- 1Laboratory Center, Xiamen University Malaysia, Sepang, Malaysia
- 2Department of Traditional Chinese Medicine, Xiamen University Malaysia, Sepang, Malaysia
- 3Department of Pediatrics, Emory Vaccine Center, Atlanta, GA, United States
- 4Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 5Department of Microbiology and Immunology, Emory Vaccine Center, Atlanta, GA, United States
- 6School of Health Sciences, College of Health and Medicine, University of Tasmania, Launceston, TAS, Australia
- 7Division of Molecular Virology, Department of Clinical and Experimental Medicine, Linkoping University, Linkoping, Sweden
- 8Division of Infection Biology and Medical Microbiology, Department of Life Sciences, Central University of Tamil Nadu (CUTN), Thiruvarur, India
Tuberculosis (TB) treatment monitoring is paramount to clinical decision-making and the host biomarkers appears to play a significant role. The currently available diagnostic technology for TB detection is inadequate. Although GeneXpert detects total DNA present in the sample regardless live or dead bacilli present in clinical samples, all the commercial tests available thus far have low sensitivity. Humoral responses against Mycobacterium tuberculosis (Mtb) antigens are generally low, which precludes the use of serological tests for TB diagnosis, prognosis, and treatment monitoring. Mtb-specific CD4+ T cells correlate with Mtb antigen/bacilli burden and hence might serve as good biomarkers for monitoring treatment progress. Omics-based techniques are capable of providing a more holistic picture for disease mechanisms and are more accurate in predicting TB disease outcomes. The current review aims to discuss some of the recent advances on TB biomarkers, particularly host biomarkers that have the potential to diagnose and differentiate active TB and LTBI as well as their use in disease prognosis and treatment monitoring.
Introduction
Tuberculosis (TB) represents a major public health problem worldwide, and is transmitted via inhalation of aerosolized droplets carrying Mycobacterium tuberculosis (Mtb). It is estimated that approximately one-third (∼2 billion) of the global population is living with latent TB (LTBI) (World Health Organization [WHO], 2002), of which ∼10% likely progress to develop active TB within 2 years after initial exposure to the tubercle bacilli (Corbett et al., 2003). The risk of reactivation of latent TB is remarkably high among individuals infected with the human immunodeficiency virus (HIV) as well as in individuals on long-term immunosuppressive treatment with TNF-α inhibitors (Selwyn et al., 1989; Getahun et al., 2010; Day et al., 2018; Amelio et al., 2019). According to the latest WHO Global TB Report, Mtb has led to 10 million cases of TB in 2017, and is also one of the top 10 causes of mortality with ∼1.6 million (1.3 and 0.3 million in HIV-negative and HIV-positive individuals, respectively) deaths in 2017, which translates to ∼4000 deaths each day (Tiberi et al., 2018; World Health Organization [WHO], 2018). Although universal Bacillus Calmette-Guérin (BCG) is administered in many countries, the vaccine is only effective against disseminated TB in children, and it’s efficacy in adults largely remains controversial (Colditz et al., 1994; Zwerling et al., 2011; Amelio et al., 2017).
Tuberculosis treatment monitoring is paramount to clinical decision-making and the host biomarkers appears to play a significant role. Patients diagnosed with TB are generally put under a four drugs regimen (isoniazid, rifampicin, pyrazinamide, and ethambutol) for 2 months, known as the intensive phase; followed by 4 months maintenance phase with isoniazid and rifampicin. In spite of 6-long-months of anti-TB therapy, some patients however, will experience recurrence of infection and have an increased risk of M/XDR-TB. This 6-month duration may lead to prohibitive delay for clinical management. Exposure of Mtb to suboptimal drug concentrations risks robust bacterial replication and dissemination, increased rates of transmission and development of drug resistance. WHO reported an estimated 558,000 cases of rifampicin-resistant TB in 2017, of which 82% are infected with MDR-TB (World Health Organization [WHO], 2018). This has caused significant complications to the patients as they are to be treated with second-line drugs for an even longer duration (18–24 months) (World Health Organization [WHO], 2016), despite that their survival rate was merely <50% (World Health Organization [WHO], 2017). Hence, biomarkers that indicate an efficacious treatment at the early therapeutic phase as well as at the end of treatment, which predicts relapse could enormously improve clinical prognosis.
In order to achieve global control of TB disease, development of an effective novel vaccine (Zwerling et al., 2011) and novel drugs with shortened treatment duration (Abu-Raddad et al., 2009; Argun et al., 2016; Tiberi et al., 2018), as well as simpler and more accurate diagnostic tests (Walzl et al., 2011, 2018; Goletti et al., 2016, 2018) are of upmost importance. Hence, there is a pressing need to develop a low cost, minimal-invasive, non-sputum-based, highly sensitive and specific TB diagnostic test that uses easily accessible biological specimens such as blood and urine (Strimbu and Tavel, 2010; Nalejska et al., 2014; World Health Organization [WHO], 2014; Ballman, 2015; Buschmann et al., 2016; Goletti et al., 2016, 2018). Biomarkers can generally be divided into: (i) Mtb components, (ii) antibody responses to Mtb antigens, (iii) cellular immune responses to Mtb antigens, and (iv) unbiased “omics” approach (i.e., transcriptomics, proteomics and metabolomics). Here, we discussed some of the recent advances on TB biomarkers, particularly host biomarkers that have the potential to diagnose and differentiate active TB and LTBI as well as their use in disease prognosis and treatment monitoring. References for this review were identified through searches of PubMed for articles published from January 2005 to December 2018, by use of the terms “Mtb,” “LTBI,” “diagnosis,” “biomarkers,” “prognosis,” “monitoring,” “transcriptomics,” and “proteomics.” Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English alone were included.
Prospects of Detection of Biomarkers Associated With Mtb
Recent Advances in the Detection of Mtb by Conventional Methods
It is widely accepted that the currently available diagnostic technology for TB detection is simply inadequate (Wallis et al., 2010). The most widely used diagnostic test to date is the microscopic detection of acid-fast bacilli (AFB) in sputum, which suffers from poor sensitivity ranging from 34–80% (Davies and Pai, 2008). This is because the AFB test requires at least 10,000 bacilli/ml of sputum to produce a positive result. If the concentration of bacilli falls below the cut-off, the chance to produce an AFB positive result is merely <10% (World Health Organization [WHO], 2004; Moro et al., 2010; Desikan, 2013). Sputum culture is relatively more sensitive than sputum AFB test but has a turnaround time of a few weeks. Furthermore, culture of Mtb requires Biosafety Level 3 facilities (World Health Organization [WHO], 2007), which are seldom available across TB endemic areas.
The recently developed PCR-based technique to amplify Mtb gene namely GeneXpert MTB/RIF represents a major breakthrough in TB diagnostics. The test is not only easy to operate, requiring less training for laboratory personnel, but is also capable of “killing two birds with one stone” by detecting Mtb and rifampicin-resistance simultaneously within 2 h (Zeka et al., 2011; Kwak et al., 2013), thereby significantly improving the rates of detection of Mtb. However, its use is only limited to active pulmonary TB (PTB), and not LTBI. Besides, all the sputum-based diagnostic methods have their own intrinsic limitations in that they are seldom useful in the detection of extra-pulmonary TB (EPTB) disease. Hence, the diagnosis of EPTB is reliant on sampling of site-specific tissues as well as other biological fluids such as pleural fluid and cerebral spinal fluid (CSF) which often involve invasive procedures (Goletti et al., 2016). This could be a real problem as the incidence of EPTB in some developing countries ranges from 13% to as high as 37% (Arora and Chopra, 2007; Gomes et al., 2014; Gaifer, 2017), and sampling often involves invasive procedures. Therefore, the use of host biomarkers that reflect the pathological process or host immune responses to active TB, EPTB, and LTBI could be a better choice.
Developments in the Detection of Mtb DNA
From the pathogen perspective, detection of Mtb product such as Mtb DNA has been widely used as a diagnostic tool. GeneXpert has been shown to detect Mtb in a wide variety of clinical specimens including blood, urine, and CSF with better sensitivity and specificity as compared to Mtb culture (Cannas et al., 2008; da Cruz et al., 2011; Maynard-Smith et al., 2014; Theron et al., 2014). Sputum culture conversion either using solid and liquid media at the 2nd month post-initiation of TB therapy has long been used to monitor the efficacy of TB treatment, although this method usually take weeks (World Health Organization [WHO], 2013). GeneXpert MTB/RIF offers rapid detection in this regard and has shown good sensitivity (97%) and correlation time to culture positivity, but suffers from poor specificity ranging from 49 to 72% (Marlowe et al., 2011; Friedrich et al., 2013). This is in part due to GeneXpert, which is a PCR-based technique that detects total DNA present in the sample regardless live or dead bacilli present in clinical samples. Nonetheless, others have shown that patients who are positive for Mtb in blood are at an increased risk for death (Feasey et al., 2013). By using digital PCR (dPCR), a theoretically ten-fold more sensitive technique than real-time quantitative PCR, Li et al. developed a MTB detection test based on the MTB insertional sequence IS6110. This novel assay has been shown to have ∼twofold higher sensitivity than GeneXpert MTB/RIF assay in detecting MTB among probable and possible TB meningitis patients (Li et al., 2019).
Prospects of Detecting Miscellaneous Components of Mtb
Other Mtb components such as the 17.5 kDa Mtb cell wall lipoarabinomannan (LAM) has also been used to detect the presence of Mtb in urine. However, all the commercial tests available thus far have low sensitivity. In a meta-analysis, Minion et al. (2011) showed that the sensitivity of urine LAM test in seven studies is highly variable ranging from 13 to 93%. Hamasur et al. (2015) had further improved the assay by concentrating the LAM antigen present in urine up to 5–100 times using immunoprecipitation method. This action has enhanced the sensitivity of the urine LAM assay, but only restricted to HIV-TB co-infected patients. The sensitivity of the assay among HIV-negative TB patients remains very low (Hamasur et al., 2015). Several possible reasons might explain the higher sensitivity of LAM assay in HIV patients; (i) there might be a higher Mtb load among HIV patients since they are immunodeficient (Boehme et al., 2005; Shah et al., 2010); or (ii) there might have been HIV-associated nephropathy among these patients that increases the glomerular permeability resulting in higher levels of LAM in urine (Doublier et al., 2007; Peter et al., 2010). Nonetheless, the assay has been used to monitor anti-TB therapy responses (Drain et al., 2015) and has also been shown to predict the onset of TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) (Conesa-Botella et al., 2011) and death (Gupta-Wright et al., 2016) among TB-HIV co-infected patients.
