- 1Department of Maternal and Child Health, Xiangya School of Public Health, Central South University, Changsha, China
- 2Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China
Background: Inflammatory cytokines have been considered to be significant factors contributing to the development and progression of non-alcoholic fatty liver disease (NAFLD). However, the role of inflammatory cytokines in NAFLD remains inconclusive.
Objective: This study aimed to evaluate the association between inflammatory cytokines and NAFLD.
Methods: PubMed, Web of Science, the Cochrane Library, and EMBASE databases were searched until 31 December 2021 to identify eligible studies that reported the association of inflammatory cytokine with NAFLD and its subtypes. We pooled odds ratios (ORs) and hazard risk (HRs) with 95% confidence intervals (CIs) and conducted heterogeneity tests. Sensitivity analysis and analysis for publication bias were also carried out.
Results: The search in the databases identified 51 relevant studies that investigated the association between 19 different inflammatory cytokines and NAFLD based on 36,074 patients and 47,052 controls. The results of the meta-analysis showed significant associations for C-reactive protein (CRP), interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and intercellular adhesion molecule-1 (ICAM-1) with NAFLD (ORs of 1.41, 1.08, 1.50, 1.15 and 2.17, respectively). In contrast, we observed non-significant associations for interferon-γ (IFN-γ), insulin-like growth factor (IGF-II), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-7 (IL-7), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12 (IL-12), monocyte chemoattractant protein-1(MCP-1), and transforming growth factor-β (TGF-β) with NAFLD. Our results also showed that CRP, IL-1β, and TNF-α were significantly associated with non-alcoholic steatohepatitis (NASH) and hepatic fibrosis.
Conclusions: Our results indicated that increased CRP, IL‐1β, IL-6, TNF‐α, and ICAM-1 concentrations were significantly associated with increased risks of NAFLD. These inflammatory mediators may serve as biomarkers for NAFLD subjects and expect to provide new insights into the aetiology of NAFLD as well as early diagnosis and intervention.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease worldwide, with a global prevalence of 25% in the general population (1), and 80% in the obese population (2). NAFLD is well recognized as a hepatic manifestation of metabolic syndrome, with strong links to obesity, insulin resistance, increased systemic inflammation, and advanced atherosclerosis (3).
The pathogenesis of NAFLD has not been fully elucidated. The mechanism of progression is usually explained by the classic “multiple strikes” theory of NAFLD pathogenesis, which states that lipid accumulation triggers hepatic steatosis, leading to multiple injuries, including adipokine secretion, inflammation, lipotoxicity, and dysregulation of glucose and lipid metabolism, which may eventually cause non-alcoholic steatohepatitis (NASH) and cirrhosis (4–6).
It is widely accepted that cytokines play a critical role as mediators of inflammation, fibrosis, and cirrhosis in NAFLD (7). Previous studies have reported several inflammatory mediators involved in the development and progression of NAFLD, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) and NOD-like receptor protein 3 (NLRP3) inflammasome (8–10). Some of these inflammatory mediators with immunomodulatory functions can be used as biomarkers to assess the severity and predict the prognosis of NAFLD (8). However, previous studies reported an inconsistent association between inflammatory cytokines and NAFLD. Some studies found positive associations between inflammatory cytokines and NAFLD (11, 12), while other studies found negative or null associations (13, 14).
Several studies have reviewed inflammatory mediators and molecular pathways involved in the development and progression of NAFLD (15–18). However, to our knowledge, few meta-analyses have comprehensively assessed the association between inflammatory cytokines and NAFLD. For instance, one previous review summarized the beneficial effects and detrimental effects of inflammatory mediators and inflammatory cells in NAFLD (18). However, this review was restricted to six inflammatory cytokines and did not review the role of other inflammatory cytokines in NAFLD. In addition, there was no quantification of the effect of inflammatory cytokines on NAFLD. In recent years, a growing number of studies have been conducted in various countries to analyze this topic (19), thus it is essential to conduct a meta-analysis to assess the association between inflammatory cytokines and NAFLD.
The aim of this study was to provide a complete review of existing cross-sectional studies, case-control studies, and cohort studies on the association between inflammatory cytokines and NAFLD. Furthermore, we classified NAFLD into different types by disease spectrum (e.g., NASH and hepatic steatosis), and performed subgroup analyses to elucidate the essential association between inflammatory cytokines and NAFLD.
Methods
This review and meta‐analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (20).
Search Strategy
Potentially relevant articles were searched using electronic databases. We systematically searched PubMed, Web of Science, the Cochrane Library, and EMBASE databases for articles published from 1 January 1960 to 31 December 2021. The search strategy included all possible combinations of keywords from the three groups related to non-alcoholic fatty liver disease, inflammatory cytokines (including interleukin, interferon, tumor necrosis factor, growth factor, and chemokine), and their associated outcomes. The specific search strategy is provided in Supplementary Table 1.
Selection Criteria
Studies that met all of the following criteria were included in the study: (a) the patients in the studies were diagnosed with NAFLD, including hepatic steatosis, NASH, and hepatic fibrosis; (b) observational studies (cross-sectional studies, case-control studies, and cohort studies) that reported the estimates of the effect of inflammatory cytokines on NAFLD, including odds ratios (ORs), relative risks (RRs) or hazard ratios (HRs), and 95% confidence intervals (CIs); (c) study subjects were human populations; and (d) articles were written in English.
Studies that met any of the following criteria were excluded from the study: (a) only conducted in vivo or in vitro cell experiments; (b) with incomplete data in the original studies; (c) duplicated studies retrieved from various databases; and (d) reviews, meta-analyses, clinical guidelines, comments, letters to the editor, or case reports.
Data Extraction
A standardized data extraction form was used to extract data from each eligible study by two reviewers (YD and XP) independently. Any further inconsistencies were addressed by a joint discussion. The following data were recorded: name of the first author, publication year, country, sample size, type of case/control, mean age (years), BMI (kg/m2), sample type, subtypes of NAFLD, the effect of inflammatory cytokines on the ORs/RRs/HRs and 95% CIs of NAFLD, diagnostic methods for NAFLD, and measurements of inflammatory cytokines.
Quality Assessment
The methodological quality of the eligible studies was assessed using the National Institutes of Health’s Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies (NIHQAT) (21).This assessment tool rates each study based on 14 criteria (Supplementary Table 2). A study‐specific global score ranging from zero to 14 was calculated by summing up scores across all criteria. The study quality assessment helped measure the strength of scientific evidence but was not used to determine the inclusion of studies.
Statistical Analysis
Each inflammatory cytokine with more than two identified studies was included in the meta-analysis. The ORs with 95% CIs were used to describe the effect of inflammatory cytokines on NAFLD. The heterogeneity was evaluated by I-squared (I2). The level of heterogeneity measured by I2 was interpreted as modest (I2 ≤ 25%), moderate (25%< I2 ≤50%), substantial (50% < I2 ≤ 75%), or considerable (I2 > 75%). Q-statistic tests were also conducted, where P < 0.1 indicates the presence of heterogeneity among studies. If I2 ≥ 50% or P < 0.1, the random effect model was used, otherwise the fixed effect model was applied. Subgroup analyses were used to explore the potential sources of heterogeneity. Sensitivity analyses were performed to evaluate the availability and reliability of the results by deleting one study at a time and combining the effect values of the remaining studies. Funnel plots with Begg’s tests and Egger’s tests were used to assess publication bias. All statistical tests were two-sided; P < 0.05 was considered statistically significant except for the evaluation of heterogeneity (P < 0.1). All meta‐analyses were performed using Stata v12.0 (StataCorp, College Station, TX, USA).
