- 1Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Cell Biology, 2011 Collaborative Innovation Center of Tianjin for Medical Epigenetics, Tianjin Key Laboratory of Medical Epigenetics, Tianjin Medical University, Tianjin, China
- 3Department of Neurosurgery, Tianjin Medical University General Hospital and Laboratory of Neuro-Oncology, Tianjin Neurological Institute, Tianjin, China
Glioblastoma Multiforme (GBM) is the most common and aggressive form of intracranial tumors with poor prognosis. In recent years, tumor immunotherapy has been an attractive strategy for a variety of tumors. Currently, most immunotherapies take advantage of the adaptive anti-tumor immunity, such as cytotoxic T cells. However, the predominant accumulation of tumor-associated microglia/macrophages (TAMs) results in limited success of these strategies in the glioblastoma. To improve the immunotherapeutic efficacy for GBM, it is detrimental to understand the role of TAM in glioblastoma immunosuppressive microenvironment. In this review, we will discuss the roles of CD47-SIRPα axis in TAMs infiltration and activities and the promising effects of targeting this axis on the activation of both innate and adaptive antitumor immunity in glioblastoma.
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults and is characterized by invasive growth and frequent recurrence. Despite of advances in surgical resection, radiotherapy, and chemotherapy, the median survival time of patients is only 12 to 15 months; the 3-year survival rate is approximately 10% (1, 2). Great progress has been made in the development of immunotherapy for extracranial tumors. However, most clinical trials of immunotherapy for GBM have shown only a moderate response and no significant improvement in over survival (OS) (3).
Currently, immunotherapy for GBM includes immune checkpoint blockade therapy, vaccination therapy, oncolytic virus therapy, and CAR-T therapy (4–6), which mainly take advantage of the adaptive anti-tumor immunity (Figure 1). Accumulating evidence suggests that the GBM microenvironment is characterized by high myeloid cell content, relatively few tumor-infiltrating lymphocytes (TILs) (7, 8)and T cell dysfunction (9). In contrast, tumor-associated microglia/macrophages (TAMs) account for 30% to 40% in GBM (10, 11). Approximately 85% of them are bone marrow-derived infiltrating macrophages/monocytes while the remaining fractions are locally resident microglia (12, 13), which engage in reciprocal interactions with GBM and adaptive immune cell to mediate tumor immune escape (14–16), promote tumor growth and progression (17–21). Therefore, reeducating, reactivating, and reconstructing the TAMs functions in GBM immunosuppressive microenvironment makes them superior again is a promising field.
