- Institute and Policlinic for Occupational Medicine, Environmental Medicine and Prevention Research, University Hospital of Cologne, Cologne, Germany
Circadian disruption is associated with sleep, mood, and metabolic disorders, and—according to the International Agency for Research on Cancer—even with cancer. Mechanistically, the source of disease may be circadian system instability which likely arises during development. In animal experiments, both low perinatal light:dark ratios and chronic perinatal photoperiod phase shifting yield enduring, detrimental effects on neuroendocrine physiology via circadian system instability. Certainly, accumulating disturbances to neuroendocrine physiology and detrimental downstream effects could predispose to internal cancers. Epidemiologically, either season of birth or latitude of birth, both of which co-determine perinatal photoperiod-zeitgeber strengths, have been utilized independently as proxies for other environmental co-etiologies of cancer. Both have been independently associated with cancer; however, the evidence is inconclusive. We hypothesize that time of birth and location of birth, together determining perinatal photoperiod, contribute to cancer development through Perinatal Light Imprinting of Circadian Clocks and Systems.
Background
Plants and animals match their physiology and behavior to the 24-h solar photoperiod. Neuroendocrine rhythmicity, the internal form of communication about—and response to—external time, must constantly re-align to photoperiods which change over days and seasons. When confined to rather constant geographical locations, this constant re-alignment is very gradual and consciously imperceptible. An abrupt circadian disruption such as a trans-meridian flight, whereby the endogenously anticipated photoperiod dramatically changes, has perceptible effects on physiology which we know as jet-lag, and it can take several days for our internal (biological) time to re-align with external (environmental) time. Circadian disruption can affect sleep, mood, and the temporal organization of physiology. Accumulated circadian disruption to physiology is associated with sleep, mood, metabolic disorders, and even cancer (1). As to use of the term cancer generically, there are two reasons why we should not restrict to specific types of cancer in the present manuscript: (1) circadian clocks and systems pervade almost every cell in the human body governing growth and metabolic processes, and (2) many endocrine signaling factors with different organ and tissue targets are under circadian control.
Experimental Links Between Perinatal Photoperiods and the Development of Circadian Clocks and Systems
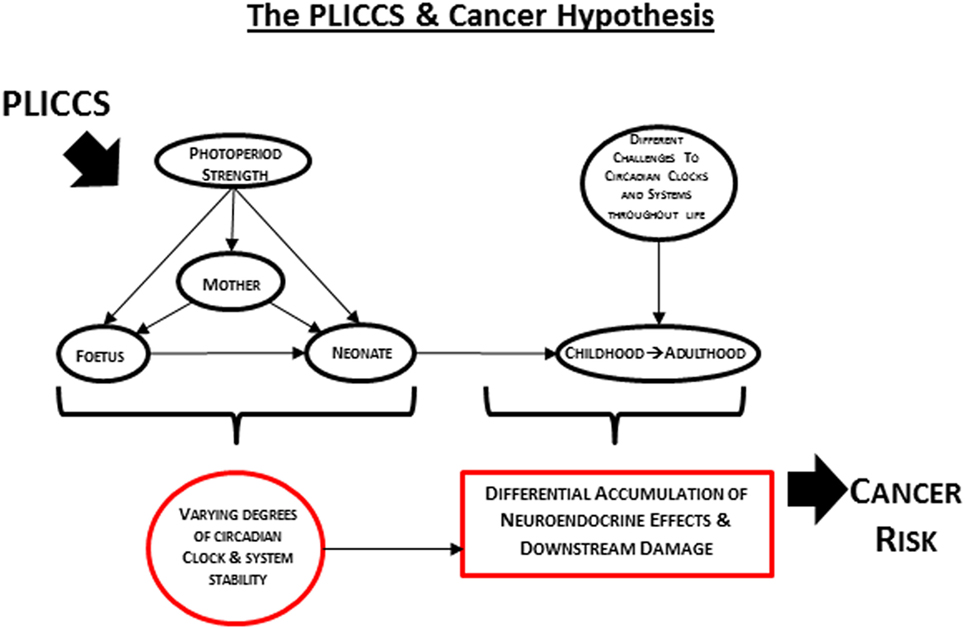
The development of circadian clocks and systems is susceptible to environmental factors, particularly light (2). In mice, different perinatal photoperiod exposures have been shown to effect differential neuronal firing parameters and circadian rhythms of gene expression of the suprachiasmatic nuclei (the internal master circadian clock) (3), circadian activity behavior, and the ability to anticipate dusk—effects that persist regardless of the continuation photoperiod (4, 5). Perinatal light affects development of mouse and rat circadian motor activities and adult rat behavior (6–8). Growth and behavior in the Siberian hamster is affected by the interaction between the perinatal and continuation photoperiods in a sex-dependent manner (9). Recent studies have shown that perinatal light environments affect how adult mouse and rat circadian behaviour is shaped by different photoperiods and, further, that perinatal photoperiod challenges that abruptly disrupt and phase shift endogenous circadian rhythms cause neuroendocrine and metabolic derangement in adulthood in Wistar rats. These changes are observed despite being maintained on controlled lighting schedules for the next 12 months and some are sex dependent (2, 10–14). Many endocrine signaling factors that are under circadian control and have a prominent role in transmitting circadian information are key to