Results
Study Selection
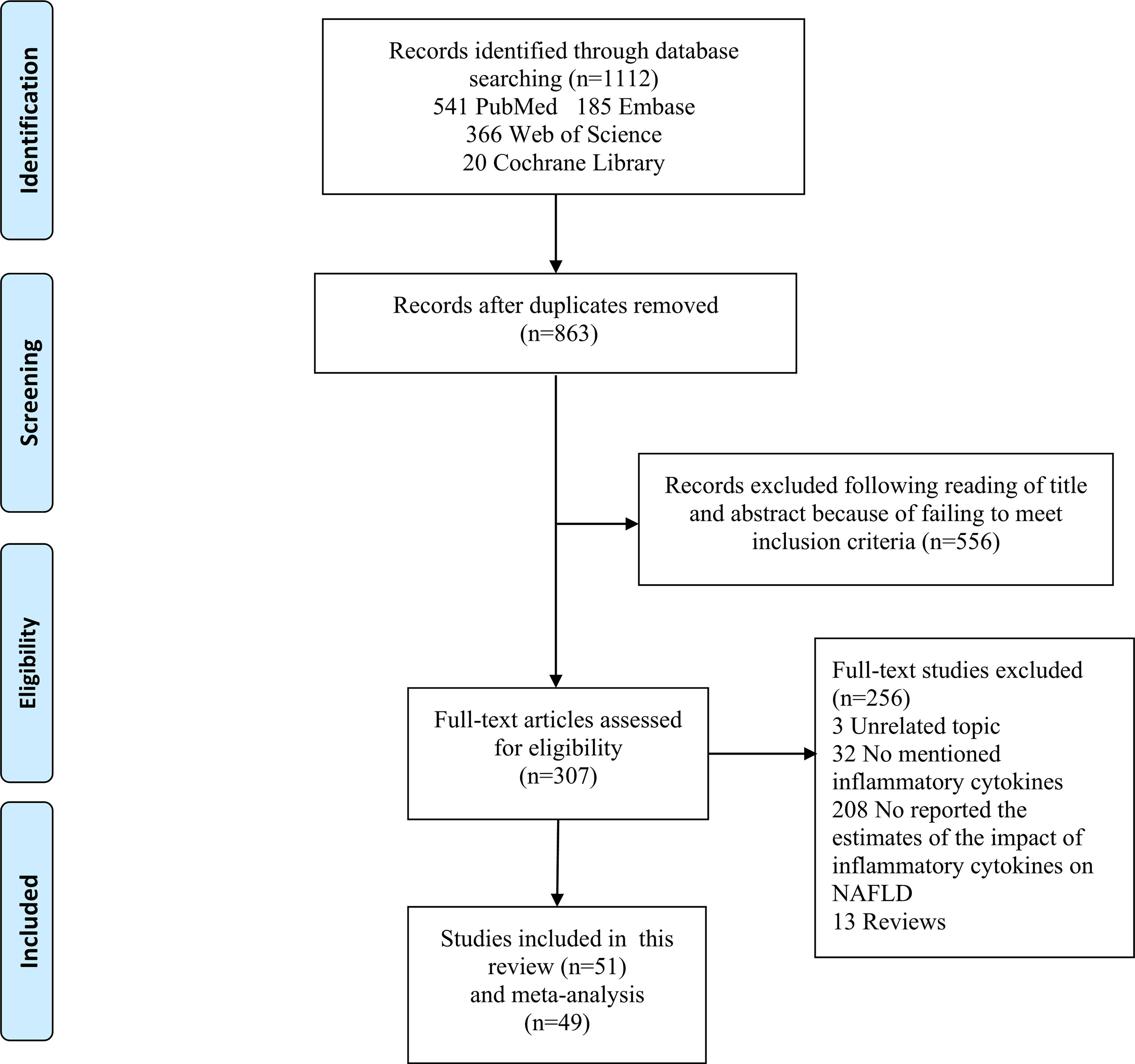
Figure 1 shows the flow chart of study selection. The search strategy identified a total of 1,112 articles in the databases and 863 studies remained after excluding duplicated articles. Title and abstract screening excluded 556 articles. Full texts of the remaining 307 articles were reviewed according to the selection criteria, and this process excluded 256 articles. The remaining 51 studies (22–72) were included in this study, and 49 of them were included in the meta‐analyses.
Study Characteristics
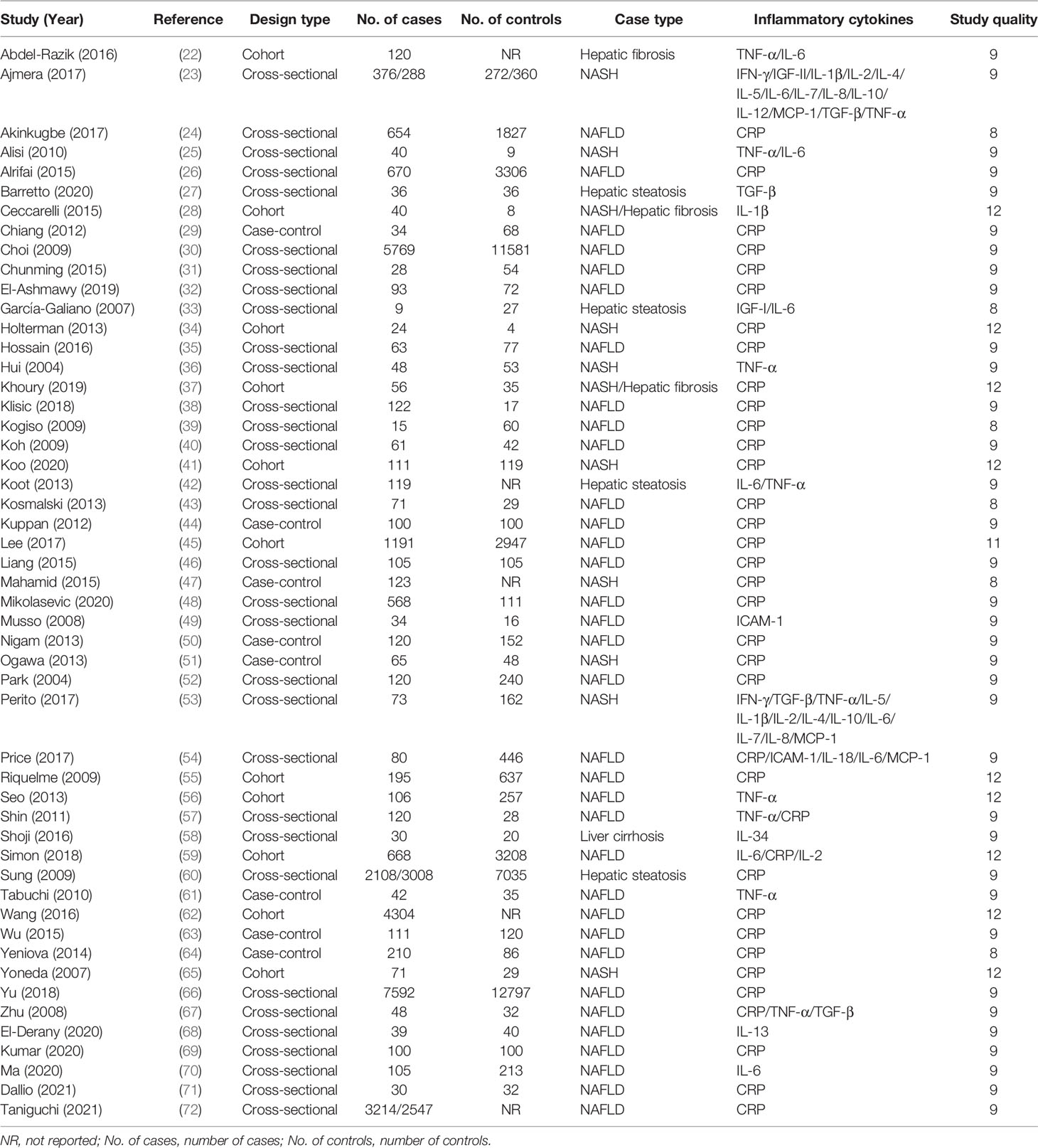
Characteristics of the 51 eligible studies are summarized in Table 1 and Supplementary Table 3–5. All the studies were published between 1960 and 2021, with 32 cross‐sectional studies, eight case‐control studies, and 11 cohort studies. A total of 36,074 NAFLD patients and 47,052 controls were included in these studies. Of these 51 studies, 49 studied on CRP, six on IL‐1β, five on interleukin-2 (IL-2), four on interleukin-4 (IL-4), four on interleukin-5 (IL-5), 12 on IL‐6, four on interleukin-7 (IL-7), four on interleukin-8 (IL-8), four on interleukin-10 (IL-10), three on interleukin-12 (IL-12), 11 on TNF-α, 10 on transforming growth factor-β (TGF-β), six on monocyte chemoattractant protein-1(MCP-1), four on interferon-γ (IFN-γ), four on insulin-like growth factor (IGF-II), and four on intercellular adhesion molecule-1 (ICAM-1). Sources of inflammatory cytokines included serum samples in 28 studies, plasma samples in 11 studies, and whole blood samples in six studies, while the source of inflammatory cytokines in six studies was unclear. 22 of these studies used an enzyme‐linked immunosorbent assay (ELISA) to detect the concentrations of inflammatory cytokines, and 29 studies used other methods, such as Luminex Multiplex, Cytokine Antibody Array, Turbidimetric inhibition immunoassay, and Latex agglutination assay. For the quality score, 51 studies scored between 8 and 14 with an average score of 9.45.