Another Mtb secretory protein, the 30–35 kDa antigen 85 complex (Ag85A, Ag85B, and Ag85c) (Kashyap et al., 2005, 2007), early secretory antigen target-6 (ESAT-6), culture filtrate protein-10 (CFP-10) (Kalra et al., 2010; Feng et al., 2011; Shen et al., 2011; Zhang et al., 2015) and MPT64 (Kumar et al., 2011; Martin et al., 2011; Arora et al., 2015) have also been evaluated for their suitability as diagnostic reagents. The Ag85 complex is present in the sputum of patients with PTB (Kashyap et al., 2007) as well as in the CSF of patients with TB meningitis (Kashyap et al., 2005); but the sensitivity is inconsistent in various studies (Bentley-Hibbert et al., 1999; Kashyap et al., 2007). Other secretory proteins such as ESAT-6, CFP-10, and MPT64 are facing a similar problem. In one study, Turbawaty et al. attempted to detect the presence of all the three antigens in urine using a cocktail of polyclonal antibodies against all the three antigens. The authors showed that this strategy increased the sensitivity to 90%, although the specificity remained poor at <30% (Turbawaty et al., 2017).
Prospects With Detection of Host Immune Biomarkers in Mtb Infection
Host Antibody Responses to Mtb Antigens
Infection and immunity are the two sides of the same coin. When an individual is infected with Mtb, the pathogen will inevitably activate the immune response of the host leading to changes in host biomarkers. Pathogen-specific antibodies are the commonly used host biomarkers for pathogen diagnostics as they are simple to perform, inexpensive and are feasible for point-of-care. Many of the available serological tests employ either the lateral-flow or the ELISA format. A number of Mtb antigen-specific antibodies against PPD, antigen 60, ESAT-6, CFP-10, lipid-derived antigens and heat shock protein have been studied extensively (Verma and Jain, 2007). Of note, several Mtb antigens such as ESAT-6 and CFP-10 are not present in the genome of BCG strain, and hence detection of an immune response specific to these antigens can distinguish between and Mtb infection from vaccination responses (Andersen et al., 2000; Arend et al., 2000). Unfortunately, these assays so far have displayed poor sensitivity (ranges from 14% – 85%) and specificity (53–98.7%) (Steingart et al., 2007, 2009, 2011; Achkar and Ziegenbalg, 2012; Lagrange et al., 2014).
More recently, several highly antigenic MTB antigens have been developed for diagnostics with improved sensitivity and specificity than the classical ESAT-6- and CFP-10-based assays such as RV0310c-E and RV1255c-E. Receiver operating characteristic (ROC) analyses have shown that serum IgG against both RV0310c-E and RV1255c-E antigens has better sensitivity and specificity (AUC = 0.8 and 0.808, respectively) in diagnosing MTB compared to ESAT-6 and CFP-10 (AUC = 0.665 and 0.623, respectively) (Luo et al., 2017). Lopez-Ramos et al. (2018) showed that the antibodies against MTB antigen P12037 has a sensitivity and specificity of 92% and 91%, respectively in diagnosing active TB when used in concert with sCD14. Other researchers have found that antibody to MTB antigens such as proline-proline-glutamic acid protein 17 (PPE17) (Abraham et al., 2018) and mycobacterial DNA binding protein (MDP-1) (Maekura et al., 2019) can differentiate between patients with LTBI and active TB. Maekura et al. (2019) further showed that MDBP-1 may also be a good monitoring tool as persistently elevated IgG against MDBP-1 post anti-TB therapy could be associated with relapse after completion of treatment. Nonetheless, studies have also found that antibody responses against Mtb antigens are generally low among children (Achkar and Ziegenbalg, 2012) therefore, the use of serological tests for TB diagnosis, prognosis, and treatment monitoring can only be effectively used among adult patients.
One interesting study by Lu et al. on “resister,” a group of individuals highly exposed to MTB but who tested negative by T-cell based interferon gamma releasing assay (IGRA) and tuberculin skin test (TST), as well as did not develop LTBI has shed some light on TB pathogenesis. The authors found that the “resister” possessing MTB-specific IgM and class switched IgG; however, displayed reduced CD4-mediated IFN-γ responses toward ESAT-6, CFP-10, Ag85A, and Ag85B. Lu et al. (2019) also showed that the IgG of “resister” has higher avidity to MTB antigens compared to LTBI and healthy controls and further analysis also showed that the “resister” had significantly higher levels of IgG1 compared to other IgG subclasses. This suggests that the level of IgG1 could potentially be a prognosis biomarker, and holds the key to development of novel MTB therapeutics.
Host Cytokine Responses to Mtb Antigens – Beyond Interferon-γ
Unlike antibody responses, the cellular immune responses against Mtb-specific antigens have shown better consistency. In the past, the TST has been widely used to diagnose active TB and LTBI. However, due to cross-reactivity, the test cannot differentiate between Mtb and other non-tuberculous mycobacterial infections as well as BCG vaccination. Besides, the TST also suffers from poor sensitivity among immunocompromised patients (Nahid et al., 2006; Frahm et al., 2011). Since the last decade, the T-cell based IGRA has emerged as the most popular tool in TB diagnostics (Ferrara et al., 2009). IGRA measure IFN-γ production after ex vivo stimulation of whole blood with Mtb-specific antigens such as ESAT-6 and CFP-10 (Lalvani et al., 2001b; Mori et al., 2004). There are two formats of IGRA assay, the ELISA-based QuantiFERON TB Gold assay (Mori et al., 2004) and the ELISPOT-based T-SPOT assay (Lalvani et al., 2001a). The T-SPOT assay has a higher sensitivity compared to QuantiFERON TB Gold assay in detecting active TB (91 and 80.2%, respectively) (Bae et al., 2016). In general, IGRA is sensitive and more specific than TST (Pai et al., 2008). Although IGRA is less affected by the HIV-status as compared to TST (Mendelson, 2007; Rangaka et al., 2007), the assay appears to perform poorly in children with advanced HIV infection (Hormi et al., 2018); however, IGRA performed using peripheral blood mononucleocytes (PBMC) isolated from specific sites of TB disease such as pleural fluid, bronchioalveolar lavage (BAL) and CSF has been found to be highly sensitive and specific (Losi et al., 2007; Thomas et al., 2008).
Some studies suggested that IGRA response is stronger in active TB than LTBI, and hence, allows the differentiation of the two forms of TB disease (Janssens, 2007). However, other studies suggested that IGRA may not be suitable for the diagnosis of active TB and LTBI in high TB-burden regions (Sharma et al., 2017). This may be attributed to the nature of the antigen used in the IGRA, i.e., ESAT-6 and CFP-10 as they are secretory proteins of MTB especially during active infection. One study by Arroyo et al. (2018) showed that the use of latency-related antigens, i.e., dormancy survival regulon (DosR) and resuscitation promoting factor (Rpf) in IGRA could be better. The DosR peptide RV2029c and the Rpf peptide RV2389c have shown to differentiate LTBI from active PTB with a sensitivity of 90 and 85%, respectively (Arroyo et al., 2018).
Given the sensitivity and specificity of IGRA, the assay has also been suggested for use as a treatment monitoring tool (Ribeiro et al., 2009; Bocchino et al., 2010; Chee et al., 2010). Several studies have shown that patients who are IGRA negative on completion of anti-TB therapy experienced complete clinical and microbiological recovery (Goletti et al., 2008; Kabeer et al., 2011; Helmy et al., 2012). Another cohort by Kaneko et al. (2015) showed that patients who were IGRA positive at the end of treatment developed TB reactivation; whilst those who were IGRA negative did not develop TB reactivation for 2 years of follow-up.
One advantage of IGRA as an ex vivo stimulation assay is that the same assay tubes can be re-used to study other biomarkers either by multiplex cytokine bead array or by flow cytometry to obtain “biosignature” that may differentiate between different stage of TB disease (Chegou et al., 2009). One biomarker at the downstream of IFN-γ, i.e., IP-10 has shown to be of good use as a biomarker. Elevation of plasma level of IP-10 in un-stimulated tubes has been associated with active TB (Azzurri et al., 2005; Whittaker et al., 2008; Lighter et al., 2009; Novel et al., 2013; Petrone et al., 2015). IP-10 not only is as sensitive as IFN-γ (Kabeer et al., 2011) in blood but also offer several additional advantages. For instance, detection of IP-10 in the urine of children (Petrone et al., 2015) and adults (Darrah et al., 2007) with active TB makes sample collection easier. Further, unlike IFN-γ, IP-10 is less affected by HIV status; making it a robust biomarker to be used in ex vivo stimulation assays (Ruhwald et al., 2008; Goletti et al., 2010a, b; Kabeer et al., 2011).
Several studies have been conducted using multiplex cytokine bead array on plasma with or without ex vivo stimulation to differentiate active TB and LTBI. These studies employ a combination of 5–15 biomarkers in their analysis whose sensitivity ranges from 82.3 to 96.7% (Mihret et al., 2013; Won et al., 2017; La Manna et al., 2018). Despite the combination of biomarkers used by different studies, IFN-γ and IP-10 are the most common biomarkers used in these studies. Besides, other studies have shown that the ratio of IFN-γ and IL-10 (Sai Priya et al., 2010), ratio of IL-2 and IFN-γ (Wu et al., 2017), IL-8, IP-10, MIP-1α, sIL-2Rα, vascular endothelial growth factor (VEGF), MCP-3 (Yao et al., 2017; Hoel et al., 2019) as well as soluble markers to TLR-4 pathway such i.e., sCD14, MD-2, and LPS (Feruglio et al., 2013) can distinguish between active TB and LTBI and can also correlate with treatment success. By screening 38 cytokines, Luo et al. (2019) found that by using a combination of three cytokines, i.e., eotaxin, CCL22 and MCP-1, they were able to discriminate LTBI from active TB with a sensitivity and specificity of 87.8 and 91.8%, respectively (AUC = 0.94). Other plasma markers that associated with treatment success (measured as time to sputum conversion) include IL-6, MCP-1 (Djoba Siawaya et al., 2009), VEGF (Riou et al., 2012), hemeoxygenase-1 (HO-1), matrix metalloproteinases (MMP) (Andrade et al., 2013), serum amyloid, proteasome activation complex subunit-1, IL-11 receptor antagonist, and 2-antiplasmin (Nahid et al., 2014). However, further studies are required to evaluate and validate these markers.
