Biologic Treatments in Interstitial Lung Diseases
- 15th Department of Pneumonology, General Hospital for Thoracic Diseases Sotiria, Athens, Greece
- 2First Academic Department of Pneumonology, Hospital for Thoracic Diseases, Sotiria Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 3Division of Rheumatology, Department of Internal Medicine, Patras University Hospital, University of Patras Medical School, Patras, Greece
Interstitial lung diseases (ILD) represent a group of heterogeneous parenchymal lung disorders with complex pathophysiology, characterized by different clinical and radiological patterns, ultimately leading to pulmonary fibrosis. A considerable proportion of these disease entities present with no effective treatment, as current therapeutic regimens only slow down disease progression, thus leaving patients, at best case, with considerable functional disability. Biologic therapies have emerged and are being investigated in patients with different forms of ILD. Unfortunately, their safety profile has raised many concerns, as evidence shows that they might cause or exacerbate ILD status in a subgroup of patients. This review article aims to summarize the current state of knowledge on their role in patients with ILD and highlight future perspectives.
Introduction
Interstitial lung diseases (ILD) are a group of heterogeneous parenchymal lung disorders, characterized by different clinical and radiological patterns (1, 2). Despite an exponential increase in our knowledge and the advent of novel therapies, treatment remains ineffective for a considerable proportion of patients (3–13). Biologic treatments comprise a wide group of compounds with natural origin produced by biotechnology and other cutting-edge technologies (14); yet, this term mainly refers to the subgroup of complex molecules representing targeted therapy, such as monoclonal antibodies and receptor fusion proteins (15). The last years have seen the emergence of biologic treatments for the treatment of several immune and oncologic disorders (16–18). The most extensively used are tumor necrosis factor-α (TNF-a) inhibitors, B-cell-targeted therapies, T cell co-stimulatory molecule blockers, and immune check point inhibitors. With regards to ILDs, there is established knowledge on the use of biologic therapies in patients with connective tissue disorders (CTD-ILDs) and sarcoidosis (12, 16, 19–21). Despite old skepticism (7, 22–27), there has been recently a shift toward targeting the immune system as a therapeutic option for different forms of interstitial lung inflammation and fibrosis (9, 28–33). Unfortunately, their safety profile has raised many concerns, as evidence shows that they might exacerbate or cause de novo development of ILD in a subgroup of patients (34–36) (Table 1). This review article aims to summarize the current state of knowledge on their role in patients with ILD and highlight future perspectives.
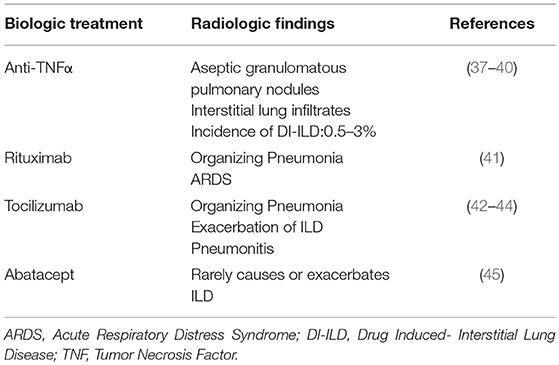
Sarcoidosis (Table 2)
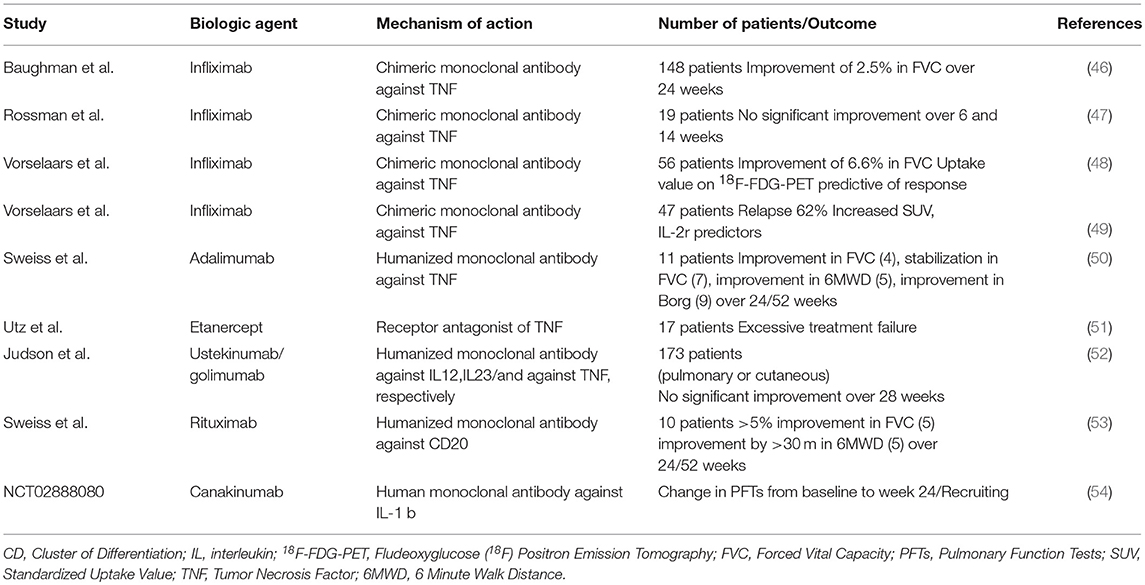
Prednisolone remains the cornerstone of sarcoidosis treatment (55). Biologic therapies currently represent a fruitful therapeutic alternative in sarcoidosis cases refractory to first line immunomodulatory agents including corticosteroids, methotrexate, azathioprine, leflunomide and mycophenolate mofetil (56). TNFα inhibitors in combination with low dose prednisolone or methotrexate have been suggested in: (i) chronic progressive pulmonary disease, (ii) debilitation by lupus pernio, (iii) persistent neurosarcoidosis, (iv) persistent cardiac sarcoidosis (55). Infliximab has shown superior response rates in pulmonary sarcoidosis compared to etanercept and adalimumab (46, 47, 50, 51, 57). In particular, a randomized controlled trial (RCT) enrolling 148 patients with chronic pulmonary sarcoidosis showed that infliximab led to a statistically significant 2.5% improvement in forced vital capacity (FVC%pred) after 24 weeks of treatment (46). Results from other non-randomized trials were rather conflicting (47, 48). Unfortunately, almost 2/3 of patients with sarcoidosis receiving infliximab demonstrated relapse following drug-cessation (49). Adalimumab has shown acceptable tolerability and efficacy profile as indicated by improvements in FVC% pred, 6 Minute-Walk-Distance (6MWD) and Borg scale over a period of 52 weeks in a small cohort of patients with refractory pulmonary sarcoidosis (50). A phase 2 trial of etanercept in patients with pulmonary sarcoidosis was prematurely terminated due to unfavorable outcomes (51). Furthermore, golimumab (TNFα inhibitor) and ustekinumab (a monoclonal antibody targeting both IL-12 and IL-23) failed to show efficacy in patients with pulmonary and/or cutaneous sarcoidosis in an RCT with 173 patients (52). Finally, rituximab had an acceptable safety profile but inconsistent efficacy in a small cohort of patients with different genetic backgrounds and refractory pulmonary sarcoidosis; thus, its use through a personalized medicine approach could be viable in the future (53).