Association Between Inflammatory Cytokines and NAFLD
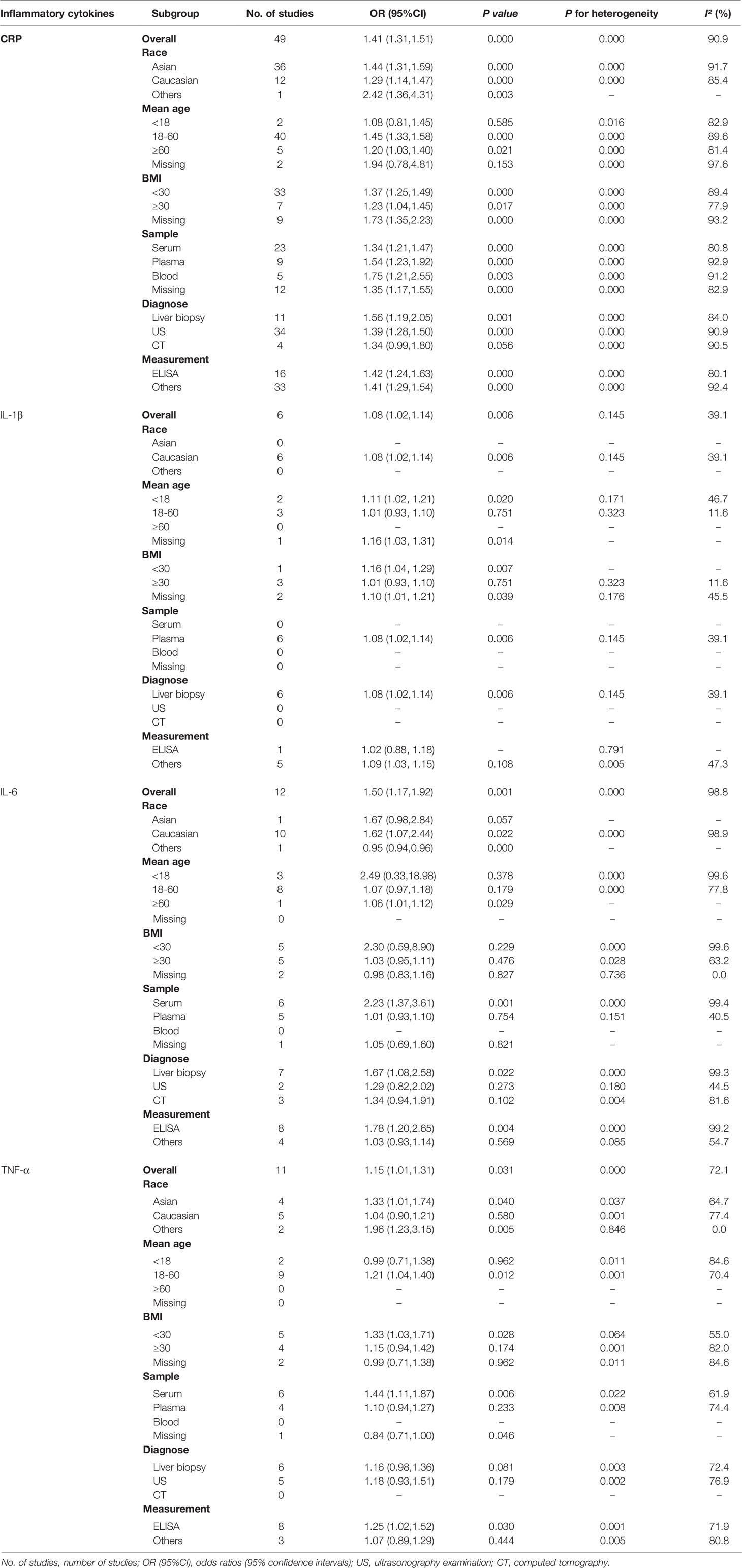
The meta-analyses were conducted for 16 inflammatory cytokines, and three inflammatory cytokines, including insulin-like growth factor I (IGF-I), interleukin-13 (IL-13), and interleukin-34 (IL-34), were excluded as they were only reported by one study. We found significant associations between CRP (OR, 1.41; 95% CI, 1.31‐1.51; P<0.001) (Figure 2A), IL-1β (OR, 1.08; 95% CI, 1.02‐1.14; P=0.006) (Figure 3A), IL-6 (OR, 1.50; 95% CI, 1.17‐1.92; P=0.001) (Figure 3B), TNF-α (OR, 1.15; 95% CI, 1.01‐1.31; P=0.031) (Figure 3C), and ICAM‐1 (OR, 2.17; 95% CI, 1.22‐3.85; P=0.008) (Figure 3D) and NAFLD. We did not find significant associations between IFN-γ, IGF-II, IL‐2, IL‐4, IL‐5, IL‐7, IL‐8, IL‐10, IL‐12, MCP‐1, and TGF-β and NAFLD (Supplementary Figures 1–11).

Figure 2 The forest plots of the association between CRP and NAFLD and subtypes of NAFLD. (A), Association between CRP and NAFLD. (B), Association between CRP and subtypes of NAFLD.
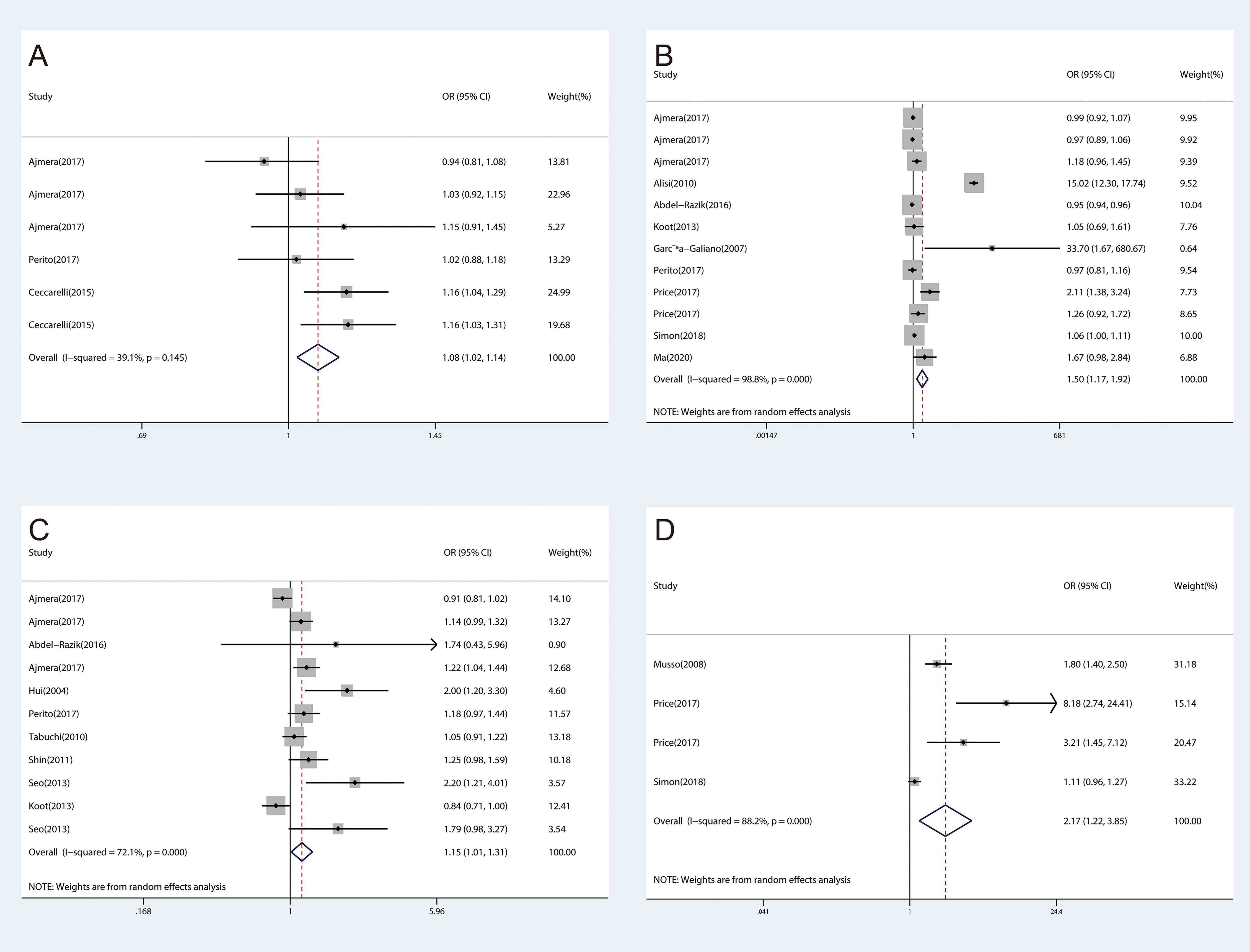
Figure 3 The forest plots of the association between inflammatory cytokines and NAFLD. (A), Association between IL-1β and NAFLD. (B), Association between IL-6 and NAFLD. (C), Association between TNF‐α and NAFLD. (D), Association between ICAM-1 and NAFLD.
To further investigate the association between inflammatory cytokines and NAFLD, subgroup analyses were performed by race, mean age, BMI, sample source, diagnostic methods for NAFLD, and the measurements of inflammatory cytokines. Subgroup analyses showed that NAFLD was significantly associated with CRP, TNF-α, and TGF-β in the Asian population. CRP, IL-1β, IL-6, and ICAM-1 were significantly associated with NAFLD in the Caucasian population. When the studies were stratified by mean age, the results showed that the association between IL-1β and NAFLD was statistically significant among studies subjects with age < 18 years. In the 18-60 years group, CRP, TNF-α, TGF-β, and ICAM-1 were significantly associated with NAFLD. In the age ≥ 60 years group, CRP and IL-6 were significantly associated with NAFLD. Besides, CRP, IL-1β, TNF-α and ICAM-1 were associated with NAFLD in the group with BMI < 30. CRP and TGF-β were associated with NAFLD in the group with BMI ≥ 30 (Table 2 and Supplementary Table 6).
