-x
32. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
33. Rangarajan K, Simmons G, Manas D, Malik H, Hamady ZZ. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: A systematic review and meta-analysis. Eur J Surg Oncol (2020) 46(4 Pt A):684–93. doi: 10.1016/j.ejso.2019.11.499
34. Park K, Kim KP, Park S, Chang HM. Comparison of gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer. Asia Pac J Clin Oncol (2017) 13(1):13–20. doi: 10.1111/ajco.12592
35. Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, et al. Combination gemcitabine plus s-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol (2019) 30(12):1950–8. doi: 10.1093/annonc/mdz402
Keywords: distal cholangiocarcinoma, laparoscopic pancreaticoduodenectomy, survival, propensity score matching, prognostic factors
Citation: Zhu Y, Zu G, Wu D, Zhang Y, Yang Y, Wu H, Chen X and Chen W (2022) Comparison of laparoscopic and open pancreaticoduodenectomy for the treatment of distal cholangiocarcinoma: A propensity score matching analysis. Front. Oncol. 12:1057337. doi: 10.3389/fonc.2022.1057337
Received: 29 September 2022; Accepted: 31 October 2022;
Published: 18 November 2022.
Edited by:
Wenbo Zou, Chinese PLA General Hospital, ChinaReviewed by:
Bao Jin, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaRenyi Qin, Huazhong University of Science and Technology, China
Copyright © 2022 Zhu, Zu, Wu, Zhang, Yang, Wu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemin Chen, tomuer@126.com; Weibo Chen, cwb_med@163.com
†These authors have contributed equally to this work
 Yuwen Zhu
Yuwen Zhu Guangchen Zu
Guangchen Zu Di Wu
Di Wu Yue Zhang
Yue Zhang Yang Yang
Yang Yang Han Wu
Han Wu Xuemin Chen
Xuemin Chen Weibo Chen
Weibo Chen