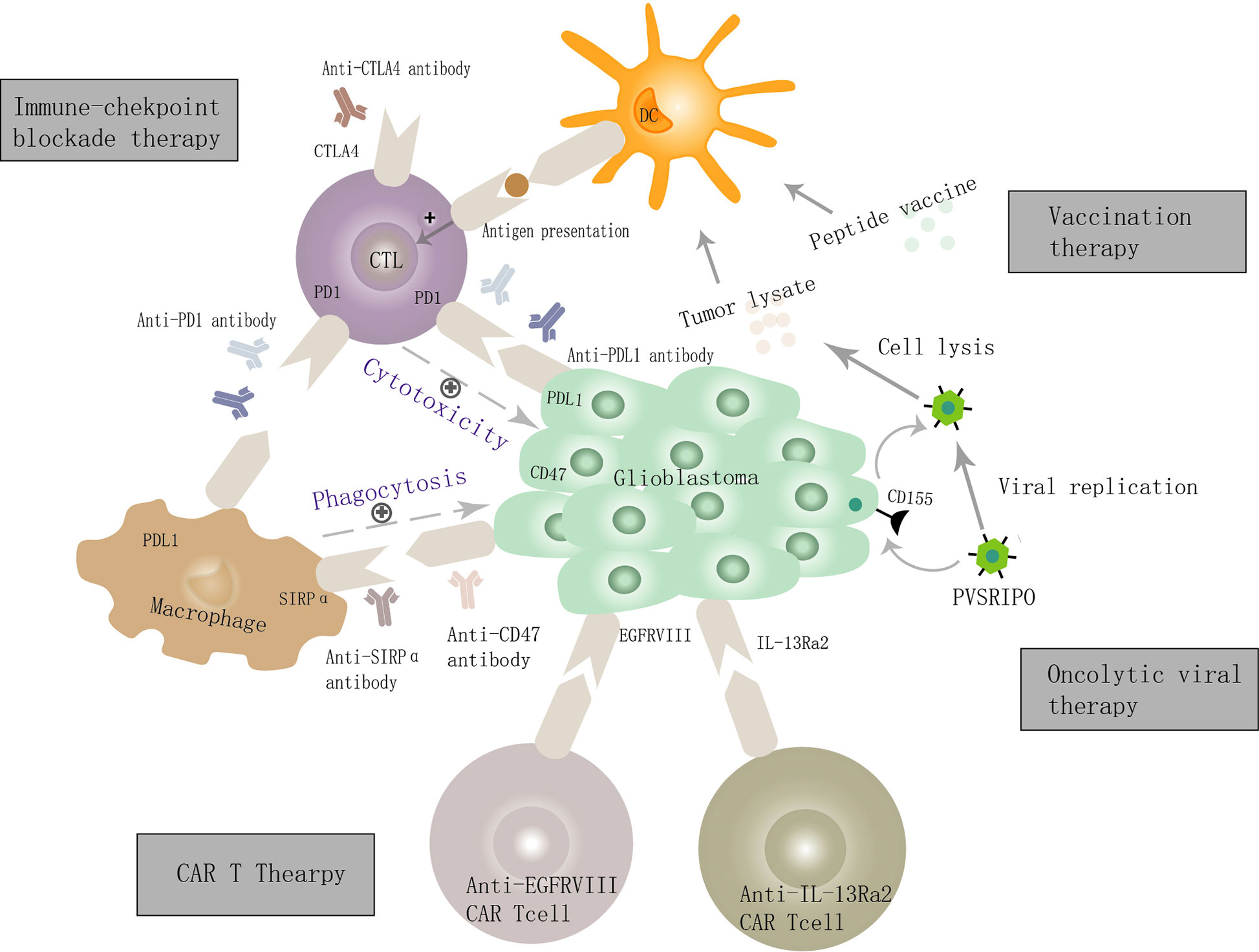
Figure 1 Cellular and molecular mechanisms of GBM immunotherapy. GBM cells overexpress PDL1, CD47, and other immunosuppressive molecules and bind the ligands present on cytotoxic T lymphocytes (CTLs) and macrophage, and thereby inhibit the innate and adaptive immune function, leading to the immune escape of GBM. Targeting immune checkpoint molecules such as PDL1, CD47, and CTLA4 can activate both innate and adaptive anti-tumor immunity. The mechanism of oncolytic virus therapy is mainly via the creation of viruses that can selectively infect GBM cells, defeat GBM cells, and enhance adaptive anti-tumor immune responses by the dendritic cell and CTL. Several tumor-related antigens (e.g., IL-13Ra2, EGFRvIII) are expressed on the surface of GBM cells and are used as specific targets for (CAR) T cell therapy to achieve a precise treatment objective. The vaccination strategy mainly mediates the activation of CTLs by antigen-presenting cells, thus killing GBM cells.
The strategies targeting TAMs fall into three main groups: 1) inhibiting recruitment of the bone marrow-derived infiltrating macrophages/monocytes (22–24); 2) promoting phagocytosis of tumor cells by TAMs and restoring its innate antitumor immunity (25, 26); 3) reprogramming TAMs to antitumor macrophages/microglial either directly through tumor cell killing or by reactivating adaptive antitumor immunity (27–30). The CD47-SIRPα Axis is currently the most widely studied innate immune checkpoint (31). Interestingly, the accumulating data shows that target the CD47- SIRPα axis bridging innate and adaptive antitumor immunity (15, 32). Targeting the CD47- SIRPα axis activates both innate and adaptive antitumor immunity (33), which is promising for GBM therapies. This review will discuss in more detail about the structure and regulation of innate immune checkpoint CD47-SIRPα and their functions in the immune-suppressive microenvironment and therapeutic potential in GBM. We would like to raise awareness of immune parameters in clinical stratification schemes and encourage discussions and improvements about innate anti-tumor immunity-oriented immunotherapies.