Elevated C-reactive protein (CRP) levels and TNFα Gly308Ala polymorphisms have been found to be predictive of response to anti-TNFα therapy, while soluble IL-2 receptor serum levels ≥4,000 pg·mL−1 at start of therapy were predictive of relapse (49, 58). Moreover, 188F-FDG-PET showed remarkable predictive accuracy in identifying patients that responded or relapsed following infliximab treatment (48, 49).
A broad spectrum of adverse events have been associated with the use of TNF-α inhibitors including anaphylactic reactions, reactivation of latent infections, neurological (i.e., demyelinating diseases) and autoimmune disorders and maybe in some cases malignancy (55, 59, 60). The paradoxical response, denominated sarcoid-like granulomatosis, has also been reported (61).
In conclusion, current evidence based on expert opinion suggests the use of biologic treatments in severe refractory pulmonary sarcoidosis. TNFα-inhibitors are preferred for patients with persistent disease despite treatment with corticosteroids and other second-line immunomodulatory compounds, especially in cases of life-threatening disease. However, such strategies need thorough pre-treatment evaluation and multidisciplinary approaches (12).
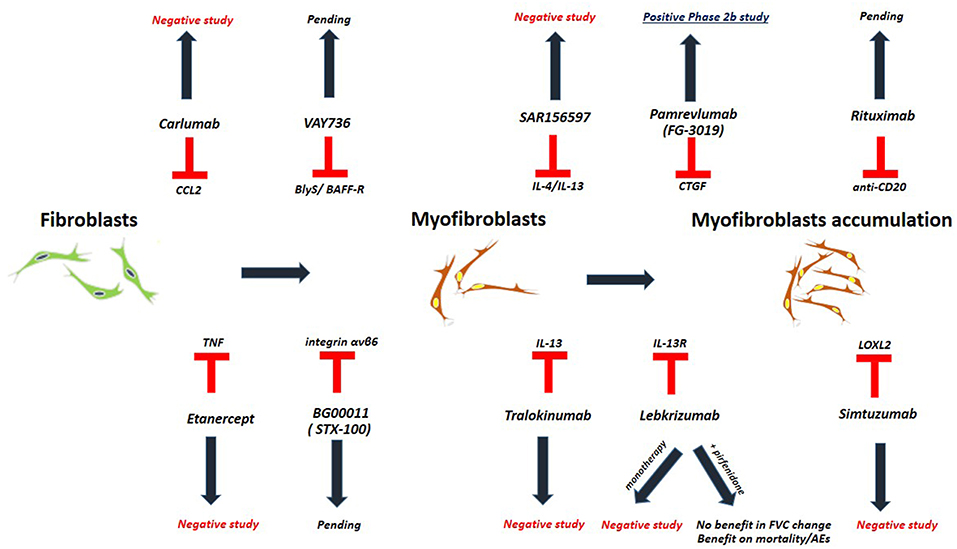
Idiopathic Pulmonary Fibrosis (Figure 1, Table 3)
The treatment of IPF has been revolutionized by the advent of two novel compounds, pirfenidone and nintedanib (3–11). Nevertheless, both compounds only slow down disease progression; thus, at best leave patients with considerable functional disability. Therefore, the need for alternative therapeutic options remains amenable (75–78).
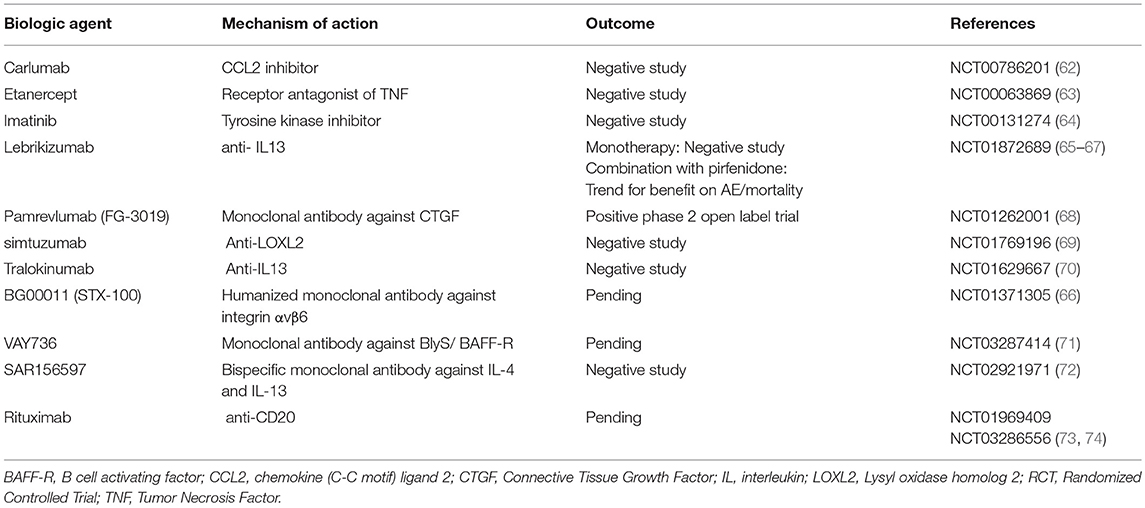
Biologic agents represent one such option, yet with disappointing results. The clinical trial of carlumab, a monoclonal antibody against CC-chemokine ligand 2 (CCL2), was stopped prematurely as patients in the carlumab-treatment-arm experienced greater functional decline compared to the patients in the placebo-treatment-arm (62). TNFa-blocking agents such as etanercept showed no efficacy in patients with IPF (63). Imatinib, a tyrosine kinase inhibitor with multiple biologic properties, did not affect survival or lung function of patients with IPF (64). The study of simtuzumab, a monoclonal antibody against lysyl oxidase-like 2 (LOXL2), was also a negative study (69). Most recently, two anti-IL-13 monoclonal antibodies have entered the pipeline of clinical trials for IPF. Tralokinumab had an acceptable safety and tolerability profile; yet, key efficacy endpoints were not met (70). Monotherapy with lebrikizumab, another anti-IL-13 monoclonal antibody, did not result in a benefit on lung function or mortality over 52 weeks (65). Combination of lebrikizumab and pirfenidone was well-tolerated but did not meet the primary endpoint of FVC% decline; yet, a trend toward beneficial effects on mortality and acute exacerbations was observed (66, 67). Furthermore, SAR156597, a monoclonal bispecific antibody targeting IL-4 and IL-13, failed to halt disease progression either as monotherapy or in combination with standard-of-care antifibrotics (72). A Phase 2 open label trial of pamrevlumab (FG-3019), a monoclonal antibody blocking the downstream effects of connective tissue growth factor (CTGF), showed an acceptable safety and efficacy profile and thus a phase III clinical trial is currently anticipated (68, 79, 80). Safety and efficacy of VAY736, a monoclonal antibody against the cytokine BlyS, a B cell activating factor, is also currently being tested in a phase 2 study (71). BG00011 (STX-100), a humanized monoclonal antibody against integrin αvβ6, demonstrated an acceptable safety profile and its efficacy is currently investigated in a phase 2b study (66, 81). Finally, rituximab ± intravenous immunoglobulin showed 1-year survival benefit in a small cohort of patients with IPF undergoing acute exacerbation compared to historical controls (82). A Phase 2 trial of rituximab in IPF aiming to reduce titers of autoantibodies to HEp-2 Cells over a 9-months period of follow up, has been recently completed (73, 83). In addition, the results of autoantibody reduction for acute exacerbations of IPF (STRIVE-IPF) are greatly anticipated (74).
Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD) Rheumatoid Arthritis
Pulmonary complications represent an important extra-articular feature of rheumatoid arthritis and a major cause of mortality and worse quality of life (16). The decision to treat them requires a multidisciplinary approach weighting: (i) the disease severity and patients' clinical status, (ii) the potential benefits of early therapy (i.e., treatment of inflammation before fibrosis is established) and (iii) the risk of adverse events (i.e., immunosuppression especially for patients with established fibrosis or severe bronchiectatic lesions). Given the lack of consensus over clinical trials, management is currently based on expert opinion. The recent emergence of novel anti-fibrotic compounds for the IPF-UIP-lung holds promise for the RA-UIP-lung (84–87) and the first randomized trial of antifibrotics in RA-ILD (TRAIL trial) is currently under investigation (84). To this end, biologic treatments may present with beneficial outcomes in a proportion of patients with refractory RA-ILD.
Rituximab represents the most widely used biologic treatment in patients with rapidly progressive RA-ILD who are unresponsive to first line therapeutic compounds including corticosteroids and methotrexate (88). Unfortunately, evidence is based on small observational studies and thus further data is required (89–97). A recent prospective, observational cohort study enrolling 43 patients on rituximab and 309 patients on TNF-α inhibitors, demonstrated better long-term survival in patients receiving rituximab than in those receiving TNF-α inhibitor, as event rates were 53.0 and 94.8 per 1,000 person years, respectively (98).
The use of TNF-α inhibitors yielded controversial safety and efficacy results in patients with RA-ILD. Caveats following their use in CTD-ILD parallel those previously described in sarcoidosis. Despite their effectiveness in improving clinical status and slowing down articular disease progression, lung toxicity remains a major concern (99–103). Small case series of patients with RA-ILD have shown that infliximab and etanercept could improve dyspnea and cough, as well as stabilize disease functional status (104–107). On the other hand, safety concerns have been raised for current TNF-α inhibitors infliximab (108–111), etanercept (112–116), adalimumab (117–121), golimumab (90), and certolizumab (37, 122, 123) considering reports for ILD exacerbation. Importantly, TNF- induced ILD could be rapidly progressive and even fatal, especially in patients with preexisting ILD (34, 124–127). Nonetheless, large cohorts of patients with RA reported no association between anti-TNF agents and ILD development or progression (128, 129). Caution should be used for elderly patients, as they represent a high-risk and frail group of patients (100).
Data for other agents including abatacept, tocilizumab and anakinra are still scarce. Abatacept has shown an acceptable safety and efficacy profile, as assessed by dyspnea, functional indicators and radiological extent of inflammation, in both large RCTs (130) and smaller case studies (45, 90, 102, 131, 132). The use of tocilizumab yielded conflicting results and it seems to be beneficial only in a small subgroup of patients with RA-ILD (42, 90, 102, 126, 133–137). Isolated cases of ILD-exacerbation following treatment with tocilizumab have been described (138). Finally, anakinra, an IL-1 receptor antagonist, is rarely, if ever, employed, in the treatment of patients with RA-ILD (126, 139).
Scleroderma
Until recently, the standard treatment for systemic sclerosis-associated ILD (SSc-ILD) was considered to be cyclophosphamide, based on the results of Scleroderma Lung Study (140). However, previously reported data from small-scale studies depicted beneficial effects of mycophenolate mofetil in SSc-ILD (141–143). The recently reported large-scale, randomized, double-blind Scleroderma Lung Study II comparing head-to-head cyclophosphamide vs. mycophenolate mofetil disclosed that mycophenolate mofetil was as effective as cyclophosphamide but with a better safety profile. Thus, mycophenolate mofetil has been established as the current standard of care for SSc-ILD (144). The statistically significant but clinically rather small benefit from the use of such treatment along with the commonly resistant nature of SSc-ILD, clearly underscores the need for novel treatments. Biologic agents, particularly rituximab, have been evaluated in small-scale studies in a minority of patients with progressive, treatment-resistant disease (145). The results of a multicenter, open label, comparative study evaluating rituximab on top of standard treatment (n = 33) vs. standard treatment alone (n = 18) showed that patients in the rituximab group had a 6% increase of FVC compared to baseline values at 2 years of treatment, a benefit that apparently was preserved later on; however, the number of patients at 7 years of treatment was too small for safe conclusions (146). Direct comparison between the rituximab group and the standard-treatment group disclosed a statistically significant benefit for the rituximab-treated patients. Other studies have reported results along the same lines (19, 20, 145, 147–149). Nevertheless, formal, multicenter, large-scale studies are clearly needed to evaluate the value of B-cell depletion treatment(s) in patients with SSc-ILD. A phase III trial evaluating the effects of the anti-IL-6 receptor monoclonal antibody tocilizumab was terminated despite relatively promising results in the earlier phase trials (150, 151) and the results from the use of belimumab, an anti-BLyS monoclonal antibody, have been evaluated only in one study with a small number of patients (n = 9) with clinically non-significant SSc-ILD (152).
Myositis/ Antisynthetase Syndrome
ILDs represent a major cause of mortality in dermatomyositis (DM), polymyositis (PM) and antisynthetase syndrome. Most common antibodies in patients with myositis-ILD include anti-EJ, anti-PL12, anti-PL7, anti-Jo1, anti-OJ and anti-KS (153). Biologics have been used in cases of myositis-associated-ILD refractory to more commonly used immunomodulatory agents such as corticosteroids, azathioprine and mycophenolate mofetil (92, 153). Data derived from case series, case reports and retrospective studies suggested clinical, functional and radiologic benefits from rituximab in patients with progressive ILD associated with PM/DM/ antisynthetase syndrome (92, 154–161). Basiliximab, a monoclonal antibody blocking the alpha chain (CD25) of the IL-2 receptor complex, resulted in radiologic and functional improvement in three out of four cases of clinically amyopathic dermatomyositis (CADM) with anti-MDA5 positivity and rapidly progressive ILD (162). However, prior to the application of such therapies, exclusion of other causes of lung function deterioration such as drug-induced pneumonitis, superimposed infection and respiratory muscle weakness is mandatory.
Future Perspectives and Concluding Remarks (Table 3)
ILDs represent disease paradigms of unknown pathogenesis, unpredictable clinical course and relatively ineffective therapeutic approaches. Biologic therapies may offer an effective alternative in progressive and refractory cases. Early identification of these patients is of paramount importance. Unfortunately, current physiologic biomarkers neither provide mechanistic insights in disease endotypes nor they predict disease clinical course. While ILDs are associated with several underlying mechanisms, currently applied regimens target specific pathways and thus there is still an amenable need for novel compounds. The development of biologics for the treatment of fibrotic lung diseases may hold promise considering the potential for disease modulation (163).