Additionally, based on the sample source, the results showed that the associations for CRP, IL-6, TNF-α, TGF‐β, and ICAM-1 with NAFLD were statistically significant among studies in serum samples. Studies using plasma samples suggested significant associations for CRP and IL-1β with NAFLD. Five studies using blood samples showed that CRP was significantly associated with NAFLD. In the subgroup analyses of diagnostic methods for NAFLD and measuring methods for inflammatory cytokines, CRP, IL-1β, and IL-6 were associated with NAFLD among studies with diagnosis by liver biopsy, and CRP, IL-6, and TNF-α were associated with NAFLD among studies with measurement by ELISA (Table 2 and Supplementary Table 6).
Significant heterogeneity was observed in studies investigating the inflammatory cytokines mentioned above (CRP, IL‐6, IL‐8, TNF‐α, TGF‐β, MCP‐1, IGF-II, and ICAM-1), with I2 above 50%, mainly attributed to patient BMI, sample source, and diagnostic methods for NAFLD. In the subgroup analysis of IL‐6, heterogeneity was reduced in the subgroups of the missing BMI group (I2 = 0.0%, P = 0.736), studies that examined the plasma samples (I2 = 40.5%, P = 0.151) and diagnosing NAFLD by ultrasonography examination (I2 = 44.5%, P = 0.180). With regard to TGF‐β, heterogeneity was reduced in the BMI ≥ 30 group (I2 = 0.0%, P = 0.934). Moreover, heterogeneity was reduced in the serum samples (I2 = 0.0%, P = 0.480) about MCP-1.
Association Between Inflammatory Cytokines and Subtypes of NAFLD
Considering NAFLD consists of different clinical subtypes, we performed subgroup analyses by classification of NAFLD as hepatic steatosis, NASH, and hepatic fibrosis. The association between inflammatory cytokines and NAFLD varied among different subtypes of NAFLD. We found significant associations for CRP with NASH (OR, 1.60; 95% CI, 1.17‐2.19; P=0.003) and hepatic fibrosis (OR, 1.18; 95% CI, 1.06‐1.32; P=0.003), which were consistent with overall effect (Figure 2B). The same associations were found in IL-1β (Figure 4A) and TNF-α (Figure 4C). However, no significant associations were found for IL-6 with NASH and hepatic fibrosis (Figure 4B). Besides, we did not find any association between CRP (Figure 2B), IL-1β (Figure 4A), and IL-6 (Figure 4B) and hepatic steatosis with pooled ORs of 1.27 (95% CI, 0.87‐1.86; P=0.206), 0.94 (95% CI, 0.81‐1.09; P=0.399), and 1.09 (95% CI, 0.71‐1.67; P=0.707), respectively.
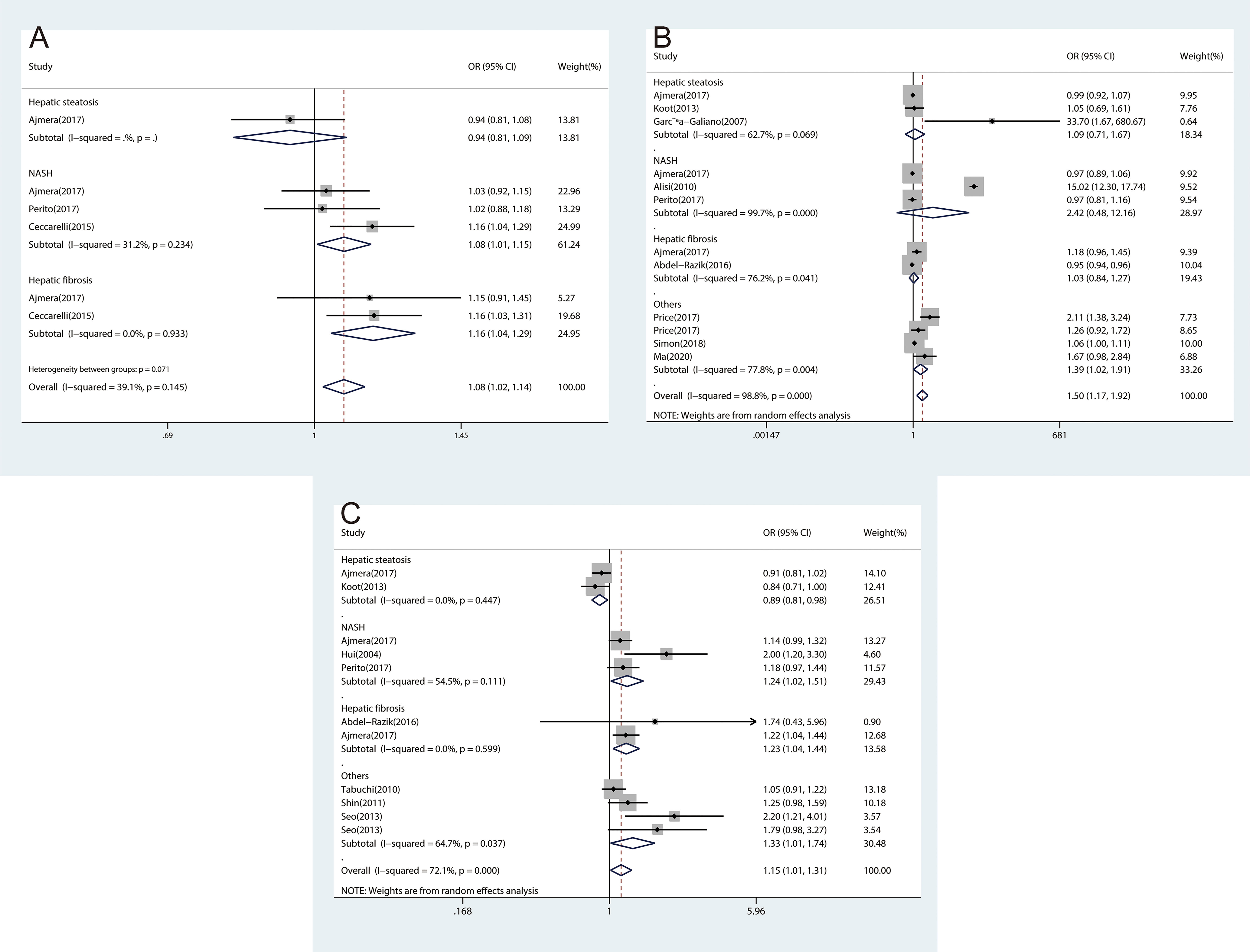
Figure 4 The forest plots of the association between inflammatory cytokines and subtypes of NAFLD. (A), Association between IL-1β and subtypes of NAFLD. (B), Association between IL-6 and subtypes of NAFLD. (C), Association between TNF‐α and subtypes of NAFLD.
Sensitivity Analysis and Publication Bias
We conducted the sensitivity analysis and examined the publication bias of the meta‐analysis on the association between inflammatory cytokines and NAFLD. No individual study markedly affected the pooled OR based on the sensitivity analysis, which indicated that the estimated OR was stable and reliable. Publication bias was assessed using funnel plots, Begg’s tests, and Egger’s tests. There was no significant publication bias detected (Supplementary Figure 12).
Discussion
In this study, 51 studies focusing on the association between inflammatory cytokines and NAFLD were selected and based on which meta‐analyses were conducted. We found significant associations for CRP, IL-1β, IL-6, TNF-α, and ICAM-1 with NAFLD. In contrast, the associations for IFN-γ, IGF-II, IL‐2, IL‐4, IL‐5, IL‐7, IL‐8, IL‐10, IL‐12, MCP‐1, and TGF-β with NAFLD were not significant. For different subtypes of NAFLD, we found significant associations of CRP, IL-1β, and TNF-α with NASH and hepatic fibrosis. And the associations of CRP, IL-1β, and IL-6 with hepatic steatosis were not significant. To the best of our knowledge, this meta‐analysis was the first to comprehensively evaluate the association between inflammatory cytokines and NAFLD.
It has been shown that the inflammatory cytokines played an important role in the development of NAFLD by activating various inflammatory pathways that interfered with insulin signalling (73). The inhibitor kappa B kinase beta/nuclear factor kappa B (IKK/NF-κB) pathway and the c-Jun N-terminal kinase/activator protein 1 (JNK/AP1) pathway were two well-known pathways (74, 75). Pro-inflammatory cytokines secreted by adipose tissue, such as TNF-α, IL-1β, and IL-6, were involved in the above pathways by activating intracellular kinases, and they also stimulated the production of CRP in the liver (74). Various inflammatory cytokines interacted and inhibited insulin signalling together (73). Figure 5 illustrates the mechanism of inflammatory cytokines and NLRP3 inflammasome in the development of NAFLD. Among the identified inflammatory cytokines associated with NAFLD, CRP is a classical non-specific acute phase protein produced by the liver (27). Previous study found that CRP can upregulate NF-κB activity, and the activated NF-κB can then join the pathway that interfered with insulin signalling (76). Consistent with the evidence described above, our findings suggested that CRP is closely associated with NAFLD.