growth and development processes. Hormone and metabolic imbalance can be linked to various types of internal cancers and thus, by extension, so can circadian instability. Additionally, circadian instability in expression of genes and proteins governing cell growth, metabolism, and homeostasis could also predispose to cancer. In agreement with the concepts of “environmental imprinting of the mammalian circadian clock and its response to subsequent seasonal change under seasonal light cycles” by Ciarleglio et al. (4) and “increased vulnerability to circadian disruption” by Ohta et al. (5), Perinatal Light Imprinting of Circadian Clocks and Systems (PLICCS) results in higher or lower susceptibilities to exogenous and endogenous circadian challenges later in life which may result in sex-dependent differences in neuroendocrine, metabolic, and growth disruption, and therefore potentially different types of internal cancer.
Epidemiological Links Between Season or Latitude of Birth and Cancer
Season of birth or latitude of birth which together determine perinatal photoperiods and zeitgeber strengths [zeitgebers are external factors that synchronize an individual’s biological rhythms with the environment; first defined by Aschoff (15, 16); in this case, referring to light:dark ratio and light intensities entraining circadian clocks] have been studied each on its own with regard to different types of cancer but have not been studied together.
In effect, evidence for either being associated with cancer development is conflicting, the relevance of photoperiod is not sufficiently discussed, and authors have attributed findings to other environmental factors (17–21). Candidates to explain conflicting findings [for example, evidence of seasonal clustering of births in cancer patients as opposed to no evidence of seasonal clustering (18, 19, 21)] likely relate to differences in studying combined neoplasms as opposed to differentiating specific cancers, genetic susceptibility (or lack thereof) within small regions or distinct populations, and heterogeneous challenges presented to circadian clocks and systems. Importantly, there is evidence which is compatible with the PLICCS rationale, such as a winter birth clustering for acute myeloid leukemia in Sweden or a latitudinal gradient for lymphoma subtypes in Australia (18, 20). Sex differences have also been observed.
Hypothesis
We hypothesize that—after taking sex, specific cancer types, and post-perinatal challenges into account—time and location of birth contribute to—and may predict—cancer development in later life through PLICCS.
In 2012, it was postulated that humans born and raised postnatally (and we propose here that PLICCS incorporates 3 months prior to and after birth) under conditions of low zeitgeber strength are at greater risk of developing internal cancer than those born and raised under conditions of high zeitgeber strength (22). Based on the supporting evidence described above, we build here on this previous postulate to propose the PLICCS and cancer hypothesis: sex-specific associations of different cancer types with different perinatal zeitgeber strength exposures will be increasingly positive with greater degrees of post-perinatal challenges and accumulation of neuroendocrine effects and downstream damage mediated through disruptions of circadian clocks and systems stability (Figure 1).
Testing the Hypothesis
For epidemiological testing of this hypothesis, three portions of information are necessary: (a) The perinatal zeitgeber strength; (b) (Co-)determinants of cancer—both established and suspected; (c) Ancillary information (covariates, including potential confounders and effect modifiers).
In regard to (a)–(c), it is clear that data concerning season of birth and latitude of birth, as well as cancer type, progression, age, and sex of patient must be acquired. Equally clearly, relevant confounding data such as exposure to other known cancer risk factors (e.g., smoking, family history of cancer, alcohol, infection, exposure to cancerous agents, and chemicals) need to be taken into account.