Biologic agents have shown to have a major impact in severe refractory cases of sarcoidosis. Furthermore, canakinumab, a human monoclonal antibody against IL-1 b, has entered the pipeline of clinical trials for sarcoidosis and the results are greatly anticipated (54). Unfortunately, the majority of biologic agents in IPF have, so far, led to disappointing results mainly due to the fact that they target immune-mediated inflammation and not fibrosis. Application of oncologic and personalized medicine approaches represent crucial steps toward successful implementation of biologic agents in lung fibrosis (164). The advent and implementation of high-throughput computational tools could identify biomarkers able to distinguish patients' endotypes and thus predict the subgroup of patients which are more likely to benefit from specific biologic interventions (165, 166). Biologic enrichment of future clinical trials and implementation of biomarkers as end-points could have a crucial impact toward this direction. Systematic pre-treatment assessment for latent infections and immunocompromise is mandatory prior treatment initiation to avoid undesirable adverse-events. Thoughtful monitoring and multi-disciplinary care with rheumatologists and pulmonologists are strongly encouraged.
Author Contributions
TK and AV wrote the manuscript. The manuscript was significantly modified by DB, S-NL, and AT. All authors offered intellectual contribution.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Resp Critic Care Med. (2013) 188:733–48. doi: 10.1164/rccm.201308-1483ST
2. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Resp Critic Care Med. (2015) 192:e3–19. doi: 10.1164/rccm.201506-1063ST
3. Tzouvelekis A, Karampitsakos T, Kontou M, Granitsas A, Malliou I, Anagnostopoulos A, et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: a real-life observational study. Pulm Pharmacol Ther. (2018) 49:61–6. doi: 10.1016/j.pupt.2018.01.006
4. Tzouvelekis A, Karampitsakos T, Ntolios P, Tzilas V, Bouros E, Markozannes E, et al. Longitudinal “real-world” outcomes of pirfenidone in idiopathic pulmonary fibrosis in greece. Front Med. (2017) 4:213. doi: 10.3389/fmed.2017.00213
5. Tzouvelekis A, Ntolios P, Karampitsakos T, Tzilas V, Anevlavis S, Bouros E, et al. Safety and efficacy of pirfenidone in severe Idiopathic Pulmonary Fibrosis: a real-world observational study. Pulm Pharmacol Ther. (2017) 46:48–53. doi: 10.1016/j.pupt.2017.08.011
6. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. (2011) 377:1760–9. doi: 10.1016/S0140-6736(11)60405-4
7. Fletcher S, Jones MG, Spinks K, Sgalla G, Marshall BG, Limbrey R, et al. The safety of new drug treatments for idiopathic pulmonary fibrosis. Expert Opin Drug Safety. (2016) 15:1483–9. doi: 10.1080/14740338.2016.1218470
8. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2083–92. doi: 10.1056/NEJMoa1402582
9. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. (2011) 365:1079–87. doi: 10.1056/NEJMoa1103690
10. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2071–82. doi: 10.1056/NEJMoa1402584
11. Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS® trials. Respir Med. (2016) 113:74–9. doi: 10.1016/j.rmed.2016.02.001
12. Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. (2018) 6:389–402. doi: 10.1016/S2213-2600(18)30064-X
13. Wells AU, Denton CP. Interstitial lung disease in connective tissue disease–mechanisms and management. Nat Rev Rheumatol. (2014) 10:728–39. doi: 10.1038/nrrheum.2014.149
14. https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cber/ucm133077.htm.
15. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. (2017) 18:18112297. doi: 10.3390/ijms18112297
16. Sfikakis PP, Bournia VK, Sidiropoulos P, Boumpas DT, Drosos AA, Kitas GD, et al. Biologic treatment for rheumatic disease: real-world big data analysis from the Greek country-wide prescription database. Clin Exp Rheumatol. (2017) 35:579–85.
17. Zugazagoitia J, Molina-Pinelo S, Lopez-Rios F, Paz-Ares L. Biological therapies in nonsmall cell lung cancer. Eur Respir J. (2017) 49:2016. doi: 10.1183/13993003.01520-2016
18. Noel MS. Biologics in bowel cancer. J Gastrointestinal Oncol. (2017) 8:449–56. doi: 10.21037/jgo.2017.05.03
19. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology. (2010) 49:271–80. doi: 10.1093/rheumatology/kep093
20. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C, et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. (2012) 30:S17–22.
21. Daoussis D, Liossis SN, Yiannopoulos G, Andonopoulos AP. B-cell depletion therapy in systemic sclerosis: experimental rationale and update on clinical evidence. Int J Rheumatol. (2011) 2011:214013. doi: 10.1155/2011/214013
22. Herazo-Maya JD, Sun J, Molyneaux PL, Li Q, Villalba JA, Tzouvelekis A, et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med. (2017) 5:857–68. doi: 10.1016/S2213-2600(17)30349-1
23. Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukocyte Biol. (2008) 83:237–44. doi: 10.1189/jlb.0707504
24. Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. (2012) 366:1968–77. doi: 10.1056/NEJMoa1113354
25. Antoniou KM, Nicholson AG, Dimadi M, Malagari K, Latsi P, Rapti A, et al. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J. (2006) 28:496–504. doi: 10.1183/09031936.06.00032605
26. Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. (2004) 350:125–33. doi: 10.1056/NEJMoa030511
27. Bouros D, Antoniou KM, Tzouvelekis A, Siafakas NM. Interferon-gamma 1b for the treatment of idiopathic pulmonary fibrosis. Expert Opin Biol Ther. (2006) 6:1051–60. doi: 10.1517/14712598.6.10.1051
28. Karampitsakos T, Woolard T, Bouros D, Tzouvelekis A. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol. (2017) 808:35–43. doi: 10.1016/j.ejphar.2016.06.045
29. Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. (2013) 41:1207–18. doi: 10.1183/09031936.00073012
30. Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. (2017) 45:1–10. doi: 10.1016/j.pupt.2017.03.016
31. Karampitsakos T, Tzouvelekis A, Chrysikos S, Bouros D, Tsangaris I, Fares WH. Pulmonary hypertension in patients with interstitial lung disease. Pulm Pharmacol Ther. (2018) 22:292–301. doi: 10.1016/j.pupt.2018.03.002
32. Papaioannou O, Karampitsakos T, Barbayianni I, Chrysikos S, Xylourgidis N, Tzilas V, et al. Metabolic disorders in chronic lung diseases. Front Med. (2017) 4:246. doi: 10.3389/fmed.2017.00246
33. Tzouvelekis A, Zacharis G, Oikonomou A, Mikroulis D, Margaritopoulos G, Koutsopoulos A, et al. Increased incidence of autoimmune markers in patients with combined pulmonary fibrosis and emphysema. BMC Pulm Med. (2013) 13:31. doi: 10.1186/1471-2466-13-31
34. Panopoulos ST, Sfikakis PP. Biological treatments and connective tissue disease associated interstitial lung disease. Curr Opin Pulm Med. (2011) 17:362–7. doi: 10.1097/MCP.0b013e3283483ea5