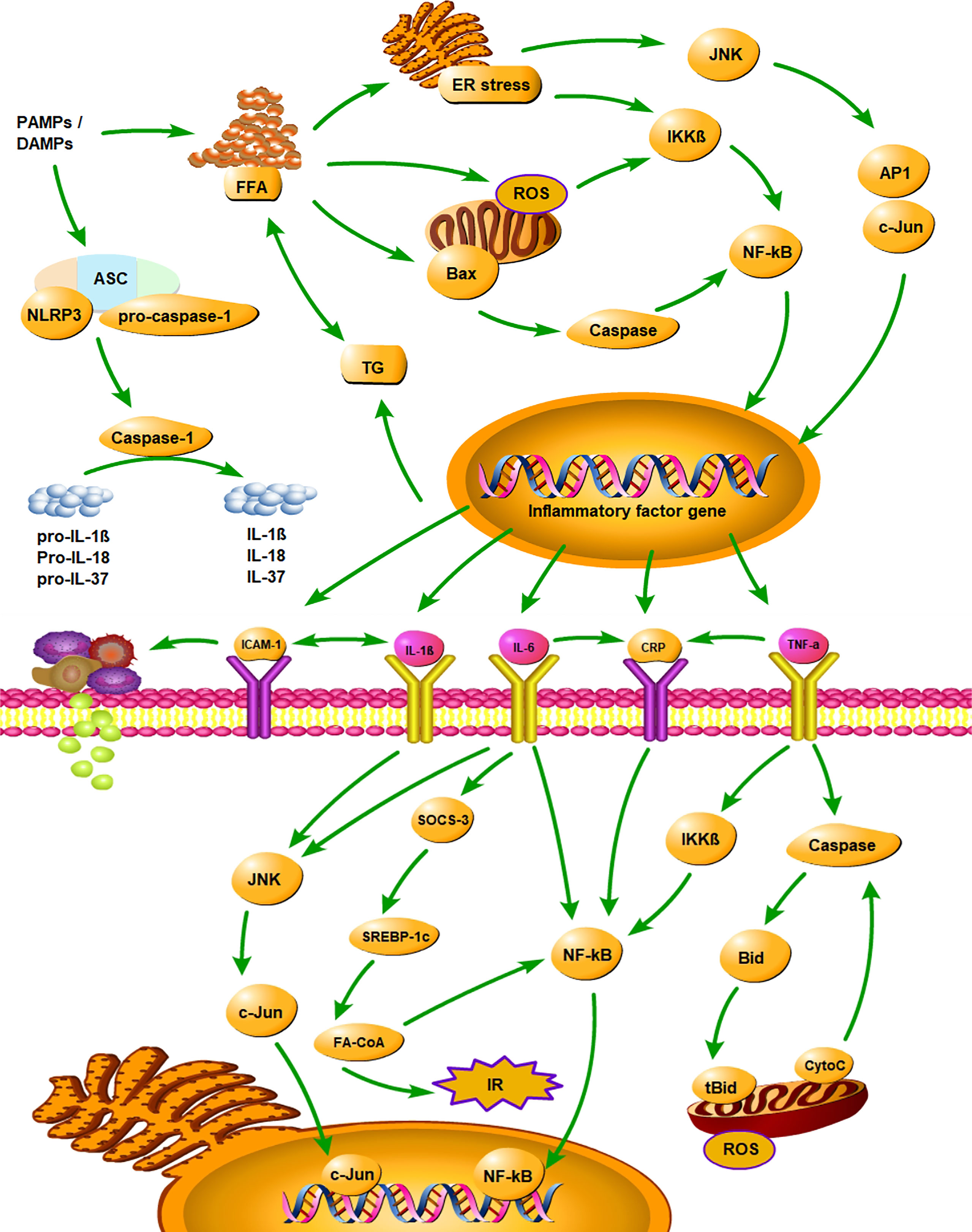
Figure 5 Summarizes the mechanism of inflammatory cytokines and NLRP3 inflammasome in the development of NAFLD. PAMPs, pathogen-associated molecular patterns; DAMPs, danger associated molecular patterns; ASC, apoptosis-associated speck-like protein containing a CARD; CARD, caspase recruitment domain; FFA, free fatty acid; TG, triglyceride; ER stress, endoplasmic reticulum stress; ROS, reactive oxygen Species; IR, insulin resistance; IKKβ, inhibitor kappa B kinase beta; NF-κB, nuclear factor κB; JNK, c-Jun N-terminal kinase; AP1, activator protein 1; SOCS-3, suppressor of cytokine signalling-3; SREBP-1c, sterol regulatory element binding protein-1c; FA-CoA, fatty acyl-CoA; Bid, BH3 interacting-domain death agonist; tBid, truncated BH3 interacting-domain death agonist; CytoC, Cytochrome-C; CRP, C‐reactive protein; IL-1β, interleukin‐1β; IL-6, interleukin‐6; IL-18, interleukin‐18; IL-37, interleukin‐37; TNF-α, tumor necrosis factor‐α; ICAM‐1, intercellular adhesion molecule-1.
TNF-α is a pro-inflammatory cytokine, primarily produced by monocytes and macrophages (77). The release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, et al) and reactive oxygen species (ROS) occurred through the pathogen-associated molecular patterns (PAMPs)-toll-like receptors (TLRs)-JNK/NF-κB pathway (78). Hepatic steatosis can lead to an increase in the transcription factor NF-κβ signalling pathway through the upstream activation. Activation of NF-κβ induced production of TNF-α, which helped recruit and activate Kupffer cells to mediate inflammation in NASH (79, 80). Many pieces of evidence indicated a positive association between TNF-α and NAFLD (81). Moreover, studies have shown that TNF-α can be used as a predictor for the development of NAFLD (56, 82). Consistent with these studies (56, 81, 82), the results of the meta-analysis showed that TNF-α can increase the risk of NAFLD.
We also found that IL‐1β, IL‐6, and ICAM-1 were significantly associated with NAFLD, which indicated that these inflammatory cytokines can promote the development of NAFLD. The pro-inflammatory effect of IL-1β may be attributed to its synergistic effect with TLRs signalling, which significantly amplifies inflammation through lipopolysaccharide (LPS) - induced inflammatory mediator (83). The pro-inflammatory effect of IL-6 may be through the suppressor of cytokine signalling-3 (SOCS-3) - sterol regulatory element binding protein-1c (SREBP-1c)- fatty acyl-CoA (FA-CoA) pathway that inhibited insulin signalling and acted to regulate the acute stage response and chronic inflammation (84, 85). Previous research found that macrophages secreted IL-1β and IL-6, which promoted hepatocyte injury and liver fibrosis (18). It has been shown that ICAM-1 was expressed in adipose tissue in addition to lipid-containing hepatocytes and may be a marker of endothelial cell activation (54). In summary, CRP, IL-1β, IL-6, TNF-α, and ICAM-1 may be reliable biomarkers to describe the risk of NAFLD.
Our results showed that there were no significant associations between IFN-γ, IGF-II, IL‐2, IL‐4, IL‐5, IL‐7, IL‐8, IL‐10, IL‐12, MCP‐1, and TGF-β and NAFLD. The main reason for this result may be the limited number of included studies. Besides, the negative findings were strongly linked to multiple characteristics of these inflammatory cytokines involved in the progression of NAFLD. Notably, although the non‐significant associations were observed in the overall population, opposite associations were observed in subgroup analyses. These results may suggest that the true association between inflammatory cytokines and NAFLD was masked or diluted when analyzing the overall population. Therefore, further studies are necessary to establish the true association. Remarkably, the meta-analysis showed that the association between IL-10 and NAFLD reached borderline significance. Due to the limited number of included studies and previous findings, we still considered IL-10 to be an important anti-inflammatory cytokine. IL-10 may have a role by affecting the myeloid-differentiation factor 88 (MyD88)-TLRs-NF-κB signalling pathway (86). Previous research found that IL-10 was a negative regulator in inflammatory response, and it can inhibit the secretion of various inflammatory cytokines by T cells, including IL-2, IL-6, TNF-α, et al (87). Thus, we suggested that this anti-inflammatory cytokine has great potential to help the assessment of NAFLD.
Moreover, recent studies suggested that inflammasome is an intracellular multi-protein complex that is thought to be a trigger for inflammatory cytokines in NAFLD (Supplementary Table 7) (88). Although there are different types of inflammasomes, most studies have focused on NLRP3 inflammasomes in NAFLD. It is reported that the activation of NLRP3 inflammasome led to the maturation of caspase-1. On the one hand, caspase-1 may cleave inactive pro-IL-1β, pro-IL-18, and pro-IL-37 to mature IL-1β, IL-18, and IL-37 (17). Increased IL-1β may promote fibrosis directly and induce activation of NF-κB, which may regulate the synthesis of pro-IL-1β, MCP-1, and other inflammatory mediators, leading to a vicious cycle of pro-inflammatory signalling. On the other hand, caspase-1 may generate an N-terminal cleavage product (GSDMD-NT) by cleaving gasdermin-D (GSDMD) specifically, triggering pyroptosis and the release of inflammatory cytokines (89, 90). Therefore, NLRP3 inflammasome is closely associated with the function of inflammatory cytokines in NAFLD and may be a key point in the progression of hepatic steatosis to NASH and hepatic fibrosis.