In specific regard to (a), different zeitgeber strengths according to season and latitude at and around birth must be adequately defined. For example, how does 16 h of lower intensity sunlight closer to the Arctic compare to 10 h of higher intensity sunlight closer to the tropics? Ideally, average lux for a certain perinatal period, such as 3 months before and after birth, and place would be determined from meteorological data. Were this data not available, other suggestions to overcome the relative zeitgeber strength problem might be to use three-dimensional models for analysis (x, y, and z for latitude of birth, season of birth, and cancer variable, respectively). Based on the empirical information on possible links between perinatal zeitgeber strength and an imprinting of circadian system stability, L:D ratios examined by Ciarleglio et al. and Ohto et al. (4, 5), i.e., 8:16, 12:12, 16:8, and24:0, would take on the value range 0.5 ≤ 1 ≤ 2 < 24. While it may take many years to understand the details, L:D 12:12 may be a reference point in humans as has been in the study by Ciarleglio et al. for mice. This would render low and high zeitgeber strengths to correspond with L:D ratios below or above 1 and close to 0.5 and 2, respectively.
In specific regard to (b) and (c), beyond other challenges, such as shift-work, to circadian clocks and systems which are more or less stable, experimental evidence suggests that the post-perinatal photoperiod challenge should be considered where possible in relation to the PLICCS cancer hypothesis (4). A less stable circadian system resultant from PLICCS may cope with gradual seasonal changes of photoperiods and less extremes between seasons equally well as a more stable circadian system with more extreme differences between seasons. With regard to cancer, pineal melatonin is secreted in greater amounts in humans living at higher latitudes in winter due to the prolonged dark period and may play a role in shaping correlations of season and latitude of birth with cancer occurrence due to suggested potential oncostatic properties (17). It may be that different PLICCS and cancer relationships are observed within ranges of latitudes rather than across entire hemispheres.
Importantly, if there are increased risks for specific types of PLICCS-induced cancers rather than internal cancers in general, there are no data available to help us reliably differentiate between all internal cancer types. Indeed, it is only with studies such as PLICCS and internal cancer generally that we may identify specific PLICCS-causing types of cancer.
One relevant question is “could photic exposures beyond natural light alter the perinatal zeitgeber strength”? We expect that natural light with intensities of 20,000 to 100,000 lux from winter through summer determines zeitgeber strength; insofar we expect to identify PLICCS effects—if they exist—despite man-made light ranging from few lux in residential settings to 500 lux as a standard requirement in many occupational settings. Empirically, a recent study by Bauer and colleagues, while being focused on a psychiatric endpoint, has been the first epidemiological study to conclusively report data targeted at the PLICCS rationale (23). In principle, cohort studies can be used to systematically examine whether psychiatric disease rates in individuals born in winter months, adjusted for latitudinal photoperiod, are higher than in individuals born at other times of the year (22, 24, 25); case–control studies can explore whether the likelihood of having been born in winter months is higher in cases with psychiatric diseases than in controls without the disease. That this type of study can be performed for a psychiatric endpoint means similar pooled cohort data can be employed for a cancer endpoint. With specific regard to cancer endpoints, we are currently investigating options to test predictions based on the PLICCS hypothesis within large international cohort collaborations. International cohort collaborations such as the I4C (The International Childhood Cancer Cohort Consortium) (26) offer large-scale prospective information which may be used for (a)–(c) above; note that enrollment of people at birth for one cohort began as early as 1964. The I4C is a highly reputable consortium of cohort representatives who published hundreds of epidemiological studies into possible consequences of early environmental and other exposures for study individuals’ cancer or other diseases in later life stages. These databases can allow exploring whether certain variables, both environmental and genetic, can explain and possibly predict differential onsets and courses of cancer in humans who are followed from their perinatal period.
Moreover, researchers who studied relationships of either season or latitude with cancer occurrence could re-visit completed studies as a time- and cost-efficient research option or launch novel studies, which employ the time and location of birth as a variable to explain, and possibly predict, differential onsets and courses of disease. Provided that epidemiological studies were to yield results compatible with the PLICCS hypothesis and associated predictions, note that studies in individuals with perinatal light exposures at or around the equator could serve as control populations; indeed, homogeneous exposures to L:D 12:12 at the equator would imply that PLICCS metrics should not explain or predict differential cancer occurrence later in life which we hypothesize for individuals who experience gradients of perinatal light-associated zeitgeber strength.