35. Chen J, Chi S, Li F, Yang J, Cho WC, Liu X. Biologics-induced interstitial lung diseases in rheumatic patients: facts and controversies AU - Chen, Juan. Exp Opin Biol Ther. (2017) 17:265–83. doi: 10.1080/14712598.2017.1287169
36. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheumat Dis Clin N Am. (2015) 41:225–36. doi: 10.1016/j.rdc.2014.12.004
37. Glaspole IN, Hoy RF, Ryan PF. A case of certolizumab-induced interstitial lung disease in a patient with rheumatoid arthritis. Rheumatology. (2013) 52:2302–4. doi: 10.1093/rheumatology/ket175
38. Atzeni F, Boiardi L, Salli S, Benucci M, Sarzi-Puttini P. Lung involvement and drug-induced lung disease in patients with rheumatoid arthritis. Exp Rev Clin Immunol. (2013) 9:649–57. doi: 10.1586/1744666X.2013.811173
39. Toussirot E, Berthelot JM, Pertuiset E, Bouvard B, Gaudin P, Wendling D, et al. Pulmonary nodulosis and aseptic granulomatous lung disease occurring in patients with rheumatoid arthritis receiving tumor necrosis factor-alpha-blocking agent: a case series. J Rheumatol. (2009) 36:2421–7. doi: 10.3899/jrheum.090030
40. Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest. (2002) 122:1858–60. doi: 10.1378/chest.122.5.1858
41. Liote H, Liote F, Seroussi B, Mayaud C, Cadranel J. Rituximab-induced lung disease: a systematic literature review. Eur Resp J. (2010) 35:681–7. doi: 10.1183/09031936.00080209
42. Kawashiri SY, Kawakami A, Sakamoto N, Ishimatsu Y, Eguchi K. A fatal case of acute exacerbation of interstitial lung disease in a patient with rheumatoid arthritis during treatment with tocilizumab. Rheum Int. (2012) 32: 4023–6. doi: 10.1007/s00296-010-1525-z
43. Ikegawa K, Hanaoka M, Ushiki A, Yamamoto H, Kubo K. A case of organizing pneumonia induced by tocilizumab. Internal Med. (2011) 50:2191–3. doi: 10.2169/internalmedicine.50.5497
44. Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthrit Rheum. (2004) 50:1761–9. doi: 10.1002/art.20303
45. Wada T, Akiyama Y, Yokota K, Sato K, Funakubo Y, Mimura T. A case of rheumatoid arthritis complicated with deteriorated interstitial pneumonia after the administration of abatacept. Japan J Clin Immunol. (2012) 35:433–8.
46. Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Critic Care Med. (2006) 174:795–802. doi: 10.1164/rccm.200603-402OC
47. Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller W Jr, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2006) 23:201–8.
48. Vorselaars AD, Crommelin HA, Deneer VH, Meek B, Claessen AM, Keijsers RG, et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J. (2015) 46:175–85. doi: 10.1183/09031936.00227014
49. Vorselaars AD, Verwoerd A, van Moorsel CH, Keijsers RG, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. (2014) 43:602–9. doi: 10.1183/09031936.00055213
50. Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2014) 31:46–54.
51. Utz JP, Limper AH, Kalra S, Specks U, Scott JP, Vuk-Pavlovic Z, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. (2003) 124:177–85. doi: 10.1378/chest.124.1.177
52. Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. (2014) 44:1296–307. doi: 10.1183/09031936.00000914
53. Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JG, Perkins D, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. (2014) 43:1525–8. doi: 10.1183/09031936.00224513
54. ClinicalTrials.gov. Study of Efficacy, Safety and Tolerability of ACZ885 (Canakinumab) in Patients With Pulmonary Sarcoidosis. Available online at: https://clinicaltrials.gov/ct2/show/NCT02888080
55. Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med. (2015) 3:813–22. doi: 10.1016/S2213-2600(15)00199-X
56. Cremers JP, Drent M, Bast A, Shigemitsu H, Baughman RP, Valeyre D, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. (2013) 19:545–61. doi: 10.1097/MCP.0b013e3283642a7a
57. Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthr Rheumat. (2012) 42:89–103. doi: 10.1016/j.semarthrit.2011.12.006
58. Wijnen PA, Cremers JP, Nelemans PJ, Erckens RJ, Hoitsma E, Jansen TL, et al. Association of the TNF-alpha G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J. (2014) 43:1730–9. doi: 10.1183/09031936.00169413
59. Pereira R, Lago P, Faria R, Torres T. Safety of anti-TNF therapies in immune-mediated inflammatory diseases: focus on infections and malignancy. Drug Dev Res. (2015) 76:419–27. doi: 10.1002/ddr.21285
60. Saketkoo LA, Baughman RP. Biologic therapies in the treatment of sarcoidosis. Expert Rev Clin Immunol. (2016) 12:817–25. doi: 10.1080/1744666X.2016.1175301
61. Tong D, Manolios N, Howe G, Spencer D. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Int Med J. (2012) 42:89–94. doi: 10.1111/j.1445-5994.2011.02612.x
62. Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, et al. CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J. (2015) 46:1740–50. doi: 10.1183/13993003.01558-2014
63. Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Critic Care Med. (2008) 178:948–55. doi: 10.1164/rccm.200709-1446OC
64. Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR, et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Critic Care Med. (2010) 181:604–10. doi: 10.1164/rccm.200906-0964OC
65. Ogura T, Scholand MB, Glaspole I, Maher TM, Kardatzke D, Kaminski J, et al. The RIFF study (cohort A): a phase II, randomized, double-blind, placebo-controlled trial of Lebrikizumab as monotherapy in patients with idiopathic pulmonary fibrosis. D12 Immunother Lung Dis. 197:A6168.
66. ClinicalTrials.gov. STX-100 in Patients With Idiopathic Pulmonary Fibrosis (IPF). Available online at: https://clinicaltrials.gov/ct2/show/NCT01371305
67. Kondoh Y, Corte TJ, Glassberg MK, Costabel U, Lancaster LH, Kardatzke D, et al. The RIFF study (Cohort B): A phase II, randomized, double-blind, placebo-controlled trial of lebrikizumab in combination with pirfenidone in patients with idiopathic pulmonary fibrosis. D12 Immunother Lung Dis. 197:A6168.
68. Raghu G, Scholand MB, de Andrade J, Lancaster L, Mageto Y, Goldin J, et al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Resp J. (2016) 47:1481–91. doi: 10.1183/13993003.01030-2015
69. Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. (2017) 5:22–32. doi: 10.1016/S2213-2600(16)30421-0
70. Parker JM, Glaspole IN, Lancaster LH, Haddad TJ, She D, Roseti SL, et al. A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Resp Crit Care Med. (2018) 197:94–103. doi: 10.1164/rccm.201704-0784OC
71. ClinicalTrials.gov. Study of Pharmacodynamics, Pharmacokinetics, Safety and Tolerability of VAY736 in Patients With Idiopathic Pulmonary Fibrosis. Available online at: https://clinicaltrials.gov/ct2/show/NCT03287414
72. Raghu G, Richeldi L, Crestani B, Wung P, Bejuit R, Esperet C, Soubrane C. Safety and efficacy of SAR156597 in idiopathic pulmonary fibrosis (IPF): a phase 2, randomized, double-blind, placebo-controlled study. A93 ILD: Clin Trail. 197:A2441.