This study has some potential limitations that should be considered. First, some inflammatory cytokines were only reported by fewer than three studies, thus more studies are needed in the future to confirm this result in different populations. Second, some of the included studies used ultrasonography examination to diagnose NAFLD, which may increase the risk of false positives compared to a diagnosis with the gold standard, i.e. liver biopsy. Finally, there were different cut-off values for inflammatory cytokines in the included studies, which may have increased heterogeneity among studies.
Conclusion
This review and meta-analysis indicated that increased CRP, IL-1β, IL-6, TNF-α, and ICAM-1 concentrations were significantly associated with increased risks of NAFLD. Furthermore, the results of subgroup analyses showed the characteristics of different inflammatory cytokines involved in the NAFLD progression progress. These inflammatory mediators may serve as biomarkers for the prediction of NAFLD. These findings may provide new insights into the aetiology of NAFLD as well as early diagnosis and intervention.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
DY, LuJ, PX and LM designed the study. DY and PX collected the data, and wrote the manuscript with support from other authors. XX and LiJ contributed to the statistical analysis and preparing the tables. LM, LuJ, DY, PX and BPL reviewed and revised the manuscript. All authors approved the final version of the article, including the authorship list.
Funding
This work was supported by the National Natural Science Foundation of China (81872641) and the Natural Science Foundation of Hunan Province (2021JJ30901).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.880298/full#supplementary-material
References
1. Powell EE, Wong VW, Rinella M. Non-Alcoholic Fatty Liver Disease. Lancet (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
2. Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis (2015) 35(3):221–35. doi: 10.1055/s-0035-1562943
3. Stols-Gonçalves D, Tristão LS, Henneman P, Nieuwdorp M. Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease. Curr Diabetes Rep (2019) 19(6):31. doi: 10.1007/s11892-019-1151-4
4. Pierantonelli I, Svegliati-Baroni G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation (2019) 103(1):e1–e13. doi: 10.1097/TP.0000000000002480
5. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat Med (2018) 24(7):908–22. doi: 10.1038/s41591-018-0104-9
6. Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-Alcoholic Steatohepatitis: A Review of Its Mechanism, Models and Medical Treatments. Front Pharmacol (2020) 11:603926. doi: 10.3389/fphar.2020.603926
7. Zhang TS, Qin HL, Wang T, Li HT, Li H, Xia SH, et al. Global Publication Trends and Research Hotspots of Nonalcoholic Fatty Liver Disease: A Bibliometric Analysis and Systematic Review. SpringerPlus (2015) 4:776. doi: 10.1186/s40064-015-1542-1
8. Auguet T, Bertran L, Binetti J, Aguilar C, Martínez S, Sabench F, et al. Relationship Between IL-8 Circulating Levels and TLR2 Hepatic Expression in Women With Morbid Obesity and Nonalcoholic Steatohepatitis. Int J Mol Sci (2020) 21(11):4189. doi: 10.3390/ijms21114189
9. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular Carcinoma is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology (2019) 69(1):107–20. doi: 10.1002/hep.30036
10. Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature (2012) 482(7384):179–85. doi: 10.1038/nature10809
11. Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, et al. Myeloid-Cell-Specific IL-6 Signaling Promotes Microrna-223-Enriched Exosome Production to Attenuate Nafld-Associated Fibrosis. Hepatology (2021) 74(1):116–32. doi: 10.1002/hep.31658
12. du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology (2015) 149(3):635–48.e14. doi: 10.1053/j.gastro.2015.05.044
13. Cabré N, Luciano-Mateo F, Fernández-Arroyo S, Baiges-Gayà G, Hernández-Aguilera A, Fibla M, et al. Laparoscopic Sleeve Gastrectomy Reverses non-Alcoholic Fatty Liver Disease Modulating Oxidative Stress and Inflammation. Metabol: Clin Exp (2019) 99:81–9. doi: 10.1016/j.metabol.2019.07.002
14. Viglino D, Jullian-Desayes I, Minoves M, Aron-Wisnewsky J, Leroy V, Zarski JP, et al. Nonalcoholic Fatty Liver Disease in Chronic Obstructive Pulmonary Disease. Eur Respir J (2017) 49(6):1601923. doi: 10.1183/13993003.01923-2016
15. Bessone F, Razori MV, Roma MG. Molecular Pathways of Nonalcoholic Fatty Liver Disease Development and Progression. Cell Mol Life Sci: CMLS (2019) 76(1):99–128. doi: 10.1007/s00018-018-2947-0
16. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Digest Dis Sci (2016) 61(5):1294–303. doi: 10.1007/s10620-016-4049-x
17. de Carvalho Ribeiro M, Szabo G. Role of the Inflammasome in Liver Disease. Annu Rev Pathol (2022) 17:345–65. doi: 10.1146/annurev-pathmechdis-032521-102529
18. Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology (2016) 150(8):1704–9. doi: 10.1053/j.gastro.2016.01.025
19. Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology (2020) 158(7):1851–64. doi: 10.1053/j.gastro.2020.01.052
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
21. Feng S, Shuxun H, Jialiang Z, Dongfeng R, Zheng C, Jiaguang T. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. PloS One (2014). doi: 10.1371/journal.pone.0111695.t001
22. Abdel-Razik A, Mousa N, Shabana W, Refaey M, ElMahdy Y, Elhelaly R, et al. A Novel Model Using Mean Platelet Volume and Neutrophil to Lymphocyte Ratio as a Marker of Nonalcoholic Steatohepatitis in NAFLD Patients: Multicentric Study. Eur J Gastroenterol Hepatol (2016) 28(1):e1–9. doi: 10.1097/MEG.0000000000000486
23. Ajmera V, Perito ER, Bass NM, Terrault NA, Yates KP, Gill R, et al. Novel Plasma Biomarkers Associated With Liver Disease Severity in Adults With Nonalcoholic Fatty Liver Disease. Hepatology (2017) 65(1):65–77. doi: 10.1002/hep.28776
24. Akinkugbe AA, Avery CL, Barritt AS, Cole SR, Lerch M, Mayerle J, et al. Do Genetic Markers of Inflammation Modify the Relationship Between Periodontitis and Nonalcoholic Fatty Liver Disease? Findings SHIP Study J Dent Res (2017) 96(12):1392–9. doi: 10.1177/0022034517720924
25. Alisi A, Manco M, Devito R, Piemonte F, Nobili V. Endotoxin and Plasminogen Activator Inhibitor-1 Serum Levels Associated With Nonalcoholic Steatohepatitis in Children. J Pediatr Gastroenterol Nutr (2010) 50(6):645–9. doi: 10.1097/MPG.0b013e3181c7bdf1
26. Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The Association of Nonalcoholic Fatty Liver Disease, Obesity, and Metabolic Syndrome, With Systemic Inflammation and Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (Mesa). Atherosclerosis (2015) 239(2):629–33. doi: 10.1016/j.atherosclerosis.2015.02.011
27. Barretto JR, Boa-Sorte N, Vinhaes CL, Malta-Santos H, Reboucas-Silva J, Ramos CF, et al. Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-Beta) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study. Nutrients (2020) 12(6):1650. doi: 10.3390/nu12061650
28. Ceccarelli S, Panera N, Mina M, Gnani D, De Stefanis C, Crudele A, et al. LPS-Induced TNF-α Factor Mediates Pro-Inflammatory and Pro-Fibrogenic Pattern in non-Alcoholic Fatty Liver Disease. Oncotarget (2015) 6(39):41434–52. doi: 10.18632/oncotarget.5163
29. Chiang CH, Huang PH, Chung FP, Chen ZY, Leu HB, Huang CC, et al. Decreased Circulating Endothelial Progenitor Cell Levels and Function in Patients With Nonalcoholic Fatty Liver Disease. PloS One (2012) 7(2):e31799. doi: 10.1371/journal.pone.0031799
30. Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, et al. The Relation Between non-Alcoholic Fatty Liver Disease and the Risk of Coronary Heart Disease in Koreans. Am J Gastroenterol (2009) 104(8):1953–60. doi: 10.1038/ajg.2009.238