Structure of CD47-SIRPα
The CD47 gene is located on chromosome 3q13 and encodes an integrin-associated protein. CD47 is an important “self-labeling” molecule in the immunoglobulin superfamily that contains an immunoglobulin variable-like amino-terminal domain, five transmembrane domains, and one carboxy-terminal intracellular tail (34, 35). Signal regulatory proteins (SIRPs) are inhibitory immune receptors encoded by a cluster of genes on chromosome 20p13, including SIRPα, SIRPβ1, SIRPγ, SIRPβ2, and SIRPδ (36). SIRPα binds to CD47 with high-affinity (37). Structurally, the extracellular domain of SIRPα consists of three immunoglobulins (Ig)-like domains (the NH2-terminal V-like domain and two C1 domains), a single transmembrane segment, and the intracellular segment containing four tyrosine residues that form two typical immune-receptor tyrosine-based inhibition motifs (ITIMs). When CD47 expressed on the surface of GBM cells binds to the NH2-terminal V-like domain of SIRPα on myeloid cells, phosphorylation of the tyrosine residue in the ITIM motif results in the recruitment and activation of tyrosine phosphatase SHP1/SHP2. This process affects the levels of downstream de-phosphorylated molecules and inhibits the phagocytosis of GBM cells by macrophages (38). Hence CD47 serves as a critical “do not eat me” signal. However, the signaling mechanisms upstream and downstream of the CD47-SIRPα axis are incompletely understood.
Expression and Regulation of CD47-SIRPα AXIS
CD47 has been found to be highly expressed in GBM cells, especially glioblastoma stem cells (39). Its expression levels are positively correlated with glioma grade and are associated with worse clinical outcomes (39–41). Hence It has been regarded as a critical biomarker for glioblastoma (42). Amounting studies have demonstrated that MYC (43), PKM2-β-catenin-BRG1-TCF4 complex (44), NF-Kβ (45), and NRF1 (46) may bind at the promoter of CD47 to regulate its transcription. SIRPα is expressed on myeloid cells, including macrophages, dendritic cells (DCs), neutrophils, and nerve cells (neurons, microglia) (36). Interestingly, SIRPγ is expressed on human activated T cells and also binds to CD47, albeit with a lower affinity than SIRPa (31), which may also play a pivotal role in the adaptive antitumor immunity. More comprehensive research into the dynamic control of the CD47-SIRP axis will be greatly helpful for us to understand its functions and optimize its targeting strategies.
The Functions of The CD47-SIRPα AXIS in Glioblastoma
The exact functions of CD47 in GBM are still in debate. The increased expression of CD47 were found to promote the proliferation and invasion of GBM cells while it did not affect the proliferation ability of normal astrocytes (47, 48). However, some other studies found that CD47 could enhance the invasion ability of GBM cells through the PI3K/AKT pathway but had no effect on proliferation (49). Moreover, CD47 positive GBM cells possessed many characteristics that associate with cancer stem cells, which implies worse clinical outcomes (50). Accumulating evidence suggests that CD47 binds SIRPα on macrophages, neutrophils, and dendritic cells, subsequently inhibiting the cytotoxicity of macrophages and neutrophils, limiting the antigen-presenting function of dendritic cells, and inhibiting both innate and adaptive immune functions (38, 50, 51).
The Significance of Targeting CD47-SIRPα AXIS in The GBM Microenvironment
Targeting the innate immune checkpoint CD47-SIRPα axis enhances the phagocytosis rate, resulting in a significant survival benefit even in the absence of peripheral macrophages (52). Therefore, when studying the effects of CD47-SIRPα immunological checkpoint inhibitors on the phagocytic function of macrophages in vitro, their impact on microglia function must be considered. Targeting the innate immunity checkpoint CD47-SIRPα axis exerts anti-GBM efficacy mainly through the following four pathways (Figure 2).