73. ClinicalTrials.gov. Autoantibody Reduction Therapy in Patients With Idiopathic Pulmonary Fibrosis (ART-IPF). (2014). Available online at: https://clinicaltrials.gov/ct2/show/NCT01969409
74. ClinicalTrials.gov. Autoantibody Reduction for Acute Exacerbations of Idiopathic Pulmonary Fibrosis (STRIVE-IPF). (2018). Available online at: https://clinicaltrials.gov/ct2/show/NCT03286556
75. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. (2018) 24:39–49. doi: 10.1038/nm.4447
76. Tzouvelekis A, Yu G, Lino Cardenas CL, Herazo-Maya JD, Wang R, Woolard T, et al. SH2 domain-containing phosphatase-2 is a novel antifibrotic regulator in pulmonary fibrosis. Am J Respir Crit Care Med. (2017) 195:500–14. doi: 10.1164/rccm.201602-0329OC
77. Albert RK, Schwartz DA. Revealing the secrets of idiopathic pulmonary fibrosis. N Engl J Med. (2019) 380:94–6. doi: 10.1056/NEJMcibr1811639
78. Spagnolo P, Tzouvelekis A, Bonella F. The management of patients with idiopathic pulmonary fibrosis. Front Med. (2018) 5:148. doi: 10.3389/fmed.2018.00148
79. Raghu G, Scholand M, Andrade JDE, Lancaster L, Mageto YN, Goldlin JG, et al. Safety and efficacy of anti-CTGF monoclonal antibody FG-3019 for the treatment of idiopathic pulmonary fibrosis (IPF): results of Phase 2 clinical trial two years after initiation. Am J Resp Crit Care Med. (2014) 189:A1426.
80. ClinicalTrials.gov. Evaluate the Safety and Efficacy of FG-3019 in Patients With Idiopathic Pulmonary Fibrosis. Available online at: https://clinicaltrials.gov/ct2/show/NCT01890265
81. Mouded M, Culver DA, Hamblin MJ, Golden JA, Veeraraghavan S, Enelow RI, et al. Randomized, double-blind, placebo-controlled, multiple dose, dose-escalation study of BG00011 (Formerly STX-100) in patients with idiopathic pulmonary fibrosis (IPF). D14 ILD Clin Res. 197:A7785.
82. Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, et al. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. PLoS ONE. (2015) 10: e0127771. doi: 10.1371/journal.pone.0127771
83. Duncan SR, Gibson KF. Rituximab Therapy in Patients with IPF. (2013). Available online at: http://grantome.com/grant/NIH/R01-HL119960-02
84. ClinicalTrials.gov. Phase ll Study of Pirfenidone in Patients With RAILD (TRAIL1). (2017). Available online at: https://clinicaltrials.gov/ct2/show/NCT02808871
85. ClinicalTrials.gov. Efficacy and Safety of Nintedanib in Patients With Progressive Fibrosing Interstitial Lung Disease (PF-ILD) (INBUILD®). (2017). Available online at: https://clinicaltrials.gov/ct2/show/NCT02999178
86. Redente EF, Aguilar MA, Black BP, Edelman B, Bahadur A, Humphries SM, et al. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L998–L1009. doi: 10.1152/ajplung.00304.2017
87. Meyer KC, Decker CA. Role of pirfenidone in the management of pulmonary fibrosis. Therap Clin Risk Manage. (2017) 13:427–37. doi: 10.2147/TCRM.S81141
88. Sharp C, Dodds N, Mayers L, Millar AB, Gunawardena H, Adamali H. The role of biologics in treatment of connective tissue disease-associated interstitial lung disease. QJM Int J Med. (2015) 108:683–8. doi: 10.1093/qjmed/hcv007
89. Franzen D, Ciurea A, Bratton DJ, Clarenbach CF, Latshang TD, Russi EW, et al. Effect of rituximab on pulmonary function in patients with rheumatoid arthritis. Pulm Pharmacol Ther. (2016) 37:24–9. doi: 10.1016/j.pupt.2016.02.002
90. Hadjinicolaou AV, Nisar MK, Bhagat S, Parfrey H, Chilvers ER, Ostor AJ. Non-infectious pulmonary complications of newer biological agents for rheumatic diseases–a systematic literature review. Rheumatology. (2011) 50:2297–305. doi: 10.1093/rheumatology/ker289
91. Matteson EBT, Ryu J, Crowson C, Hartman T, Dellaripa P. Open-label, pilot study of the safety and clinical effects of rituximab in patients with rheumatoid arthritis-associated interstitial pneumonia. Open J Rheumatol Autoimmune. (2012) 2:53–8. doi: 10.4236/ojra.2012.23011
92. Sharp C, McCabe M, Dodds N, Edey A, Mayers L, Adamali H, et al. Rituximab in autoimmune connective tissue disease-associated interstitial lung disease. Rheumatology. (2016) 55:1318–24. doi: 10.1093/rheumatology/kew195
93. Kabia AMYM, Dass S, Vital E, Beirne P, Emery P. Efficacy and safety of rituximab in rheumatoid arthritis patients with concomitant interstitial lung disease: 10-year experience at single centre. Rheumatology. (2015) 54:i86. doi: 10.1093/rheumatology/kev088.092
94. Braun-Moscovici Y, Butbul-Aviel Y, Guralnik L, Toledano K, Markovits D, Rozin A, et al. Rituximab: rescue therapy in life-threatening complications or refractory autoimmune diseases: a single center experience. Rheumatol Int. (2013) 33:1495–504. doi: 10.1007/s00296-012-2587-x
95. Hartung W, Maier J, Pfeifer M, Fleck M. Effective treatment of rheumatoid arthritis-associated interstitial lung disease by B-cell targeted therapy with rituximab. Case Reports Immunol. (2012) 2012:272303. doi: 10.1155/2012/272303
96. Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years' experience at a single centre. Rheumatology. (2017) 56:1348–57. doi: 10.1093/rheumatology/kex072
97. Fernández-Díaz C, Martin-Lopez M, Carrasco-Cubero M, Reina-Sanz D, Rubio-Mu-oz P, Urruticoechea-Arana A, et al. FRI0226 Rituximab in rheumatoid arthritis with interstitial lung disease: a multicenter study. Ann Rheum Dis. (2017) 76:569–70.
98. Druce KL, Iqbal K, Watson KD, Symmons DPM, Hyrich KL, Kelly C. Mortality in patients with interstitial lung disease treated with rituximab or TNFi as a first biologic. RMD Open. (2017) 3:e000473. doi: 10.1136/rmdopen-2017-000473
99. Perez-De-Lis M, Retamozo S, Flores-Chavez A, Kostov B, Perez-Alvarez R, Brito-Zeron P, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opin Drug Saf. (2017) 16:1255–71. doi: 10.1080/14740338.2017.1372421
100. Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthrit Rheumat. (2011) 41:256–64. doi: 10.1016/j.semarthrit.2010.11.002
101. Dixon WG, Hyrich KL, Watson KD, Lunt M, Symmons DP, BSRBR Control Centre Consortium, et al. Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. (2010) 69:1086–91. doi: 10.1136/ard.2009.120626
102. Jani M, Hirani N, Matteson EL, Dixon WG. The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol. (2014) 10:284–94. doi: 10.1038/nrrheum.2013.197
103. Komiya K, Ishii H, Fujita N, Oka H, Iwata A, Sonoda H, et al. Adalimumab-induced interstitial pneumonia with an improvement of pre-existing rheumatoid arthritis-associated lung involvement. Int Med. (2011) 50:749–51. doi: 10.2169/internalmedicine.50.4748
104. Vassallo R, Matteson E, Thomas CF Jr. Clinical response of rheumatoid arthritis-associated pulmonary fibrosis to tumor necrosis factor-alpha inhibition. Chest. (2002) 122:1093–6. doi: 10.1378/chest.122.3.1093
105. Antoniou KM, Mamoulaki M, Malagari K, Kritikos HD, Bouros D, Siafakas NM, et al. Infliximab therapy in pulmonary fibrosis associated with collagen vascular disease. Clin Exp Rheumatol. (2007) 25:23–8.