31. Chunming L, Jianhui S, Hongguang Z, Chunwu Q, Xiaoyun H, Lijun Y, et al. The Development of a Clinical Score for the Prediction of Nonalcoholic Steatohepatitis in Patients With Nonalcoholic Fatty Liver Disease Using Routine Parameters. Turk J Gastroenterol (2015) 26(5):408–16. doi: 10.5152/tjg.2015.6336
32. El-Ashmawy HM, Ahmed AM. Serum Fetuin-B Level is an Independent Marker for Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes. Eur J Gastroenterol Hepatol (2019) 31(7):859–64. doi: 10.1097/MEG.0000000000001354
33. García-Galiano D, Sánchez-Garrido MA, Espejo I, Montero JL, Costán G, Marchal T, et al. IL-6 and IGF-1 are Independent Prognostic Factors of Liver Steatosis and non-Alcoholic Steatohepatitis in Morbidly Obese Patients. Obes Surg (2007) 17(4):493–503. doi: 10.1007/s11695-007-9087-1
34. Holterman AX, Guzman G, Fantuzzi G, Wang H, Aigner K, Browne A, et al. Nonalcoholic Fatty Liver Disease in Severely Obese Adolescent and Adult Patients. Obes (Silver Spring) (2013) 21(3):591–7. doi: 10.1002/oby.20174
35. Hossain IA, Akter S, Bhuiyan FR, Shah MR, Rahman MK, Ali L. Subclinical Inflammation in Relation to Insulin Resistance in Prediabetic Subjects With Nonalcoholic Fatty Liver Disease. BMC Res Notes (2016) 9:266. doi: 10.1186/s13104-016-2071-x
36. Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond Insulin Resistance in NASH: TNF-Alpha or Adiponectin? Hepatology (2004) 40(1):46–54. doi: 10.1002/hep.20280
37. Khoury T, Mari A, Nseir W, Kadah A, Sbeit W, Mahamid M. Neutrophil-to-Lymphocyte Ratio is Independently Associated With Inflammatory Activity and Fibrosis Grade in Nonalcoholic Fatty Liver Disease. Eur J Gastroenterol Hepatol (2019) 31(9):1110–5. doi: 10.1097/MEG.0000000000001393
38. Klisic A, Isakovic A, Kocic G, Kavaric N, Jovanovic M, Zvrko E, et al. Relationship Between Oxidative Stress, Inflammation and Dyslipidemia With Fatty Liver Index in Patients With Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes (2018) 126(6):371–8. doi: 10.1055/s-0043-118667
39. Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-Sensitivity C-reactive Protein as a Serum Predictor of Nonalcoholic Fatty Liver Disease Based on the Akaike Information Criterion Scoring System in the General Japanese Population. J Gastroenterol (2009) 44(4):313–21. doi: 10.1007/s00535-009-0002-5
40. Koh JH, Shin YG, Nam SM, Lee MY, Chung CH, Shin JY. Serum Adipocyte Fatty Acid-Binding Protein Levels are Associated With Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Patients. Diabetes Care (2009) 32(1):147–52. doi: 10.2337/dc08-1379
41. Koo BK, Joo SK, Kim D, Lee S, Bae JM, Park JH, et al. Development and Validation of a Scoring System, Based on Genetic and Clinical Factors, to Determine Risk of Steatohepatitis in Asian Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol (2020) 18(11):2592–9.e10. doi: 10.1016/j.cgh.2020.02.011
42. Koot BG, van der Baan-Slootweg OH, Bohte AE, Nederveen AJ, van Werven JR, Tamminga-Smeulders CL, et al. Accuracy of Prediction Scores and Novel Biomarkers for Predicting Nonalcoholic Fatty Liver Disease in Obese Children. Obes (Silver Spring) (2013) 21(3):583–90. doi: 10.1002/oby.20173
43. Kosmalski M, Kasznicki J, Drzewoski J. Relationship Between Ultrasound Features of Nonalcoholic Fatty Liver Disease and Cardiometabolic Risk Factors in Patients With Newly Diagnosed Type 2 Diabetes. Polskie Archiwum Medycyny Wewnetrznej (2013) 123(9):436–42. doi: 10.20452/pamw.1919
44. Kuppan G, Anjana RM, Deepa M, Paramasivam P, Chandrakumar S, Kaliyaperumal V, et al. Inflammatory Markers in Relation to Nonalcoholic Fatty Liver Disease in Urban South Indians. Diabetes Technol Ther (2012) 14(2):152–8. doi: 10.1089/dia.2011.0213
45. Lee J, Yoon K, Ryu S, Chang Y, Kim HR. High-Normal Levels of hs-CRP Predict the Development of non-Alcoholic Fatty Liver in Healthy Men. PloS One (2017) 12(2):e0172666. doi: 10.1371/journal.pone.0172666
46. Liang C-C, Ding Y-N, Ji W-J, Shi J, Yeer N-E. Risk Factors for Nonalcoholic Fatty Liver Disease in Uygur People in Urumqi. World Chin J Digestol (2015) 23(25):4094–100. doi: 10.11569/wcjd.v23.i25.4094
47. Mahamid M, Kalman P, Wengrover D. P1081: Hyperplastic Colonic Polyps Link to Nonalcoholic Steatohepatitis and Vitamin D Deficiency. J Hepatol (2015) 62:S754. doi: 10.1016/S0168-8278(15)31279-4
48. Mikolasevic I, Domislovic V, Turk Wensveen T, Delija B, Klapan M, Juric T, et al. Screening for Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus Using Transient Elastography - a Prospective, Cross Sectional Study. Eur J Intern Med (2020) 82:68–75. doi: 10.1016/j.ejim.2020.08.005
49. Musso G, Gambino R, Bo S, Uberti B, Biroli G, Pagano G, et al. Should Nonalcoholic Fatty Liver Disease be Included in the Definition of Metabolic Syndrome? A Cross-Sectional Comparison With Adult Treatment Panel III Criteria in Nonobese Nondiabetic Subjects. Diabetes Care (2008) 31(3):562–8. doi: 10.2337/dc07-1526
50. Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-Alcoholic Fatty Liver Disease is Closely Associated With Sub-Clinical Inflammation: A Case-Control Study on Asian Indians in North India. PloS One (2013) 8(1):e49286. doi: 10.1371/journal.pone.0049286
51. Ogawa Y, Imajo K, Yoneda M, Kessoku T, Tomeno W, Shinohara Y, et al. Soluble CD14 Levels Reflect Liver Inflammation in Patients With Nonalcoholic Steatohepatitis. PloS One (2013) 8(6):e65211. doi: 10.1371/journal.pone.0065211
52. Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, et al. Insulin Resistance and C-reactive Protein as Independent Risk Factors for non-Alcoholic Fatty Liver Disease in non-Obese Asian Men. J Gastroenterol Hepatol (2004) 19(6):694–8. doi: 10.1111/j.1440-1746.2004.03362.x
53. Perito ER, Ajmera V, Bass NM, Rosenthal P, Lavine JE, Schwimmer JB, et al. Association Between Cytokines and Liver Histology in Children With Nonalcoholic Fatty Liver Disease. Hepatol Commun (2017) 1(7):609–22. doi: 10.1002/hep4.1068
54. Price JC, Wang R, Seaberg EC, Budoff MJ, Kingsley LA, Palella FJ, et al. The Association of Inflammatory Markers With Nonalcoholic Fatty Liver Disease Differs by Human Immunodeficiency Virus Serostatus. Open Forum Infect Dis (2017) 4(3):ofx153. doi: 10.1093/ofid/ofx153
55. Riquelme A, Arrese M, Soza A, Morales A, Baudrand R, Perez-Ayuso RM, et al. Non-Alcoholic Fatty Liver Disease and its Association With Obesity, Insulin Resistance and Increased Serum Levels of C-reactive Protein in Hispanics. Liver Int (2009) 29(1):82–8. doi: 10.1111/j.1478-3231.2008.01823.x
56. Seo YY, Cho YK, Bae JC, Seo MH, Park SE, Rhee EJ, et al. Tumor Necrosis Factor-Alpha as a Predictor for the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Follow-Up Study. Endocrinol Metab (Seoul) (2013) 28(1):41–5. doi: 10.3803/EnM.2013.28.1.41
57. Shin JY, Kim SK, Lee MY, Kim HS, Ye BI, Shin YG, et al. Serum Sex Hormone-Binding Globulin Levels are Independently Associated With Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes. Diabetes Res Clin Pract (2011) 94(1):156–62. doi: 10.1016/j.diabres.2011.07.029
58. Shoji H, Yoshio S, Mano Y, Kumagai E, Sugiyama M, Korenaga M, et al. Interleukin-34 as a Fibroblast-Derived Marker of Liver Fibrosis in Patients With non-Alcoholic Fatty Liver Disease. Sci Rep (2016) 6:28814. doi: 10.1038/srep28814
59. Simon TG, Trejo MEP, McClelland R, Bradley R, Blaha MJ, Zeb I, et al. Circulating Interleukin-6 is a Biomarker for Coronary Atherosclerosis in Nonalcoholic Fatty Liver Disease: Results From the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol (2018) 259:198–204. doi: 10.1016/j.ijcard.2018.01.046
60. Sung KC, Ryan MC, Wilson AM. The Severity of Nonalcoholic Fatty Liver Disease is Associated With Increased Cardiovascular Risk in a Large Cohort of non-Obese Asian Subjects. Atherosclerosis (2009) 203(2):581–6. doi: 10.1016/j.atherosclerosis.2008.07.024