Host Cellular Immune Responses to Mtb Antigens
Flow cytometry is a powerful technique used to analyze the characteristics of individual cells within heterogeneous populations. In principle, following Mtb antigen exposure, CD4+ and CD8+ T cells undergo several stages of differentiation from naïve T-cells (TN) progressing to central memory (TCM), effector memory (TEM) and finally to terminally differentiated T cells (TEMRA) (Harari et al., 2011; Rovina et al., 2013); and the more antigenic exposure (in quantity, antigenicity, and duration) of T cells the more advance the cellular differentiation. Based on this principle, several efforts have been made to characterize the functional signature of T cells (i.e., the combination of subsets and their cytokine production) that associated with stages of TB disease. CD69 is a co-stimulatory receptor and an early activation marker (Borrego et al., 1999; Yong et al., 2017) and increased levels of CD4+CD69+IFN-γ+ T cells is associated with early active TB or recent TB infection (Nikolova et al., 2013). Similarly, the frequency of CD137, a co-stimulatory molecule responsible to sustain effective activation, proliferation and survival of T-cells has also been shown to be associated with active TB (Yan et al., 2017).
A study done by Millington et al. (2007) reported that the polyfunctional CD4+ IFN-γ+IL-2+TNF-α+ T cells are predominantly seen in patients with active TB as compared to CD4+IL-2+IFN-γ+ T cells and CD4+IFN-γ+ only in LTBI. More detail studies found that the non-active form of TB response including LTBI or BCG vaccination and treated TB are associated with predominant CD4+IFN-γ+IL-2+ TEM and CD4+IL-2+ TCM; whilst active TB is associated with predominantly CD4+IFN-γ+ TEMRA cells (Sutherland et al., 2009; Caccamo et al., 2010; Casey et al., 2010; Sester et al., 2011). By using CD38, an immune activation marker and CD27, a maturation marker, Ahmed et al. (2018) showed that active TB was associated with increased frequency of CD38 + CD27low; whilst LTBI was associated with CD38-CD27high. Another study showed that LTBI is associated mostly with polyfunctional CD4+ T cells expressing IFN-γ, IL-2, and TNF-α and in combination; whilst active TB is predominated with CD4+ T cells expressing only TNF-α, and not IFN-γ as measured by IGRA (Harari et al., 2011). CD27, a member of TNF-α receptor superfamily was found to be useful in differentiating active TB and LTBI. Streitz et al. (2007) showed that active TB patients had significantly higher CD4+CD27+ T cells as compared to BCG vaccinees and patients with LTBI had an intermediate level of CD4+CD27+ T-cells. Other studies found that CD27 (Adekambi et al., 2012; Nikitina et al., 2012; Petruccioli et al., 2013, 2015; Portevin et al., 2014) and Mtb-specific CD4+ T cells (Adekambi et al., 2012; Nikolova et al., 2013) correlate with Mtb antigen/bacilli burden and hence might serve as good biomarkers for monitoring treatment progress. Other subpopulations of T cells such as IL-10 + Th17 T cells were found to be significantly higher among LTBI; whilst IFN-γ + Th17 was significantly higher in active TB when stimulated with DosR (Rakshit et al., 2017). Besides T-cells, dendritic cells, especially the percentage of BDCA3 + mDC and CD123 + pDCs were significantly reduced in patients with active TB; while the same subtypes were found to be significantly activated among patients with LTBI (Parlato et al., 2018).
Several other surface markers including the immune activation marker CD38 and HLA-DR, the proliferation marker Ki-67 (Adekambi et al., 2012) as well as the percentage of myeloid-derived suppressor cells (MDSCs) (El Daker et al., 2015) have also been suggested as biomarkers to monitor treatment efficacy. The expression of immune activation markers such as CD38 and HLA-DR on T cells was significantly reduced by week 9 after initiation of the anti-TB therapy. The slope of decline in the expression of these markers was correlated with the time of stable culture conversion (Ahmed et al., 2018). Study also showed that individuals had a substantial amount of TEM at the sixth month of anti-TB therapy suggesting that persistence of live Mtb may lead to relapse; while individuals who retained only TCM may hint complete clearance of Mtb (Goletti et al., 2006; Butera et al., 2009; Millington et al., 2010; Wang et al., 2010; Pollock et al., 2013; Chiacchio et al., 2014; Petruccioli et al., 2015). Similarly, follow up on the anti-TB treatment showed that patients who had significant reduction in TB load showed a shift from CD4+IFN-γ+ TEMRA cells to CD4+IFN-γ+IL-2+ TEM (Caccamo et al., 2010). In a longitudinal prospective study on active TB on anti-TB therapy, Ferrian et al. studied the association between Tregs and treatment efficacy. By using cut-off point at day 71 after initiation of anti-TB therapy, the authors classified the patients into two groups: (i) those who achieved stable sputum culture conversion faster than day 71 as rapid responders, and (ii) those achieved stable sputum culture conversion later than day 71 as slow responders. The authors found that the frequencies of Treg was significantly higher in slow responders and could predict time to stable culture conversion with the sensitivity of 81% and specificity of 85% (AUC = 0.87).
Prospects of Biomarkers by Unbiased “Omics” Approach
Omics approach, i.e., genomic, transcriptomic, proteomic and metabolomics is a high throughput method that allows thousands of biomarkers of multi-dimension, to be unbiasedly acquired in one step (Kell and Oliver, 2004). While genomics provide an overview of genetic instruction provide by DNA, transcriptomics would investigate the gene expression patterns, proteomics the dynamic of protein products, and metabolomics the interactions and understanding of the entire metabolism of an individual in a disease setting. These “omics” approaches have been used not only in TB diagnosis, monitoring treatment efficacy, predicting treatment outcomes, but also used to improve understanding the pathogenesis of TB disease.
One genomic study has investigated SP110, a gene encoded for IFN-induced nuclear protein in a large cohort of patients including 301 active TB, 68 LTBI and 278 healthy controls. From the 5 index SNPs, i.e., rs7580900, r7580912, rd9061, rs11556887, and rs2241525, the study identified that rs9061 was significantly associated with increased susceptibility to LTBI (Chang et al., 2018). Further investigations indicated that individuals bearing this SNP had decreased levels of plasma TNF-α (Leu et al., 2018).
One transcriptomic study identified a profile of 393-transcripts signatures in whole-blood characterizing active TB; and 86-transcript signature that distinguishes TB from other infections. Through modular and pathway enrichment analysis the study revealed that active TB was predominated with neutrophil-driven interferon-inducible genes, consisting of both IFN-γ and type I IFN-αβ signaling (Berry et al., 2010). These findings have been further validated by several independent cohorts (Lesho et al., 2011; Maertzdorf et al., 2011a, b; Ottenhoff et al., 2012; Bloom et al., 2013; Kaforou et al., 2013; Walter et al., 2015) and the profile of transcript signatures decreased after the initiation of treatment (Ottenhoff, 2009; Bloom et al., 2013; Cliff et al., 2016). Moreover, Anderson et al. (2014) also showed that the transcription profile was able to distinguish between active TB and LTBI.
The cytotoxic cell gene transcripts may also be used for end treatment assessment to predict TB relapse (Joosten et al., 2012; Cliff et al., 2016). Maertzdorf et al. (2011b) found that the high-affinity IgG Fc receptor IB (FcγRIB) along with other four different transcripts are differentially expressed among between active TB and LTBI. Other transcripts such as lactotransferrin, CD64 (also known as FcγR1A) also can discriminate active TB from LTBI (Sutherland et al., 2014). Another study by Lee et al. (2016) found that genes related to innate immune responses are highly expressed among patients with active TB; whilst genes related to apoptosis and natural killer (NK) cell activation are predominantly expressed in patients with LTBI. RAS and RAB interactor 3 (RIN3) also could discriminate between active TB, LTBI, and recurrent infection (Mistry et al., 2007).
In the pass, the transcriptomic studies have mainly studied the profiling of mRNA expression, but more recently, there has been a growing interest on the non-coding region of mRNA. Although these non-coding RNA does not encode for any protein, they do possess certain regulatory functions and hence may be altered by different stages of TB disease. By comparing the micro RNA (miRNA) profile of children infected with TB, adult patients with active PTB, active EPTB, TB/HIV co-infection as well as LTBI, Miotto et al. (2013) managed to identify a set of 15 miRNA signature that was common for TB infection with a sensitivity and specificity of 82 and 77%, respectively. Another study by Fu et al. has looked into the circular RNA (circRNA) and their association with TB disease. circRNA is a recently discovered, endogenous, covalently closed without free 3′- and 5′-end non-coding RNA. Being a covalently closed circular RNA, it is highly resistant to RNase degradation and hence are abundant and long-lasting in cells. The authors found that there were 171 deregulated circRNA in TB infection where circRNA_103017, circRNA_059914, circRNA_101128 were most profoundly elevated whilst circRNA_062400 was decreased. This circRNA signature could potentially be a useful marker for TB (Fu et al., 2019). Chakrabarty et al. (2019) also identified several miRNA including 2 from human, i.e., hsa-miR-146a-5p and hsa-miR-125b-5p and one miRNA from MTB, i.e., MTB-miR5 that increased among patients with active TB. miRNA has also been used to differentiate LTBI from active TB. By studying 250 miRNAs, Lyu et al. (2019) also identified the patterns where the hsa-let-7e-5p, hsa-let-7d-5p, hsa-miR-450a-5p, and has-miR-140-5p were differentially expressed among patients with LTBI; whilst hsa-miR-1246, hsa-miR-2110, hsa-miR-370-3p, hsa-miR-28-3p, hsa-miR-193b-5p were associated differentially expressed among patients with active TB.