106. Bargagli E, Galeazzi M, Rottoli P. Infliximab treatment in a patient with rheumatoid arthritis and pulmonary fibrosis. Eur Resp J. (2004) 24:708. doi: 10.1183/09031936.04.00076904
107. Wang Y, Xu SQ, Xu JH, Ding C. Treatment with etanercept in a patient with rheumatoid arthritis-associated interstitial lung disease. Clin Med Insights Case Reports. (2011) 4:49–52. doi: 10.4137/CCRep.S8150
108. Mori S, Imamura F, Kiyofuji C, Sugimoto M. Development of interstitial pneumonia in a rheumatoid arthritis patient treated with infliximab, an anti-tumor necrosis factor alpha-neutralizing antibody. Modern Rheumatol. (2006) 16:251–5. doi: 10.3109/s10165-006-0491-5
109. Ostor AJ, Chilvers ER, Somerville MF, Lim AY, Lane SE, Crisp AJ, et al. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. (2006) 33:622–8.
110. Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. (2008) 67:189–94. doi: 10.1136/ard.2007.072967
111. Taki H, Kawagishi Y, Shinoda K, Hounoki H, Ogawa R, Sugiyama E, et al. Interstitial pneumonitis associated with infliximab therapy without methotrexate treatment. Rheumatol Int. (2009) 30:275–76. doi: 10.1007/s00296-009-0931-6
112. Lindsay K, Melsom R, Jacob BK, Mestry N. Acute progression of interstitial lung disease: a complication of etanercept particularly in the presence of rheumatoid lung and methotrexate treatment. Rheumatology. (2006) 45:1048–9. doi: 10.1093/rheumatology/kel090
113. Hagiwara K, Sato T, Takagi-Kobayashi S, Hasegawa S, Shigihara N, Akiyama O. Acute exacerbation of preexisting interstitial lung disease after administration of etanercept for rheumatoid arthritis. J Rheumatol. (2007) 34: 1151–1154.
114. Tournadre A, Ledoux-Eberst J, Poujol D, Dubost JJ, Ristori JM, Soubrier M. Exacerbation of interstitial lung disease during etanercept therapy: two cases. Joint Bone Spine Revue Rhum. (2008) 75:215–8. doi: 10.1016/j.jbspin.2007.04.028
115. Koike T, Harigai M, Inokuma S, Inoue K, Ishiguro N, Ryu J, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. (2009) 36:898–906. doi: 10.3899/jrheum.080791
116. Horai Y, Miyamura T, Shimada K, Takahama S, Minami R, Yamamoto M, et al. Eternacept for the treatment of patients with rheumatoid arthritis and concurrent interstitial lung disease. J Clin Pharm Therap. (2012) 37:117–21. doi: 10.1111/j.1365-2710.2010.01234.x
117. Schoe A, van der Laan-Baalbergen NE, Huizinga TW, Breedveld FC, van Laar JM. Pulmonary fibrosis in a patient with rheumatoid arthritis treated with adalimumab. Arthrit Rheumat. (2006) 55:157–9. doi: 10.1002/art.21716
118. Dascalu C, Mrejen-Shakin K, Bandagi S. Adalimumab-induced acute pneumonitis in a patient with rheumatoid arthritis. J Clin Rheumatol. (2010) 16:172–4. doi: 10.1097/RHU.0b013e3181df8361
119. Yamazaki H, Isogai S, Sakurai T, Nagasaka K. A case of adalimumab-associated interstitial pneumonia with rheumatoid arthritis. Modern Rheumatol. (2010) 20:518–21. doi: 10.3109/s10165-010-0308-4
120. Koike T, Harigai M, Ishiguro N, Inokuma S, Takei S, Takeuchi T, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Modern Rheumatol. (2012) 22:498–508. doi: 10.3109/s10165-011-0541-5
121. Dias OM, Pereira DA, Baldi BG, Costa AN, Athanazio RA, Kairalla RA, et al. Adalimumab-induced acute interstitial lung disease in a patient with rheumatoid arthritis. J Brasil Pneumol. (2014) 40:77–81. doi: 10.1590/S1806-37132014000100012
122. Lager J, Hilberg O, Lokke A, Bendstrup E. Severe interstitial lung disease following treatment with certolizumab pegol: a case report. Eur Resp Rev. (2013) 22:414–6. doi: 10.1183/09059180.00002013
123. Pearce F, Johnson SR, Courtney P. Interstitial lung disease following certolizumab pegol. Rheumatology. (2012) 51:578–80. doi: 10.1093/rheumatology/ker309
124. Nakashita T, Ando K, Kaneko N, Takahashi K, Motojima S. Potential risk of TNF inhibitors on the progression of interstitial lung disease in patients with rheumatoid arthritis. BMJ Open. (2014) 4:e005615. doi: 10.1136/bmjopen-2014-005615
125. Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine. (2007) 86:242–51. doi: 10.1097/MD.0b013e3181441a68
126. Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthr Rheum. (2014) 43:613–26. doi: 10.1016/j.semarthrit.2013.09.005
127. Iqbal K, Kelly C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Therap Adv Musc Dis. (2015) 7:247–67. doi: 10.1177/1759720X15612250
128. Herrinton LJ, Harrold LR, Liu L, Raebel MA, Taharka A, Winthrop KL, et al. Association between anti-TNF-alpha therapy and interstitial lung disease. Pharmacoepidemiol Drug Safe. (2013) 22:394–402. doi: 10.1002/pds.3409
129. Curtis JR, Sarsour K, Napalkov P, Costa LA, Schulman KL. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor alpha agents, a retrospective cohort study. Arthrit Res Ther. (2015) 17:319. doi: 10.1186/s13075-015-0835-7
130. Fernandez-Diaz C, Loricera J, Castaneda S, Lopez-Mejias R, Ojeda-Garcia C, Olive A, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: A national multicenter study of 63 patients. Semin Arthr Rheum. (2018) 48:22–27. doi: 10.1016/j.semarthrit.2017.12.012
131. Nakashita T, Ando K, Takahashi K, Motojima S. Possible effect of abatacept on the progression of interstitial lung disease in rheumatoid arthritis patients. Resp Invest. (2016) 54:376–9. doi: 10.1016/j.resinv.2016.03.001
132. Mera-Varela A, Perez-Pampin E. Abatacept therapy in rheumatoid arthritis with interstitial lung disease. J Clin Rheumatol. (2014) 20:445–6. doi: 10.1097/RHU.0000000000000084
133. Mohr M, Jacobi AM. Interstitial lung disease in rheumatoid arthritis: response to IL-6R blockade. Scand J Rheumatol. (2011) 40:400–1. doi: 10.3109/03009742.2011.599072
134. Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. (2014) 8:349–64. doi: 10.2147/DDDT.S41437
135. Picchianti Diamanti A, Markovic M, Argento G, Giovagnoli S, Ricci A, Lagana B, et al. Therapeutic management of patients with rheumatoid arthritis and associated interstitial lung disease: case report and literature review. Ther Adv Respir Dis. (2017) 11:64–72. doi: 10.1177/1753465816668780