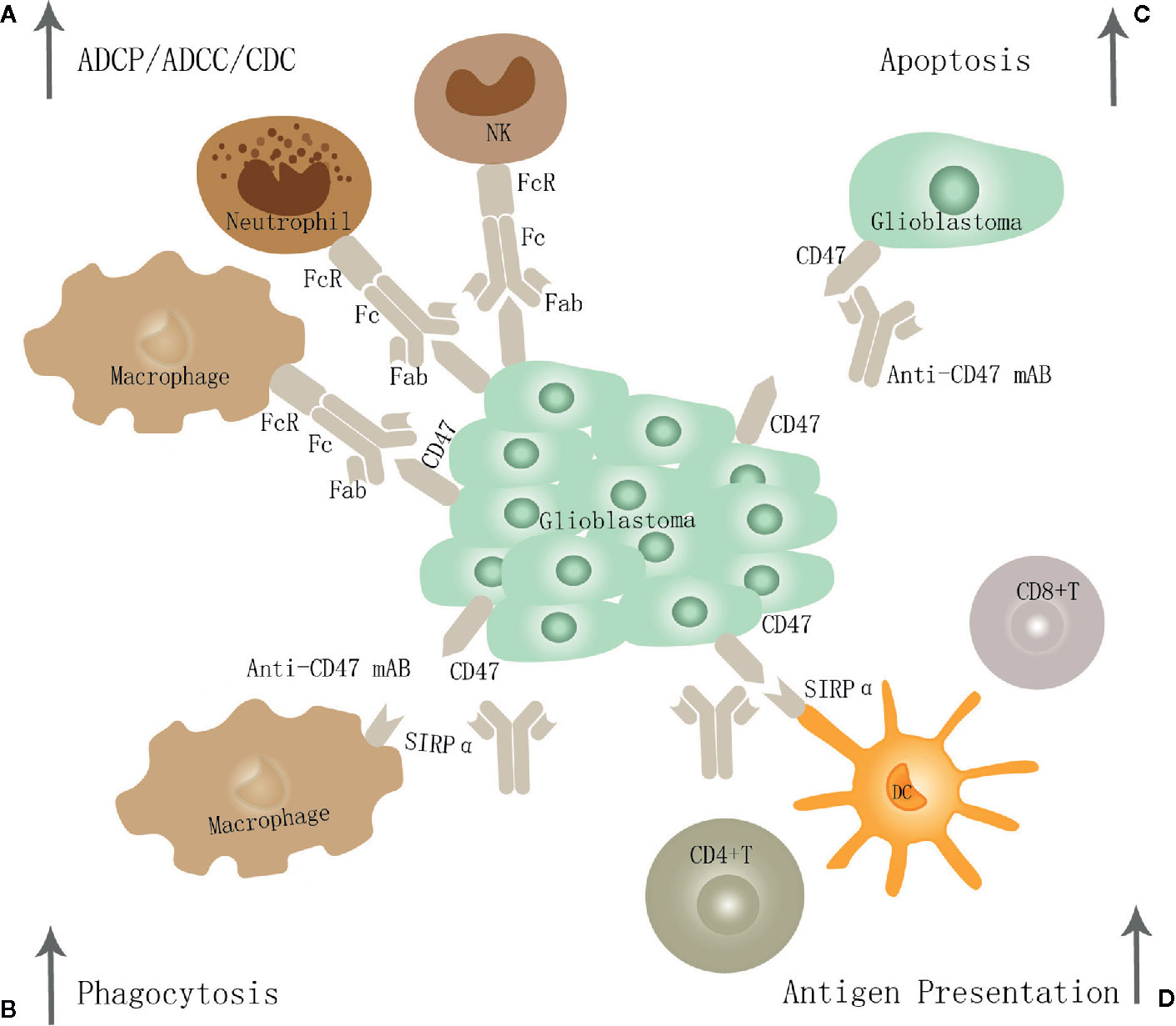
Figure 2 The potential mechanism of CD47-SIRPα inhibition in GBM. Targeting the CD47-SIRPα axis may exert anti-GBM effects through the following four pathways: (A) Eliminate GBM cells through traditional antibody Fc-dependent mechanisms, including ADCP, ADCC, and CDC. (B) it leads to enhanced tumor cell phagocytosis by macrophage through disrupting the binding of CD47 to SIRPα. (C) Promote apoptosis of GBM cells. (D) Restore dendritic cells' function to present antigen to CD4+ and CD8+T cells, thereby stimulating an anti-tumor adaptive immune response.