Besides, by using proteomic microarray method, Hai et al. screened 4262 MTB antigens and found that IgG toward 152 Mtb antigens were differentially elevated among patients with active TB when compared to patients with LTBI. Further analysis showed that RV2031c, RV1408 and RV2421c were able to discriminate between active TB and LTBI (Cao et al., 2018). By studying 1011 host serum biomarkers, Liu et al. found that 153 protein were significantly elevated among patients with severe TB. These included α-1-acid glycoprotein 2 (ORM2), IL-36α, s100 calcium-binding protein (S100-A9), and superoxide dismutase (SOD) (Liu et al., 2018). Aiming to improve understanding on TB progression, Duffy et al. investigated a cohort of household contacts of TB index cases HHCs and non-human primate challenge model. By combining both blood transcriptomic, serum metabolomics and pathway analysis, the authors identified a novel immunemetabolic signature involving cortisol, tryptophan, glutathione and tRNA acylation that associated with the progression of latent to active TB (Duffy et al., 2019).
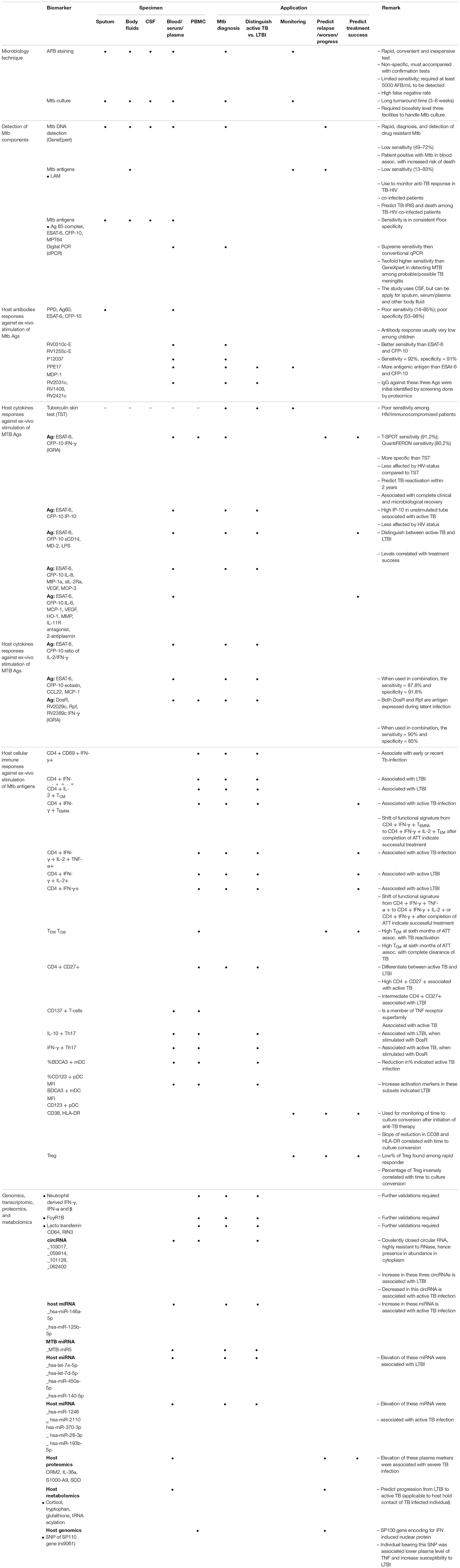
Summary of each biomarkers and their applications are given in Table 1.
Future Developments and Conclusion
As omics approach is capable to provide a more holistic picture for the disease mechanisms and hence more accurate in predicting disease outcomes (Clarke et al., 2008; Heidecker et al., 2008; Gesthalter et al., 2015; Jong et al., 2016; Kohonen et al., 2017; Lowe et al., 2017). Based on the omics profile, new hypotheses will be generated for further examination. There have already been a few successful cases in the search for TB biomarkers (Weiner et al., 2012; Kaforou et al., 2013; Goletti et al., 2016; Maertzdorf et al., 2014, 2016; Weiner and Kaufmann, 2017) and the number of study is still increasing. It is also worthwhile to point out that Mtb-specific immune responses are probably not homogenous in human populations and might be influenced by HIV-1 co-infection, heredity and several other exogenous environmental factors [183–185]. State-of-the-art data mining tools including supervised and unsupervised learning as well as new algorithms must be designed to handle such big data. Further, since the number of variables in omics studies is usually way larger than the sample size, the statistical power for detecting a few suitable biomarkers will inevitably decrease profoundly. Given that high investment is required for omics studies, which obviously may be impractical for developing countries, the well-validated omics markers should be applied for simple and rapid point-of-care tests.
Author Contributions
YY and ES wrote the first draft of the manuscript. HT, AS, WW, RV, RE, VV, and ML contributed to the writing of the manuscript. YY, HT, AS, WW, VV, ML, and ES agreed with the manuscript’s results and conclusion. All authors have read and confirmed that they meet, ICMJE criteria for authorship.
Funding
This work was supported by a grant from Xiamen University Malaysia Research Funding (XMUMRF), XMUMRF/2018-C2/ILAB/0001. ML was supported by the Swedish Research Council, the Swedish Physicians against AIDS Research Foundation; VINNMER for Vinnova, Linköping University Hospital Research Fund, ALF Grants Region Östergötland, FORSS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abraham, P. R., Devalraju, K. P., Jha, V., Valluri, V. L., and Mukhopadhyay, S. (2018). PPE17 (Rv1168c) protein of Mycobacterium tuberculosis detects individuals with latent TB infection. PLoS One 13:e0207787. doi: 10.1371/journal.pone.0207787
Abu-Raddad, L. J., Sabatelli, L., Achterberg, J. T., Sugimoto, J. D., Longini, I. M. Jr., Dye, C., et al. (2009). Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl. Acad. Sci. U.S.A. 106, 13980–13985.
Achkar, J. M., and Ziegenbalg, A. (2012). Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin. Vacc. Immunol. 19, 1898–1906.
Adekambi, T., Ibegbu, C. C., Kalokhe, A. S., Yu, T., Ray, S. M., and Rengarajan, J. (2012). Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One 7:e36046. doi: 10.1371/journal.pone.0036046
Ahmed, M. I. M., Ntinginya, N. E., Kibiki, G., Mtafya, B. A., Semvua, H., Mpagama, S., et al. (2018). Phenotypic changes on Mycobacterium tuberculosis-specific CD4 T cells as surrogate markers for tuberculosis treatment efficacy. Front. Immunol. 9:2247. doi: 10.3389/fimmu.2018.02247
Amelio, P., Portevin, D., Hella, J., Reither, K., Kamwela, L., Lweno, O., et al. (2019). HIV infection functionally impairs Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses. J. Virol. 93:e01728-18.
Amelio, P., Portevin, D., Reither, K., Mhimbira, F., Mpina, M., Tumbo, A., et al. (2017). Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLoS Negl. Trop. Dis. 11:e0005817. doi: 10.1371/journal.pntd.0005817
Andersen, P., Munk, M. E., Pollock, J. M., and Doherty, T. M. (2000). Specific immune-based diagnosis of tuberculosis. Lancet 356, 1099–1104.
Anderson, S. T., Kaforou, M., Brent, A. J., Wright, V. J., Banwell, C. M., Chagaluka, G., et al. (2014). Diagnosis of childhood tuberculosis and host RNA expression in Africa. N. Engl. J. Med. 370, 1712–1723.
Andrade, B. B., Pavan Kumar, N., Mayer-Barber, K. D., Barber, D. L., Sridhar, R., Rekha, V. V., et al. (2013). Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One 8:e62618. doi: 10.1371/journal.pone.0062618
Arend, S. M., Andersen, P., van Meijgaarden, K. E., Skjot, R. L., Subronto, Y. W., van Dissel, J. T., et al. (2000). Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181, 1850–1854.
Argun, M., Uzum, K., Sonmez, M. F., Ozyurt, A., Derya, K., Cilenk, K. T., et al. (2016). Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anat. J. Cardiol. 16, 234–241.
Arora, J., Kumar, G., Verma, A. K., Bhalla, M., Sarin, R., and Myneedu, V. P. (2015). Utility of MPT64 antigen detection for rapid confirmation of Mycobacterium tuberculosis complex. J. Glob. Infect. Dis. 7, 66–69.
Arora, V. K., and Chopra, K. K. (2007). Extra pulmonary tuberculosis. Indian J. Tuberculosis 54, 165–167.
Arroyo, L., Marin, D., Franken, K., Ottenhoff, T. H. M., and Barrera, L. F. (2018). Potential of DosR and Rpf antigens from Mycobacterium tuberculosis to discriminate between latent and active tuberculosis in a tuberculosis endemic population of Medellin Colombia. BMC Infect. Dis. 18:26. doi: 10.1186/s12879-017-2929-0
Azzurri, A., Sow, O. Y., Amedei, A., Bah, B., Diallo, S., Peri, G., et al. (2005). IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7, 1–8.
Bae, W., Park, K. U., Song, E. Y., Kim, S. J., Lee, Y. J., Park, J. S., et al. (2016). Comparison of the sensitivity of QuantiFERON-TB gold in-tube and T-SPOT. TB according to patient age. PLoS One 11:e0156917. doi: 10.1371/journal.pone.0156917
Bentley-Hibbert, S. I., Quan, X., Newman, T., Huygen, K., and Godfrey, H. P. (1999). Pathophysiology of antigen 85 in patients with active tuberculosis: antigen 85 circulates as complexes with fibronectin and immunoglobulin G. Infect. Immun. 67, 581–588.
Berry, M. P., Graham, C. M., McNab, F. W., Xu, Z., Bloch, S. A., Oni, T., et al. (2010). An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 466, 973–977.
Bloom, C. I., Graham, C. M., Berry, M. P., Rozakeas, F., Redford, P. S., Wang, Y., et al. (2013). Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS One 8:e70630. doi: 10.1371/journal.pone.0070630
Bocchino, M., Chairadonna, P., Matarese, A., Bruzzese, D., Salvatores, M., Tronci, M., et al. (2010). Limited usefulness of QuantiFERON-TB Gold In-Tube for monitoring anti-tuberculosis therapy. Respir. Med. 104, 1551–6.