61. Tabuchi M, Tomioka K, Kawakami T, Murakami Y, Hiramatsu M, Itoshima T, et al. Serum Cytokeratin 18 M30 Antigen Level and its Correlation With Nutritional Parameters in Middle-Aged Japanese Males With Nonalcoholic Fatty Liver Disease (NAFLD). J Nutr Sci Vitaminol (2010) 56(5):271–8. doi: 10.3177/jnsv.56.271
62. Wang LR, Liu WY, Wu SJ, Zhu GQ, Lin YQ, Braddock M, et al. Parabolic Relationship Between Sex-Specific Serum High Sensitive C Reactive Protein and non-Alcoholic Fatty Liver Disease in Chinese Adults: A Large Population-Based Study. Oncotarget (2016) 7(12):14241–50. doi: 10.18632/oncotarget.7401
63. Wu PB, Deng YZ, Shu YX, Tan SY, Li M, Fang G. Increased Plasma CgA Levels Associated With Nonalcoholic Fatty Liver Disease. Turk J Gastroenterol (2015) 26(5):404–7. doi: 10.5152/tjg.2015.0075
64. Yeniova AO, Küçükazman M, Ata N, Dal K, Kefeli A, Başyiğit S, et al. High-Sensitivity C-reactive Protein is a Strong Predictor of non-Alcoholic Fatty Liver Disease. Hepato-Gastroenterology (2014) 61(130):422–5. doi: 10.5754/hge13916
65. Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, et al. High-Sensitivity C-reactive Protein is an Independent Clinical Feature of Nonalcoholic Steatohepatitis (NASH) and Also of the Severity of Fibrosis in NASH. J Gastroenterol (2007) 42(7):573–82. doi: 10.1007/s00535-007-2060-x
66. Yu YY, Cai JT, Song ZY, Tong YL, Wang JH. The Associations Among Helicobacter Pylori Infection, White Blood Cell Count and Nonalcoholic Fatty Liver Disease in a Large Chinese Population. Med (Baltimore) (2018) 97(46):e13271. doi: 10.1097/MD.0000000000013271
67. Zhu QX, Deng CS. Detection and Significance of Serum TNF-α, Tgf-β1 and hs-CRP in Patients With non-Alcoholic Fatty Liver Disease. World Chin J Digestol (2008) 16(34):3910–2. doi: 10.11569/wcjd.v16.i34.3910
68. El-Derany MO. Polymorphisms in Interleukin 13 Signaling and Interacting Genes Predict Advanced Fibrosis and Hepatocellular Carcinoma Development in Non-Alcoholic Steatohepatitis. Biology (2020) 9(4):75. doi: 10.3390/biology9040075
69. Kumar R, Porwal YC, Dev N, Kumar P, Chakravarthy S, Kumawat A. Association of High-Sensitivity C-reactive Protein (hs-CRP) With non-Alcoholic Fatty Liver Disease (NAFLD) in Asian Indians: A Cross-Sectional Study. J Family Med Primary Care (2020) 9(1):390–4. doi: 10.4103/jfmpc.jfmpc_887_19
70. Ma C, Liu Y, He S, Zeng J, Li P, Ma C, et al. Association Between Leukocyte Mitochondrial Dna Copy Number and Non-alcoholic Fatty Liver Disease in a Chinese Population Is Mediated by 8-Oxo-2’-Deoxyguanosine. Front Med (2020) 7:536. doi: 10.3389/fmed.2020.00536
71. Dallio M, Masarone M, Romeo M, Tuccillo C, Morisco F, Persico M, et al. Pnpla3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front Med (2021) 8:734847. doi: 10.3389/fmed.2021.734847
72. Taniguchi H, Iwasaki Y, Aimi M, Matsushita H. Relationship Between Fatty Liver and High-Sensitivity C-reactive Protein. United Eur Gastroenterol J (2021) 9(SUPPL 8):657–8. doi: 10.1002/ueg2.12144
73. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology (2019) 70(2):711–24. doi: 10.1002/hep.30429
74. Mu W, Cheng XF, Liu Y, Lv QZ, Liu GL, Zhang JG, et al. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front Pharmacol (2018) 9:1566. doi: 10.3389/fphar.2018.01566
75. Uemura H, Katsuura-Kamano S, Yamaguchi M, Bahari T, Ishizu M, Fujioka M, et al. Relationships of Serum High-Sensitivity C-reactive Protein and Body Size With Insulin Resistance in a Japanese Cohort. PloS One (2017) 12(6):e0178672. doi: 10.1371/journal.pone.0178672
76. Bian F, Yang XY, Xu G, Zheng T, Jin S. Crp-Induced NLRP3 Inflammasome Activation Increases LDL Transcytosis Across Endothelial Cells. Front Pharmacol (2019) 10:40. doi: 10.3389/fphar.2019.00040
77. McGeehan GM, Becherer JD, Bast RC Jr., Boyer CM, Champion B, Connolly KM, et al. Regulation of Tumour Necrosis Factor-Alpha Processing by a Metalloproteinase Inhibitor. Nature (1994) 370(6490):558–61. doi: 10.1038/370558a0
78. Ntandja Wandji LC, Gnemmi V, Mathurin P, Louvet A. Combined Alcoholic and non-Alcoholic Steatohepatitis. JHEP Rep: Innovation Hepatol (2020) 2(3):100101. doi: 10.1016/j.jhepr.2020.100101
79. Nagai H, Matsumaru K, Feng G, Kaplowitz N. Reduced Glutathione Depletion Causes Necrosis and Sensitization to Tumor Necrosis Factor-Alpha-Induced Apoptosis in Cultured Mouse Hepatocytes. Hepatology (2002) 36(1):55–64. doi: 10.1053/jhep.2002.33995
80. Huang J, Hao C, Li Z, Wang L, Jiang J, Tang W, et al. Nrf2 -617 C/A Polymorphism Impacts Proinflammatory Cytokine Levels, Survival, and Transplant-Related Mortality After Hematopoietic Stem Cell Transplantation in Adult Patients Receiving Busulfan-Based Conditioning Regimens. Front Pharmacol (2020) 11:563321. doi: 10.3389/fphar.2020.563321
81. Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and Probiotic Treatment of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Nutr Rev (2018) 76(11):822–39. doi: 10.1093/nutrit/nuy031
82. Ajmal MR, Yaccha M, Malik MA, Rabbani MU, Ahmad I, Isalm N, et al. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients of Cardiovascular Diseases and its Association With hs-CRP and TNF-α. Indian Heart J (2014) 66(6):574–9. doi: 10.1016/j.ihj.2014.08.006
83. Szabo G, Petrasek J. Inflammasome Activation and Function in Liver Disease. Nat Rev Gastroenterol Hepatol (2015) 12(7):387–400. doi: 10.1038/nrgastro.2015.94
84. Cobbina E, Akhlaghi F. Non-Alcoholic Fatty Liver Disease (NAFLD) - Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab Rev (2017) 49(2):197–211. doi: 10.1080/03602532.2017.1293683
85. Lima-Cabello E, García-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillón J, Lozano-Rodríguez T, Fernández-Bermejo M, et al. Enhanced Expression of Pro-Inflammatory Mediators and Liver X-receptor-regulated Lipogenic Genes in non-Alcoholic Fatty Liver Disease and Hepatitis C. Clin Sci (Lond Engl: 1979) (2011) 120(6):239–50. doi: 10.1042/CS20100387
86. Li H, Hu D, Fan H, Zhang Y, LeSage GD, Caudle Y, et al. β-Arrestin 2 Negatively Regulates Toll-like Receptor 4 (TLR4)-Triggered Inflammatory Signaling Via Targeting P38 MAPK and Interleukin 10. J Biol Chem (2014) 289(33):23075–85. doi: 10.1074/jbc.M114.591495
87. Holan V, Zajicova A, Javorkova E, Trosan P, Chudickova M, Pavlikova M, et al. Distinct Cytokines Balance the Development of Regulatory T Cells and interleukin-10-producing Regulatory B Cells. Immunology (2014) 141(4):577–86. doi: 10.1111/imm.12219
88. Wan X, Xu C, Yu C, Li Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can J Gastroenterol Hepatol (2016) 2016:6489012. doi: 10.1155/2016/6489012
89. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-Activated Gasdermin D Causes Pyroptosis by Forming Membrane Pores. Nature (2016) 535(7610):153–8. doi: 10.1038/nature18629
Keywords: inflammatory cytokines, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, hepatic steatosis, hepatic fibrosis
Citation: Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL and Luo M (2022) Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 13:880298. doi: 10.3389/fimmu.2022.880298
Received: 21 February 2022; Accepted: 11 April 2022;
Published: 06 May 2022.
Edited by:
Sadiq Umar, University of Illinois at Chicago, United StatesReviewed by:
Mohammad Faraz Zafeer, University of Miami, United StatesMohd Salman, University of Tennessee Health Science Center (UTHSC), United States
Copyright © 2022 Duan, Pan, Luo, Xiao, Li, Bestman and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miyang Luo, miyangluo@csu.edu.cn
†These authors have contributed equally to this work and share the first authorship
 Yamei Duan1†
Yamei Duan1† Jingya Li
Jingya Li Miyang Luo
Miyang Luo