Conclusion—What if the Hypothesis is Valid?
Let us assume that PLICCS, depending on low vs. high zeitgeber strength, can be either a cancer risk or protective factor. In that case, we could arrange for perinatal summer light exposure conditions irrespective of time of year and geographical location. Indeed, light represents a very amenable environmental agent for preventative measures against noxious-photic stimuli (27). Pregnant and breast-feeding mothers could utilize anthropogenic light to mimic a summer photoperiod during winter for themselves and their children. Furthermore, it has already been proposed to provide cyclical lighting for newborn intensive care units (28, 29). These lighting conditions could potentially be modified to reduce the risk of developing cancer later in life. Further studies would be required to determine optimum perinatal light conditions for preventative measures.
Associations between circadian disruption and cancer and between light and cancer have been reported ~half a century ago (30, 31). Somewhat scattered data from animal and human studies followed which is compatible with the PLICCS and cancer hypothesis such that the hypothesis warrants systematic testing. Should the PLICCS and cancer hypothesis hold true, simple preventative steps against cancer development would be at minimal cost while contributing to decrease what is a very large, and growing, global health system strain and economic burden.
Author Contribution
PL and TE contributed equally to this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Drs. Fritschi and Groß and journal reviewers for comments.
References
1. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol (2007) 8(12):1065–6.
2. Brooks E, Canal MM. Development of circadian rhythms: role of postnatal light environment. Neurosci Biobehav Rev (2013) 37:551–60. doi:10.1016/j.neubiorev.2013.02.012
3. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science (1990) 247:975–8. doi:10.1126/science.2305266
4. Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG. Perinatal photoperiod imprints the circadian clock. Nat Neurosci (2011) 14:25–7. doi:10.1038/nn.2699
5. Ohta H, Mitchell AC, McMahon DG. Constant light disrupts the developing mouse biological clock. Pediatr Res (2006) 60:304–8. doi:10.1203/01.pdr.0000233114.18403.66
6. Canal-Corretger MM, Vilaplana J, Cambras T, Diez-Noguera A. Functioning of the rat circadian system is modified by light applied in critical postnatal days. Am J Physiol Regul Integr Comp Physiol (2001) 280:R1023–30.
7. Canal-Corretger MM, Vilaplana J, Cambras T, Diez-Noguera A. Effect of light on the development of the circadian rhythm of motor activity in the mouse. Chronobiol Int (2001) 18:683–96. doi:10.1081/CBI-100106081
8. Cambras T, Canal MM, Cernuda-Cernuda R, Garcia-Fernandez JM, Diez-Noguera A. Darkness during early postnatal development is required for normal circadian patterns in the adult rat. Chronobiol Int (2015) 32:178–86. doi:10.3109/07420528.2014.960048
9. Pyter LM, Nelson RJ. Enduring effects of photoperiod on affective behaviors in Siberian hamsters (Phodopus sungorus). Behav Neurosci (2006) 120:125–34. doi:10.1037/0735-7044.120.1.125
10. Vilches N, Spichiger C, Mendez N, Abarzua-Catalan L, Galdames HA, Hazlerigg DG, et al. Gestational chronodisruption impairs hippocampal expression of NMDA receptor subunits Grin1b/Grin3a and spatial memory in the adult offspring. PLoS One (2014) 9:e91313. doi:10.1371/journal.pone.0091313
11. Mendez N, Halabi D, Spichiger C, Salazar ER, Vergara K, Alonso-Vasquez P, et al. Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology (2016) 157(12):4654–68. doi:10.1210/en.2016-1282
12. Varcoe TJ, Boden MJ, Voultsios A, Salkeld MD, Rattanatray L, Kennaway DJ. Characterisation of the maternal response to chronic phase shifts during gestation in the rat: implications for fetal metabolic programming. PLoS One (2013) 8:e53800. doi:10.1371/journal.pone.0053800