136. Wendling D, Vidon C, Godfrin-Valnet M, Rival G, Guillot X, Prati C. Exacerbation of combined pulmonary fibrosis and emphysema syndrome during tocilizumab therapy for rheumatoid arthritis. Joint Bone Spine Revue du Rhum. (2013) 80:670–1. doi: 10.1016/j.jbspin.2013.03.009
137. Fernández-Díaz C, Narvaez-García J, Martín-Lόpez M, Rubio-Mu-oz P, Casta-eda-Sanz S, Vegas-Revenga N, et al. THU0134 Interstitial lung disease and rheumatoid arthritis. multicenter study with tocilizumab. Ann Rheum Dis. (2017) 76:251–2. doi: 10.1136/annrheumdis-2017-eular.3580
138. Akiyama M, Kaneko Y, Yamaoka K, Kondo H, Takeuchi T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study. Rheumatol Int. (2016) 36:881–9. doi: 10.1007/s00296-016-3478-3
139. Cohen SB. The use of anakinra, an interleukin-1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. (2004) 30:365–80. doi: 10.1016/j.rdc.2004.01.005
140. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. (2006) 354:2655–66. doi: 10.1056/NEJMoa055120
141. Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology. (2006) 45:1005–8. doi: 10.1093/rheumatology/kei211
142. Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. (2013) 40:640–6. doi: 10.3899/jrheum.121043
143. Kowal-Bielecka O, Landewe R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. (2009) 68:620–8. doi: 10.1136/ard.2008.096677
144. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Resp Med. (2016) 4:708–19. doi: 10.1016/S2213-2600(16)30152-7
145. Giuggioli D, Lumetti F, Colaci M, Fallahi P, Antonelli A, Ferri C. Rituximab in the treatment of patients with systemic sclerosis. Our experience and review of the literature. Autoimmun Rev. (2015) 14:1072–8. doi: 10.1016/j.autrev.2015.07.008
146. Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthr Rheum. (2017) 46:625–31. doi: 10.1016/j.semarthrit.2016.10.003
147. Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, et al. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Resp J. (2012) 40:641–8. doi: 10.1183/09031936.00163911
148. Bosello SL, De Luca G, Rucco M, Berardi G, Falcione M, Danza FM, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthr Rheum. (2015) 44:428–36. doi: 10.1016/j.semarthrit.2014.09.002
149. Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. (2015) 74:1188–94. doi: 10.1136/annrheumdis-2013-204522
150. Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. (2016) 387:2630–40. doi: 10.1016/S0140-6736(16)00232-4
151. Khanna D, Denton CP, Lin CJF, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. (2018) 77:212–20. doi: 10.1136/annrheumdis-2017-211682
152. Gordon JK, Martyanov V, Franks JM, Bernstein EJ, Szymonifka J, Magro C, et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial. Arthr Rheumatol. (2018) 70:308–16. doi: 10.1002/art.40358
153. Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Noma S, et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med. (2011) 105:1238–47. doi: 10.1016/j.rmed.2011.03.022
154. Lambotte O, Kotb R, Maigne G, Blanc FX, Goujard C, Delfraissy JF. Efficacy of rituximab in refractory polymyositis. J Rheumatol. (2005) 32:1369–70.
155. Brulhart L, Waldburger JM, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis. (2006) 65:974–5. doi: 10.1136/ard.2005.045898
156. Andersson H, Sem M, Lund MB, Aalokken TM, Gunther A, Walle-Hansen R, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology. (2015) 54:1420–8. doi: 10.1093/rheumatology/kev004
157. Zappa MC, Trequattrini T, Mattioli F, Rivitti R, Vigliarolo R, Marcoccia A, et al. Rituximab treatment in a case of antisynthetase syndrome with severe interstitial lung disease and acute respiratory failure. Multidiscip Res Med. (2011) 6:183–8. doi: 10.1186/2049-6958-6-3-183
158. Vandenbroucke E, Grutters JC, Altenburg J, Boersma WG, ter Borg EJ, van den Bosch JM. Rituximab in life threatening antisynthetase syndrome. Rheumatol Int. (2009) 29:1499–502. doi: 10.1007/s00296-009-0859-x
159. Dasa O, Ruzieh M, Oraibi O. Successful Treatment of life-threatening interstitial lung disease secondary to antisynthetase syndrome using rituximab: a case report and review of the literature. Am J Ther. (2016) 23:e639–45. doi: 10.1097/MJT.0000000000000245
160. Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. (2012) 106:581–7. doi: 10.1016/j.rmed.2012.01.001
161. Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. (2014) 19:353–9. doi: 10.1111/resp.12214
162. Zou J, Li T, Huang X, Chen S, Guo Q, Bao C. Basiliximab may improve the survival rate of rapidly progressive interstitial pneumonia in patients with clinically amyopathic dermatomyositis with anti-MDA5 antibody. Ann Rheum Dis. (2014) 73:1591–3. doi: 10.1136/annrheumdis-2014-205278
163. Karampitsakos T CS, Tzilas V, Dimakou K, Bouros D, Tzouvelekis A. Idiopathic pulmonary fibrosis. Time to get personal. Pneumon. (2018) 31:71–80.
164. Doyle TJ, Lee JS, Dellaripa PF, Lederer JA, Matteson EL, Fischer A, et al. A roadmap to promote clinical and translational research in rheumatoid arthritis-associated interstitial lung disease. Chest. (2014) 145:454–63. doi: 10.1378/chest.13-2408
165. Spagnolo P, Tzouvelekis A, Maher TM. Personalized medicine in idiopathic pulmonary fibrosis: facts and promises. Curr Opin Pulm Med. (2015) 21:470–8. doi: 10.1097/MCP.0000000000000187
Keywords: interstitial lung diseases, biologic treatments, pulmonary fibrosis, treatment, safety
Citation: Karampitsakos T, Vraka A, Bouros D, Liossis S-N and Tzouvelekis A (2019) Biologic Treatments in Interstitial Lung Diseases. Front. Med. 6:41. doi: 10.3389/fmed.2019.00041
Received: 06 January 2019; Accepted: 13 February 2019;
Published: 13 March 2019.
Edited by:
Mehdi Mirsaeidi, University of Miami, United StatesReviewed by:
Paolo Spagnolo, University of Padova, ItalyMarilyn K. Glassberg, Leonard M. Miller School of Medicine, United States
Copyright © 2019 Karampitsakos, Vraka, Bouros, Liossis and Tzouvelekis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Argyris Tzouvelekis, argyrios.tzouvelekis@fleming.gr
 Theodoros Karampitsakos
Theodoros Karampitsakos Argyro Vraka2
Argyro Vraka2  Stamatis-Nick Liossis
Stamatis-Nick Liossis Argyris Tzouvelekis
Argyris Tzouvelekis