Boehme, C., Molokova, E., Minja, F., Geis, S., Loscher, T., Maboko, L., et al. (2005). Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 99, 893–900.
Borrego, F., Robertson, M. J., Ritz, J., Pena, J., and Solana, R. (1999). CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology 97, 159–165.
Buschmann, D., Haberberger, A., Kirchner, B., Spornraft, M., Riedmaier, I., Schelling, G. et al. (2016). Toward reliable biomarker signatures in the age of liquid biopsies - how to standardize the small RNA-Seq workflow. Nucleic acids Res. 44, 5995–6018.
Butera, O., Chiacchio, T., Carrara, S., Casetti, R., Vanini, V., Meraviglia, S. et al. (2009). New tools for detecting latent tuberculosis infection: evaluation of RD1-specific long-term response. BMC Infect. Dis. 9:182. doi: 10.1186/1471-2334-9-182
Caccamo, N., Guggino, G., Joosten, S. A., Gelsomino, G., Di Carlo, P., Titone, L. et al. (2010). Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40, 2211–2220.
Cannas, A., Goletti, D., Girardi, E., Chiacchio, T., Calvo, L., Cuzzi, G. et al. (2008). Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int. J. Tuberculosis Lung Dis. 12, 146–151.
Cao, S. H., Chen, Y. Q., Sun, Y., Liu, Y., Zheng, S. H., Zhang, Z. G. et al. (2018). Screening of serum biomarkers for distinguishing between latent and active tuberculosis using proteome microarray. Biomed. Environ. Sci. 31, 515–526.
Casey, R., Blumenkrantz, D., Millington, K., Montamat-Sicotte, D., Kon, O. M., Wickremasinghe, M. et al. (2010). Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One 5:e15619. doi: 10.1371/journal.pone.0015619
Chakrabarty, S., Kumar, A., Raviprasad, K., Mallya, S., Satyamoorthy, K., and Chawla, K. (2019). Host and MTB genome encoded miRNA markers for diagnosis of tuberculosis. Tuberculosis 116, 37–43.
Chang, S. Y., Chen, M. L., Lee, M. R., Liang, Y. C., Lu, T. P., Wang, J. Y. et al. (2018). SP110 polymorphisms are genetic markers for vulnerability to latent and active tuberculosis infection in Taiwan. Dis. Mark. 2018:4687380.
Chee, C. B., KhinMar, K. W., Gan, S. H., Barkham, T. M., Koh, C. K., Shen, L. et al. (2010). Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 36, 355–361.
Chegou, NN., Black, GF., Kidd, M., van Helden, PD., and Walzl, G. (2009). Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulmon. Med. 9:21. doi: 10.1186/1471-2466-9-21
Chiacchio, T., Petruccioli, E., Vanini, V., Cuzzi, G., Pinnetti, C., Sampaolesi, A. et al. (2014). Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J. Infect. 69, 533–545.
Clarke, R., Ressom, H. W., Wang, A., Xuan, J., Liu, M. C., Gehan, E. A. et al. (2008). The properties of high-dimensional data spaces: implications for exploring gene and protein expression data. Nat. Rev. Cancer 8, 37–49.
Cliff, J. M., Cho, J. E., Lee, J. S., Ronacher, K., King, E. C., van Helden, P. et al. (2016). Excessive cytolytic responses predict tuberculosis relapse after apparently successful treatment. J. Infect. Dis. 213, 485–495. doi: 10.1093/infdis/jiv447
Colditz, G. A., Brewer, T. F., Berkey, C. S., Wilson, M. E., Burdick, E., Fineberg, H. V. et al. (1994). Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271, 698–702. doi: 10.1001/jama.271.9.698
Conesa-Botella, A., Loembe, M. M., Manabe, Y. C., Worodria, W., Mazakpwe, D., Luzinda, K. et al. (2011). Urinary lipoarabinomannan as predictor for the tuberculosis immune reconstitution inflammatory syndrome. J. Acq. Immune Defic. Syndromes 58, 463–468. doi: 10.1097/qai.0b013e31823801de
Corbett, E. L., Watt, C. J., Walker, N., Maher, D., Williams, B. G., Raviglione, M. C. et al. (2003). The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021.
da Cruz, H. L., de Albuquerque Montenegro, R., de Araujo Lima, J. F., da Rocha Poroca, D., da Costa Lima, J. F., Maria Lapa Montenegro, L. et al. (2011). Evaluation of a nested-PCR for mycobacterium tuberculosis detection in blood and urine samples. Braz. J. Microbiol. 42, 321–329. doi: 10.1590/s1517-83822011000100041
Darrah, PA., Patel, DT., De Luca, PM., Lindsay, RW., Davey, DF., Flynn, BJ. et al. (2007). Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850. doi: 10.1038/nm1592
Davies, P. D., and Pai, M. (2008). The diagnosis and misdiagnosis of tuberculosis. Int. J. Tuberculosis Lung Dis. 12, 1226–1234.
Day, C. L., Abrahams, D. A., Bunjun, R., Stone, L., de Kock, M., Walzl, G. et al. (2018). PD-1 expression on Mycobacterium tuberculosis-specific CD4 T cells is associated with bacterial load in human tuberculosis. Front. Immunol. 9:1995. doi: 10.3389/fimmu.2018.01995
Desikan, P. (2013). Sputum smear microscopy in tuberculosis: is it still relevant? Indian J. Med. Res. 137, 442–444.
Djoba Siawaya, J. F., Beyers, N., van Helden, P., and Walzl, G. (2009). Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin. Exp. Immunol. 156, 69–77. doi: 10.1111/j.1365-2249.2009.03875.x
Doublier, S., Zennaro, C., Spatola, T., Lupia, E., Bottelli, A., Deregibus, M. C. et al. (2007). HIV-1 Tat reduces nephrin in human podocytes: a potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. Aids 21, 423–432. doi: 10.1097/qad.0b013e328012c522
Drain, P. K., Gounder, L., Grobler, A., Sahid, F., Bassett, I. V., and Moosa, M. Y. (2015). Urine lipoarabinomannan to monitor antituberculosis therapy response and predict mortality in an HIV-endemic region: a prospective cohort study. BMJ Open 5:e006833. doi: 10.1136/bmjopen-2014-006833
Duffy, F. J., Weiner, J.III., Hansen, S., Tabb, D. L., Suliman, S., Thompson, E. et al. (2019). Immunometabolic signatures predict risk of progression to active tuberculosis and disease outcome. Front. Immunol. 10:527. doi: 10.3389/fimmu.2019.00527
El Daker, S., Sacchi, A., Tempestilli, M., Carducci, C., Goletti, D., Vanini, V. et al. (2015). Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PLoS One 10:e0123772. doi: 10.1371/journal.pone.0123772
Feasey, N. A., Banada, P. P., Howson, W., Sloan, D. J., Mdolo, A., Boehme, C. et al. (2013). Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J. Clin. Microbiol. 51, 2311–2316. doi: 10.1128/jcm.00330-13
Feng, T. T., Shou, C. M., Shen, L., Qian, Y., Wu, Z. G., Fan, J. et al. (2011). Novel monoclonal antibodies to ESAT-6 and CFP-10 antigens for ELISA-based diagnosis of pleural tuberculosis. Int. J. Tuberculosis Lung Disease 15, 804–810. doi: 10.5588/ijtld.10.0393
Ferrara, G., Losi, M., Fabbri, L. M., Migliori, G. B., Richeldi, L., and Casali, L. (2009). Exploring the immune response against Mycobacterium tuberculosis for a better diagnosis of the infection. Arch. Immunol. Ther. Exp. 57, 425–433. doi: 10.1007/s00005-009-0050-9
Feruglio, S. L., Troseid, M., Damas, J. K., Kvale, D., and Dyrhol-Riise, A. M. (2013). Soluble markers of the Toll-like receptor 4 pathway differentiate between active and latent tuberculosis and are associated with treatment responses. PLoS One 8:e69896. doi: 10.1371/journal.pone.0069896
Frahm, M., Goswami, N. D., Owzar, K., Hecker, E., Mosher, A., Cadogan, E. et al. (2011). Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis 91, 250–256. doi: 10.1016/j.tube.2011.02.006
Friedrich, S. O., Rachow, A., Saathoff, E., Singh, K., Mangu, C. D., Dawson, R. et al. (2013). Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir. Med. 1, 462–470. doi: 10.1016/s2213-2600(13)70119-x
Fu, Y., Wang, J., Qiao, J., and Yi, Z. (2019). Signature of circular RNAs in peripheral blood mononuclear cells from patients with active tuberculosis. J. Cell. Mol. Med. 23, 1917–1925. doi: 10.1111/jcmm.14093
Gaifer, Z. (2017). Epidemiology of extrapulmonary and disseminated tuberculosis in a tertiary care center in Oman. Int. J. Mycobacteriol. 6, 162–166.
Gesthalter, Y. B., Vick, J., Steiling, K., and Spira, A. (2015). Translating the transcriptome into tools for the early detection and prevention of lung cancer. Thorax 70, 476–481. doi: 10.1136/thoraxjnl-2014-206605
Getahun, H., Gunneberg, C., Granich, R., and Nunn, P. (2010). HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 50(Suppl 3), S201–S207.
Goletti, D., Butera, O., Bizzoni, F., Casetti, R., Girardi, E., and Poccia, F. (2006). Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J. Infect. Dis. 194, 984–992. doi: 10.1086/507427
Goletti, D., Carrara, S., Mayanja-Kizza, H., Baseke, J., Mugerwa, M. A., Girardi, E. et al. (2008). Response to M. tuberculosis selected RD1 peptides in Ugandan HIV-infected patients with smear positive pulmonary tuberculosis: a pilot study. BMC Infect. Dis. 8:11. doi: 10.1186/1471-2334-8-11
Goletti, D., Lee, M. R., Wang, J. Y., Walter, N., and Ottenhoff, T. H. M. (2018). Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology 23, 455–466. doi: 10.1111/resp.13272
Goletti, D., Petruccioli, E., Joosten, S. A., and Ottenhoff, T. H. (2016). Tuberculosis biomarkers: from diagnosis to protection. Infect. Dis. Rep. 8:6568.