13. Varcoe TJ, Voultsios A, Gatford KL, Kennaway DJ. The impact of prenatal circadian rhythm disruption on pregnancy outcomes and long-term metabolic health of mice progeny. Chronobiol Int (2016) 33:1171–81. doi:10.1080/07420528.2016.1207661
14. Varcoe TJ, Wight N, Voultsios A, Salkeld MD, Kennaway DJ. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One (2011) 6:e18504. doi:10.1371/journal.pone.0018504
15. Aschoff J. Die 24-Stunden-Periodik der Maus unter konstanten Umgebungsbedingungen. Naturwissenschaften (1951) 38:506–7. doi:10.1007/BF00628863
16. Aschoff J. Zeitgeber der tierischen Tagesperiodik. Naturwissenschaften (1954) 41:49–56. doi:10.1007/BF00634164
17. Erren TC, Piekarski C. Does winter darkness in the Artic protect against cancer? The melatonin hypothesis revisited. Med Hypotheses (1999) 53:1–5. doi:10.1054/mehy.1999.0810
18. Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K. Perinatal risk factors for acute myeloid leukemia. Eur J Epidemiol (2015) 30:1277–85. doi:10.1007/s10654-015-0063-0
19. Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K. Season of birth and risk of Hodgkin and non-Hodgkin lymphoma. Int J Cancer (2014) 135:2735–9. doi:10.1002/ijc.28909
20. van Leeuwen MT, Turner JJ, Falster MO, Meagher NS, Joske DJ, Grulich AE, et al. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int J Cancer (2013) 133:944–51. doi:10.1002/ijc.28081
21. van Laar M, Kinsey SE, Picton SV, Feltbower RG. First description of seasonality of birth and diagnosis amongst teenagers and young adults with cancer aged 15-24 years in England, 1996-2005. BMC Cancer (2013) 13:365. doi:10.1186/1471-2407-13-365
22. Erren TC, Koch MS, Gross JV, Reiter RJ, Meyer-Rochow VB. A possible role of perinatal light in mood disorders and internal cancers: reconciliation of instability and latitude concepts. Neuro Endocrinol Lett (2012) 33:314–7.
23. Bauer M, Glenn T, Alda M, Andreassen OA, Angelopoulos E, Ardau R, et al. Influence of light exposure during early life on the age of onset of bipolar disorder. J Psychiatr Res (2015) 64:1–8. doi:10.1016/j.jpsychires.2015.03.013
24. Erren TC, Nise MS, Meyer-Rochow VB. Latitude, light, clocks and mood. Psychopharmacology (Berl) (2011) 216:147–8. doi:10.1007/s00213-011-2205-8
25. Erren TC, Gross JV, Meyer-Rochow VB. Light, clocks, mood, and cancer: consolidation and novel tests of latitude and instability hypotheses. Chronobiol Int (2011) 28:471–3; author reply p. 473. doi:10.3109/07420528.2011.577542
26. Brown RC, Dwyer T, Kasten C, Krotoski D, Li Z, Linet MS, et al. Cohort profile: the International Childhood Cancer Cohort Consortium (I4C). Int J Epidemiol (2007) 36:724–30. doi:10.1093/ije/dyl299
27. Lewy AJ, Kern HA, Rosenthal NE, Wehr TA. Bright artificial light treatment of a manic-depressive patient with a seasonal mood cycle. Am J Psychiatry (1982) 139:1496–8. doi:10.1176/ajp.139.11.1496
28. Erren TC, Trautmann K, Salz MM, Reiter RJ. Newborn intensive care units and perinatal healthcare: on light’s imprinting role on circadian system stability for research and prevention. J Perinatol (2013) 33:824–5. doi:10.1038/jp.2013.107
29. Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev (2013) 8:CD006982. doi:10.1002/14651858.CD006982.pub2
30. Harker JE. Experimental production of midgut tumours in Periplaneta americana L. J Exp Biol (1958) 35:251–9.
Keywords: circadian, perinatal, photoperiod, chronodisruption, season, latitude, epidemiology, cancer prevention
Citation: Lewis P and Erren TC (2017) Perinatal Light Imprinting of Circadian Clocks and Systems (PLICCS): The PLICCS and Cancer Hypothesis. Front. Oncol. 7:44. doi: 10.3389/fonc.2017.00044
Received: 13 January 2017; Accepted: 06 March 2017;
Published: 20 March 2017
Edited by:
Jianguang Ji, Lund University, SwedenReviewed by:
Valeria Edefonti, Università degli Studi di Milano, ItalyWagner Ricardo Montor, Faculdade de Ciências Médicas da Santa Casa de São Paulo, Brazil
Copyright: © 2017 Lewis and Erren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip Lewis, philip.lewis@uk-koeln.de
 Philip Lewis
Philip Lewis Thomas C. Erren
Thomas C. Erren