Goletti, D., Raja, A., Ahamed Kabeer, B. S., Rodrigues, C., Sodha, A., Butera, O. et al. (2010a). IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected peptides associates to active tuberculosis. J. Infect. 61, 133–143. doi: 10.1016/j.jinf.2010.05.002
Goletti, D., Raja, A., Syed Ahamed Kabeer, B., Rodrigues, C., Sodha, A., Carrara, S. et al. (2010b). Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS One 5:e12577. doi: 10.1371/journal.pone.0012577
Gomes, T., Reis-Santos, B., Bertolde, A., Johnson, J. L., Riley, L. W., and Maciel, E. L. (2014). Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect. Dis. 14:9. doi: 10.1186/1471-2334-14-9
Gupta-Wright, A., Peters, J. A., Flach, C., and Lawn, S. D. (2016). Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med. 14:53. doi: 10.1186/s12916-016-0603-9
Hamasur, B., Bruchfeld, J., van Helden, P., Kallenius, G., and Svenson, S. (2015). A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS One 10:e0123457. doi: 10.1371/journal.pone.0123457
Harari, A., Rozot, V., Bellutti Enders, F., Perreau, M., Stalder, J. M., Nicod, L. P. et al. (2011). Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat. Med. 17, 372–376. doi: 10.1038/nm.2299
Heidecker, B., Kasper, E. K., Wittstein, I. S., Champion, H. C., Breton, E., Russell, S. D. et al. (2008). Transcriptomic biomarkers for individual risk assessment in new-onset heart failure. Circulation 118, 238–246. doi: 10.1161/circulationaha.107.756544
Helmy, N., Abdel, SL., Kamela, M. M., Ashoura, W., and El Kattan, E. (2012). Role of Quantiferon TB gold assays in monitoring the efficacy of antituberculosis therapy. Egypt. J. Chest Dis. Tuberculosis 61, 329–336. doi: 10.1016/j.ejcdt.2012.09.011
Hoel, I. M., Jorstad, M. D., Marijani, M., Ruhwald, M., Mustafa, T., and Dyrhol-Riise, A. M. (2019). IP-10 dried blood spots assay monitoring treatment efficacy in extrapulmonary tuberculosis in a low-resource setting. Sci. Rep. 9:3871.
Hormi, M., Guerin-El Khourouj, V., Pommelet, V., Jeljeli, M., Pedron, B., Diana, J. S., et al. (2018). Performance of the QuantiFERON-TB gold assay among HIV-infected children with active tuberculosis in France. Pediatr. Infect. Dis. J. 37, 339–344. doi: 10.1097/inf.0000000000001774
Janssens, J. P. (2007). Interferon-gamma release assay tests to rule out active tuberculosis. Eur. Respir. J. 30, 183–184; author reply 4–5.
Jong, V. L., Ahout, I. M., van den Ham, H. J., Jans, J., Zaaraoui-Boutahar, F., Zomer, A., et al. (2016). Transcriptome assists prognosis of disease severity in respiratory syncytial virus infected infants. Sci. Rep. 6:36603.
Joosten, S. A., Goeman, J. J., Sutherland, J. S., Opmeer, L., de Boer, K. G., Jacobsen, M., et al. (2012). Identification of biomarkers for tuberculosis disease using a novel dual-color RT-MLPA assay. Genes Immun. 13, 71–82.
Kabeer, B. S., Raja, A., Raman, B., Thangaraj, S., Leportier, M., Ippolito, G., et al. (2011). IP-10 response to RD1 antigens might be a useful biomarker for monitoring tuberculosis therapy. BMC Infect. Dis. 11:135. doi: 10.1186/1471-2334-11-135
Kaforou, M., Wright, V. J., Oni, T., French, N., Anderson, S. T., Bangani, N., et al. (2013). Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 10:e1001538. doi: 10.1371/journal.pmed.1001538
Kalra, M., Khuller, G. K., Grover, A., Behera, D., Wanchu, A., and Verma, I. (2010). Utility of a combination of RD1 and RD2 antigens as a diagnostic marker for tuberculosis. Diagn. Microbiol. Infect. Dis. 66, 153–161. doi: 10.1016/j.diagmicrobio.2009.09.005
Kaneko, Y., Nakayama, K., Kinoshita, A., Kurita, Y., Odashima, K., Saito, Z., et al. (2015). Relation between recurrence of tuberculosis and transitional changes in IFN-gamma release assays. Am. J. Respir. Crit. Care Med. 191, 480–483. doi: 10.1164/rccm.201409-1590le
Kashyap, R. S., Dobos, K. M., Belisle, J. T., Purohit, H. J., Chandak, N. H., Taori, G. M., et al. (2005). Demonstration of components of antigen 85 complex in cerebrospinal fluid of tuberculous meningitis patients. Clin. Diagn. Lab. Immunol. 12, 752–758. doi: 10.1128/cdli.12.6.752-758.2005
Kashyap, R. S., Rajan, A. N., Ramteke, S. S., Agrawal, V. S., Kelkar, S. S., Purohit, H. J., et al. (2007). Diagnosis of tuberculosis in an Indian population by an indirect ELISA protocol based on detection of Antigen 85 complex: a prospective cohort study. BMC Infect. Dis. 7:74. doi: 10.1186/1471-2334-7-74
Kell, D. B., and Oliver, S. G. (2004). Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. BioEssays 26, 99–105. doi: 10.1002/bies.10385
Kohonen, P., Parkkinen, J. A., Willighagen, E. L., Ceder, R., Wennerberg, K., Kaski, S., et al. (2017). A transcriptomics data-driven gene space accurately predicts liver cytopathology and drug-induced liver injury. Nat. Commun. 8:15932.
Kumar, V. G., Urs, T. A., and Ranganath, R. R. (2011). MPT 64 antigen detection for rapid confirmation of M.tuberculosis isolates. BMC Res. Notes 4:79. doi: 10.1186/1756-0500-4-79
Kwak, N., Choi, S. M., Lee, J., Park, Y. S., Lee, C. H., Lee, S. M., et al. (2013). Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One 8:e77456. doi: 10.1371/journal.pone.0077456
La Manna, M. P., Orlando, V., Li Donni, P., Sireci, G., Di Carlo, P., Cascio, A., et al. (2018). Identification of plasma biomarkers for discrimination between tuberculosis infection/disease and pulmonary non tuberculosis disease. PLoS One 13:e0192664. doi: 10.1371/journal.pone.0192664
Lagrange, P. H., Thangaraj, S. K., Dayal, R., Deshpande, A., Ganguly, N. K., Girardi, E., et al. (2014). A toolbox for tuberculosis (TB) diagnosis: an Indian multi-centric study (2006-2008); evaluation of serological assays based on PGL-Tb1 and ESAT-6/CFP10 antigens for TB diagnosis. PLoS One 9:e96367. doi: 10.1371/journal.pone.0096367
Lalvani, A., Nagvenkar, P., Udwadia, Z., Pathan, A. A., Wilkinson, K. A., Shastri, J. S., et al. (2001a). Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183, 469–477. doi: 10.1086/318081
Lalvani, A., Pathan, A. A., Durkan, H., Wilkinson, K. A., Whelan, A., Deeks, J. J., et al. (2001b). Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357, 2017–2021. doi: 10.1016/s0140-6736(00)05115-1
Lee, S. W., Wu, L. S., Huang, G. M., Huang, K. Y., Lee, T. Y., and Weng, J. T. (2016). Gene expression profiling identifies candidate biomarkers for active and latent tuberculosis. BMC Bioinform. 17(Suppl 1):3. doi: 10.1186/s12859-015-0848-x
Lesho, E., Forestiero, F. J., Hirata, M. H., Hirata, R. D., Cecon, L., Melo, F. F., et al. (2011). Transcriptional responses of host peripheral blood cells to tuberculosis infection. Tuberculosis 91, 390–399. doi: 10.1016/j.tube.2011.07.002
Leu, J. S., Chang, S. Y., Mu, C. Y., Chen, M. L., and Yan, B.S. (2018). Functional domains of SP110 that modulate its transcriptional regulatory function and cellular translocation. J. Biomed. Sci. 25:34.
Li, Z., Pan, L., Lyu, L., Li, J., Jia, H., Du, B., et al. (2019). Diagnostic accuracy of droplet digital PCR analysis of cerebrospinal fluid for tuberculous meningitis in adult patients. Clin. Microbiol. Infect. doi: 10.1016/j.cmi.2019.07.015 [Epub ahead of print].
Lighter, J., Rigaud, M., Huie, M., Peng, C. H., and Pollack, H. (2009). Chemokine IP-10: an adjunct marker for latent tuberculosis infection in children. Int. J. Tuberculosis Lung Dis. 13, 731–736.
Liu, Q., Pan, L., Han, F., Luo, B., Jia, H., Xing, A., et al. (2018). Proteomic profiling for plasma biomarkers of tuberculosis progression. Mol. Med. Rep. 18, 1551–1559.
Lopez-Ramos, J. E., Macias-Segura, N., Cuevas-Cordoba, B., Araujo-Garcia, Z., Bastian, Y., Castaneda-Delgado, J. E., et al. (2018). Improvement in the diagnosis of tuberculosis combining Mycobacterium tuberculosis immunodominant peptides and serum host biomarkers. Arch. Med. Res. 49, 147.e1–53 e1.
Losi, M., Bossink, A., Codecasa, L., Jafari, C., Ernst, M., Thijsen, S., et al. (2007). Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur. Respir. J. 30, 1173–1179. doi: 10.1183/09031936.00067307
Lowe, R., Shirley, N., Bleackley, M., Dolan, S., and Shafee, T. (2017). Transcriptomics technologies. PLoS Comput. Biol. 13:e1005457. doi: 10.1371/journal.pcbi.1005457
Lu, L. L., Smith, M. T., Yu, K. K. Q., Luedemann, C., Suscovich, T. J., Grace, P. S., et al. (2019). IFN-gamma-independent immune markers of Mycobacterium tuberculosis exposure. Nat. Med. 25, 977–987.
Luo, J., Zhang, M., Yan, B., Li, F., Guan, S., Chang, K., et al. (2019). Diagnostic performance of plasma cytokine biosignature combination and MCP-1 as individual biomarkers for differentiating stages Mycobacterium tuberculosis infection. J. Infection. 78, 281–291. doi: 10.1016/j.jinf.2018.10.017
Luo, L., Zhu, L., Yue, J., Liu, J., Liu, G., Zhang, X., et al. (2017). Antigens Rv0310c and Rv1255c are promising novel biomarkers for the diagnosis of Mycobacterium tuberculosis infection. Emerg. Microbes Infect. 6:e64.
Lyu, L., Zhang, X., Li, C., Yang, T., Wang, J., Pan, L., et al. (2019). Small RNA profiles of serum exosomes derived from individuals with latent and active tuberculosis. Front. Microbiol. 10:1174. doi: 10.3389/fmicb.2019.01174
Maekura, R., Kitada, S., Osada-Oka, M., Tateishi, Y., Ozeki, Y., Fujicawa, T., et al. (2019). Serum antibody profiles in individuals with latent Mycobacterium tuberculosis infection. Microbiol. Immunol. 63, 130–138. doi: 10.1111/1348-0421.12674
Maertzdorf, J., Kaufmann, S. H., and Weiner, J. III (2014). Toward a unified biosignature for tuberculosis. Cold Spring Harbor Perspect. Med. 5:a018531.
Maertzdorf, J., McEwen, G., Weiner, J. III, Tian, S., Lader, E., Schriek, U., et al. (2016). Concise gene signature for point-of-care classification of tuberculosis. EMBO Mol. Med. 8, 86–95. doi: 10.15252/emmm.201505790
Maertzdorf, J., Ota, M., Repsilber, D., Mollenkopf, H. J., Weiner, J., Hill, P. C., et al. (2011a). Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS One 6:e26938. doi: 10.1371/journal.pone.0026938
Maertzdorf, J., Repsilber, D., Parida, S. K., Stanley, K., Roberts, T., Black, G., et al. (2011b). Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 12, 15–22. doi: 10.1038/gene.2010.51
Marlowe, E. M., Novak-Weekley, S. M., Cumpio, J., Sharp, S. E., Momeny, M. A., Babst, A., et al. (2011). Evaluation of the cepheid xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J. Clin. Microbiol. 49, 1621–1623. doi: 10.1128/jcm.02214-10
Martin, A., Bombeeck, D., Mulders, W., Fissette, K., De Rijk, P., and Palomino, J. C. (2011). Evaluation of the TB Ag MPT64 Rapid test for the identification of Mycobacterium tuberculosis complex. Int. J. Tubercu. Lung Dis. 15, 703–705.
Maynard-Smith, L., Larke, N., Peters, J. A., and Lawn, S. D. (2014). Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect. Dis. 14:709. doi: 10.1186/s12879-014-0709-7
Mendelson, M. (2007). Diagnosing tuberculosis in HIV-infected patients: challenges and future prospects. Br. Med. Bull. 81-82, 149–165. doi: 10.1093/bmb/ldm009
Mihret, A., Bekele, Y., Bobosha, K., Kidd, M., Aseffa, A., Howe, R., et al. (2013). Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J. Infect. 66, 357–365. doi: 10.1016/j.jinf.2012.11.005
Millington, K. A., Gooding, S., Hinks, T. S., Reynolds, D. J., and Lalvani, A. (2010). Mycobacterium tuberculosis-specific cellular immune profiles suggest bacillary persistence decades after spontaneous cure in untreated tuberculosis. J. Infect. Dis. 202, 1685–1689. doi: 10.1086/656772
Millington, K. A., Innes, J. A., Hackforth, S., Hinks, T. S., Deeks, J. J., Dosanjh, D. P., et al. (2007). Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178, 5217–5226. doi: 10.4049/jimmunol.178.8.5217
Minion, J, Leung, E, Talbot, E, Dheda, K, Pai, M, and Menzies, D. (2011). Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur. Respir. J. 38, 1398–1405. doi: 10.1183/09031936.00025711
Miotto, P., Mwangoka, G., Valente, I. C., Norbis, L., Sotgiu, G., Bosu, R., et al. (2013). miRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS One 8:e80149. doi: 10.1371/journal.pone.0080149
Mistry, R., Cliff, J. M., Clayton, C. L., Beyers, N., Mohamed, Y. S., Wilson, P. A., et al. (2007). Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J. Infect. Dis. 195, 357–365. doi: 10.1086/510397
Mori, T., Sakatani, M., Yamagishi, F., Takashima, T., Kawabe, Y., Nagao, K., et al. (2004). Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170, 59–64. doi: 10.1164/rccm.200402-179oc
Moro, M. L., Nascetti, S., Morsillo, F., and Morandi, M. Italian TB-SORV Project Working Group, (2010). Laboratory procedures for the diagnosis of tuberculosis: a survey in ten Italian Regions. Annali dell’Istituto superiore di sanita. 46, 178–184.
Nahid, P., Bliven-Sizemore, E., Jarlsberg, L. G., De Groote, M. A., Johnson, J. L., Muzanyi, G., et al. (2014). Aptamer-based proteomic signature of intensive phase treatment response in pulmonary tuberculosis. Tuberculosis 94, 187–196. doi: 10.1016/j.tube.2014.01.006
Nahid, P., Pai, M., and Hopewell, P. C. (2006). Advances in the diagnosis and treatment of tuberculosis. Proc. Am. Thoracic Soc. 3, 103–110.
Nalejska, E., Maczynska, E., and Lewandowska, M. A. (2014). Prognostic and predictive biomarkers: tools in personalized oncology. Mol. Diagn. Ther. 18, 273–284. doi: 10.1007/s40291-013-0077-9
Nikitina, I. Y., Kondratuk, N. A., Kosmiadi, G. A., Amansahedov, R. B., Vasilyeva, I. A., Ganusov, V. V., et al. (2012). Mtb-specific CD27low CD4 T cells as markers of lung tissue destruction during pulmonary tuberculosis in humans. PLoS One 7:e43733. doi: 10.1371/journal.pone.0043733
Nikolova, M., Markova, R., Drenska, R., Muhtarova, M., Todorova, Y., Dimitrov, V., et al. (2013). Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn. Microbiol. Infect. Dis. 75, 277–281. doi: 10.1016/j.diagmicrobio.2012.11.023
Novel, N. C., Lani, T., Elisabetta, W., Anna, M. M., Anneke, C. H., Gerhard, W., et al. (2013). Utility of host markers detected in quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS One 8:e64226. doi: 10.1371/journal.pone.0064226
Ottenhoff, T. H. (2009). Overcoming the global crisis: “yes, we can”, but also for TB. ? Eur. J. Immunol. 39, 2014–2020. doi: 10.1002/eji.200939518
Ottenhoff, T. H., Ellner, J. J., and Kaufmann, S. H. (2012). Ten challenges for TB biomarkers. Tuberculosis 92(Suppl 1), S17–S20.
Pai, M., Zwerling, A., and Menzies, D. (2008). Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Int. Med. 149, 177–184.
Parlato, S., Chiacchio, T., Salerno, D., Petrone, L., Castiello, L., Romagnoli, G., et al. (2018). Impaired IFN-alpha-mediated signal in dendritic cells differentiates active from latent tuberculosis. PLoS One 13:e0189477. doi: 10.1371/journal.pone.0189477
Peter, J., Green, C., Hoelscher, M., Mwaba, P., Zumla, A., and Dheda, K. (2010). Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr. Opin. Pulm. Med. 16, 262–270. doi: 10.1097/mcp.0b013e328337f23a
Petrone, L., Cannas, A., Aloi, F., Nsubuga, M., Sserumkuma, J., Nazziwa, R. A., et al. (2015). Blood or urine IP-10 cannot discriminate between active tuberculosis and respiratory diseases different from tuberculosis in children. BioMed Res. Int. 2015, 589471.
Petruccioli, E., Petrone, L., Vanini, V., Cuzzi, G., Navarra, A., Gualano, G., et al. (2015). Assessment of CD27 expression as a tool for active and latent tuberculosis diagnosis. J. Infect. 71, 526–533. doi: 10.1016/j.jinf.2015.07.009
Petruccioli, E., Petrone, L., Vanini, V., Sampaolesi, A., Gualano, G., Girardi, E., et al. (2013). IFNgamma/TNFalpha specific-cells and effector memory phenotype associate with active tuberculosis. J. Infect. 66, 475–486. doi: 10.1016/j.jinf.2013.02.004
Pollock, K. M., Whitworth, H. S., Montamat-Sicotte, D. J., Grass, L., Cooke, G. S., Kapembwa, M. S., et al. (2013). T-cell immunophenotyping distinguishes active from latent tuberculosis. J. Infect. Dis. 208, 952–968. doi: 10.1093/infdis/jit265
Portevin, D., Moukambi, F., Clowes, P., Bauer, A., Chachage, M., Ntinginya, N. E., et al. (2014). Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect. Dis. 14, 931–938. doi: 10.1016/s1473-3099(14)70884-9
Rakshit, S., Adiga, V., Nayak, S., Sahoo, P. N., Sharma, P. K., van Meijgaarden, K. E., et al. (2017). Circulating Mycobacterium tuberculosis DosR latency antigen-specific, polyfunctional, regulatory IL10(+) Th17 CD4 T-cells differentiate latent from active tuberculosis. Sci. Rep. 7:11948.
Rangaka, M. X., Wilkinson, K. A., Seldon, R., Van Cutsem, G., Meintjes, G. A., Morroni, C., et al. (2007). Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am. J. Respir. Crit. Care Med. 175, 514–520. doi: 10.1164/rccm.200610-1439oc
Ribeiro, S., Dooley, K., Hackman, J., Loredo, C., Efron, A., Chaisson, R. E., et al. (2009). T-SPOT. TB responses during treatment of pulmonary tuberculosis. BMC Infect. Dis. 9:23. doi: 10.1186/1471-2334-9-23
Riou, C., Perez Peixoto, B., Roberts, L., Ronacher, K., Walzl, G., Manca, C., et al. (2012). Effect of standard tuberculosis treatment on plasma cytokine levels in patients with active pulmonary tuberculosis. PLoS One 7:e36886. doi: 10.1371/journal.pone.0036886
Rovina, N., Panagiotou, M., Pontikis, K., Kyriakopoulou, M., Koulouris, N. G., and Koutsoukou, A. (2013). Immune response to mycobacterial infection: lessons from flow cytometry. Clin. Dev. Immunol. 2013, 464039.
Ruhwald, M., Petersen, J., Kofoed, K., Nakaoka, H., Cuevas, L. E., Lawson, L., et al. (2008). Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One 3:e2858. doi: 10.1371/journal.pone.0002858
Sai Priya, V. H., Latha, G. S., Hasnain, S. E., Murthy, K. J., and Valluri, V. L. (2010). Enhanced T cell responsiveness to Mycobacterium bovis BCG r32-kDa Ag correlates with successful anti-tuberculosis treatment in humans. Cytokine 52, 190–193. doi: 10.1016/j.cyto.2010.07.001
Selwyn, P. A., Hartel, D., Lewis, V. A., Schoenbaum, E. E., Vermund, S. H., Klein, R. S., et al. (1989). A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Eng. J. Med. 320, 545–550. doi: 10.1056/nejm198903023200901
Sester, U., Fousse, M., Dirks, J., Mack, U., Prasse, A., Singh, M., et al. (2011). Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS One 6:e17813. doi: 10.1371/journal.pone.0017813
Shah, M., Martinson, N. A., Chaisson, R. E., Martin, D. J., Variava, E., and Dorman, S. E. (2010). Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J. Clin. Microbiol. 48, 2972–2974. doi: 10.1128/jcm.00363-10
Sharma, S. K., Vashishtha, R., Chauhan, L. S., Sreenivas, V., and Seth, D. (2017). Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLoS One 12:e0169539. doi: 10.1371/journal.pone.0169539
Shen, G. H., Chiou, C. S., Hu, S. T., Wu, K. M., and Chen, J. H. (2011). Rapid identification of the Mycobacterium tuberculosis complex by combining the ESAT-6/CFP-10 immunochromatographic assay and smear morphology. J. Clin. Microbiol. 49, 902–907. doi: 10.1128/jcm.00592-10
Steingart, K. R., Dendukuri, N., Henry, M., Schiller, I., Nahid, P., Hopewell, P. C., et al. (2009). Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16, 260–276. doi: 10.1128/cvi.00355-08
Steingart, K. R., Flores, L. L., Dendukuri, N., Schiller, I., Laal, S., Ramsay, A., et al. (2011). Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 8:e1001062. doi: 10.1371/journal.pmed.1001062
Steingart, K. R., Henry, M., Laal, S., Hopewell, P. C., Ramsay, A., Menzies, D., et al. (2007). Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. doi: 10.1371/journal.pmed.0040202
Streitz, M., Tesfa, L., Yildirim, V., Yahyazadeh, A., Ulrichs, T., Lenkei, R., et al. (2007). Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS One. 2:e735. doi: 10.1371/journal.pone.0000735
Sutherland, J. S., Adetifa, I. M., Hill, P. C., Adegbola, R. A., and Ota, M. O. (2009). Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 39, 723–729. doi: 10.1002/eji.200838693
Sutherland, J. S., Loxton, A. G., Haks, M. C., Kassa, D., Ambrose, L., Lee, J. S., et al. (2014). Differential gene expression of activating Fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin. Microbiol. Infect. 20:O230–O238.
Theron, G., Peter, J., Calligaro, G., Meldau, R., Hanrahan, C., Khalfey, H., et al. (2014). Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci. Rep. 4:5658.
Thomas, M. M., Hinks, T. S., Raghuraman, S., Ramalingam, N., Ernst, M., Nau, R., et al. (2008). Rapid diagnosis of Mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int. J. Tuberc. Lung Dis. 12, 651–657.
Tiberi, S., du Plessis, N., Walzl, G., Vjecha, M. J., Rao, M., Ntoumi, F., et al. (2018). Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 18:e183–e98.
Turbawaty, D. K., Sugianli, A. K., Soeroto, A. Y., Setiabudiawan, B., and Parwati, I. (2017). Comparison of the performance of urinary Mycobacterium tuberculosis antigens cocktail (ESAT6, CFP10, and MPT64) with culture and microscopy in pulmonary tuberculosis patients. Int. J. Microbiol. 2017:3259329.
Verma, R. K., and Jain, A. (2007). Antibodies to mycobacterial antigens for diagnosis of tuberculosis. FEMS Immunol. Med. Microbiol. 51, 453–461.
Wallis, R. S., Pai, M., Menzies, D., Doherty, T. M., Walzl, G., and Perkins, M. D, et al. (2010). Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375, 1920–1937. doi: 10.1016/s0140-6736(10)60359-5
Walter, N. D., Dolganov, G. M., Garcia, B. J., Worodria, W., Andama, A., Musisi, E., et al. (2015). Transcriptional adaptation of drug-tolerant Mycobacterium tuberculosis during treatment of human tuberculosis. J. Infect. Dis. 212, 990–998.
Walzl, G., McNerney, R., du Plessis, N., Bates, M., McHugh, T. D., Chegou, N. N., et al. (2018). Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect. Dis. 18, e199–e210. doi: 10.1016/s1473-3099(18)30111-7
Walzl, G., Ronacher, K., Hanekom, W., Scriba, T. J., and Zumla, A. (2011). Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11, 343–354. doi: 10.1038/nri2960
Wang, X., Cao, Z., Jiang, J., Niu, H., Dong, M., Tong, A., et al. (2010). Association of mycobacterial antigen-specific CD4(+) memory T cell subsets with outcome of pulmonary tuberculosis. J. Infect. 60, 133–139. doi: 10.1016/j.jinf.2009.10.048
Weiner, J. III., Parida, S. K., Maertzdorf, J., Black, G. F., Repsilber, D., Telaar, A., et al. (2012). Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS One 7:e40221. doi: 10.1371/journal.pone.0040221
Weiner, J., and Kaufmann, S. H. (2017). High-throughput and computational approaches for diagnostic and prognostic host tuberculosis biomarkers. Int. J. Infect. Dis. 56, 258–262. doi: 10.1016/j.ijid.2016.10.017
Whittaker, E., Gordon, A., and Kampmann, B. (2008). Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One 3:e3901. doi: 10.1371/journal.pone.0003901
Won, E. J., Choi, J. H., Cho, Y. N., Jin, H. M., Kee, H. J., and Park, Y. W, et al. (2017). Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J. Infect. 74, 281–293. doi: 10.1016/j.jinf.2016.11.010
World Health Organization [WHO], (2002). WHO Report Global Tuberculosis Control: Surveillence, Planning, Financing. Geneva: WHO.
World Health Organization [WHO], (2004). Toman’s Tuberculosis. Case Detection, Treatment, and Monitoring. Geneva: WHO
World Health Organization [WHO], (2013). Systemetic Screening for Active Tuberculosis: Principle and Recommendation. Geneva: WHO.
World Health Organization [WHO], (2014). Meeting Report. High-Priority Target Product profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Geneva: WHO.
World Health Organization [WHO], (2016). WHO Treatment Guidelines for Drugresistant Tuberculosis. Geneva: WHO.
Wu, J., Wang, S., Lu, C., Shao, L., Gao, Y., Zhou, Z., et al. (2017). Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis 102, 68–75. doi: 10.1016/j.tube.2016.06.001
Yan, Z. H., Zheng, X. F., Yi, L., Wang, J., Wang, X. J., Wei, P. J., et al. (2017). CD137 is a useful marker for identifying CD4(+) T Cell responses to Mycobacterium tuberculosis. Scand. J. Immunol. 85, 372–380. doi: 10.1111/sji.12541
Yao, X., Liu, Y., Liu, Y., Liu, W., Ye, Z., Zheng, C., et al. (2017). Multiplex analysis of plasma cytokines/chemokines showing different immune responses in active TB patients, latent TB infection and healthy participants. Tuberculosis 107, 88–94. doi: 10.1016/j.tube.2017.07.013
Yong, Y. K., Tan, H. Y., Saeidi, A., Rosmawati, M., Atiya, N., Ansari, A. W., et al. (2017). Decrease of CD69 levels on TCR Valpha7.2(+)CD4(+) innate-like lymphocytes is associated with impaired cytotoxic functions in chronic hepatitis B virus-infected patients. Innate Immun. 23, 459–467. doi: 10.1177/1753425917714854
Zeka, A. N., Tasbakan, S., and Cavusoglu, C. (2011). Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J. Clin. Microbiol. 49, 4138–4141. doi: 10.1128/jcm.05434-11
Zhang, C., Song, X., Zhao, Y., Zhang, H., Zhao, S., Mao, F., et al. (2015). Mycobacterium tuberculosis secreted proteins as potential biomarkers for the diagnosis of active tuberculosis and latent tuberculosis infection. J. Clin. Lab. Anal. 29, 375–382. doi: 10.1002/jcla.21782
Keywords: biomarkers, diagnostics, treatment monitoring, tuberculosis, HIV
Citation: Yong YK, Tan HY, Saeidi A, Wong WF, Vignesh R, Velu V, Eri R, Larsson M and Shankar EM (2019) Immune Biomarkers for Diagnosis and Treatment Monitoring of Tuberculosis: Current Developments and Future Prospects. Front. Microbiol. 10:2789. doi: 10.3389/fmicb.2019.02789
Received: 06 June 2019; Accepted: 18 November 2019;
Published: 18 December 2019.
Edited by:
Aleksandra Barac, University of Belgrade, SerbiaReviewed by:
Joel Fleury Djoba Siawaya, Centre Hospitalier Universitaire Mère-enfant Fondation Jeanne EBORI, GabonMatthieu Perreau, Lausanne University Hospital (CHUV), Switzerland
Cecilia Lindestam Arlehamn, La Jolla Institute for Immunology (LJI), United States
Copyright © 2019 Yong, Tan, Saeidi, Wong, Vignesh, Velu, Eri, Larsson and Shankar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esaki M. Shankar, shankarem@cutn.ac.in
 Yean K. Yong
Yean K. Yong Hong Y. Tan1,2
Hong Y. Tan1,2 Won F. Wong
Won F. Wong Vijayakumar Velu
Vijayakumar Velu Marie Larsson
Marie Larsson Esaki M. Shankar
Esaki M. Shankar