In the first pathway, it leads to enhanced tumor cell phagocytosis by both M1 and M2 macrophage subtypes and shifts the phenotype of macrophages toward the M1 subtype in vivo (53). And the phagocytic potential of M1 was similar to that of M2 in vitro. Phagocytosis by M1 increased in a CD47-dependent manner by the neutralizing antibody and siRNA against CD47 but not in M2 (54). In line with previous studies, Zhu et al. suggest that surgical resection combined with anti-CD47 immunotherapy was shown to promote the recruitment of macrophages and promote phagocytosis of glioblastoma (25). Li et al. come to a similar conclusion that humanized CD47 antibody HU5F9-G4 inhibits CD47 expression, enhanced tumor cell phagocytosis by macrophage, improves the survival time of animals, and has nontoxic effects on neurons and other tissues in a xenograft model derived from the malignant brain tumor (50).
In the second pathway, it enhances the antigen presentation ability of DC to generate potent T-cell priming and adaptive antitumor immune responses (32, 33). Christina et al. suggest that anti-CD47 treatment alone has limited anti-tumor effects and is inefficient in inducing changes within the tumor immune microenvironment or eradicating murine GBMs in immune-competent hosts. Instead, combined TMZ and CD47 blockade activates the cGAS-STING pathway, increases T-cell priming, and thereby activates both innate and adaptive immune responses in vivo. Hence the combination treatment is further augmented by adjuvant PD-1 blockade (33). In addition, radiotherapy was demonstrated to enhance the anti-CD47 therapeutic effects (55).
In the third pathway, glioblastoma cells may be eliminated via traditional antibody Fc-dependent mechanisms, including neutrophil cell-mediated antibody-dependent cellular cytotoxicity (ADCC) and macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) (56, 57). Recent studies have demonstrated that neutrophil ADCC toward cancer cells occurs through a mechanism called trogocytosis, which can be further improved by targeting CD47-SIRPα interactions (58). The bispecific antibodies targeting the membrane-proximal epitope of MSLN improve ADCC activity by augmenting FcγR-IIIA activation and enhanced ADCP via a more efficient blockade of the CD47/SIRPα axis (59).
In the fourth pathway, it can induce apoptosis of tumor cells directly (60). It has been shown that CD47 antibody-induced apoptosis of cancer cells is due to neither ADCC nor CDC. Instead, such antitumor activity by bivalent scFv is presumably attributable to cell death caused by the ligation of CD47 (61, 62). And tumor cells may be eliminated through direct induction of apoptosis by a novel pathway involving regulation of cAMP levels by heterotrimeric Gi with subsequent effects mediated by PKA (63, 64). However, its specific functions and mechanism in GBM require further studies.
Collectively, targeting the immune checkpoint complex CD47-SIRPα has been shown as a promising anti-tumor strategy that may remodel the GBM microenvironment, restore innate and adaptive immunity functions, and improve the prognosis of patients with GBM. Notably, these promising strategies still need considerable refinement before becoming the standard clinical treatment options for GBM.
Immunological Checkpoint Inhibitors Targeting CD47-SIRPα Axis
Currently, inhibitors targeting CD47-SIRPα immunological checkpoints are in preclinical and clinical study phases. These inhibitors include 1) monoclonal antibodies (CD47 monoclonal antibody Hu5F9-G4, human IgG4 subclass; SIRPα monoclonal antibody FSI-189), which are mainly to block the anti-phagocytosis signal and reactive macrophages to attack and destroy tumor cells (65, 66); 2) recombinant fusion proteins (TTI-621, SIRPα-Fc fusion protein, human IgG1 subclass; TTI-622, SIRPα-Fc fusion protein, human IgG4 subclass), which are composed of the N-terminal V domain of human SIRPα and the human IgG Fc region. The N-terminal V domain of human SIRPα bind human CD47 on tumor cells and prevent it from delivering inhibitory signals to macrophages. At the same time, The IgG Fc region of SIRPαFc can bind to the high-affinity receptor FcγRI (CD64) as well as to the low-affinity receptors FcγRII (CD32) and FcγRIII (CD16) on macrophages to further enhance macrophage-mediated ADCP, tumor antigen presentation, and effective anti-tumor activity. Lower affinities for normal red blood cells and reduced side effects are important advantages of recombinant fusion protein therapies (67); 3) bispecific antibodies (NI-1701, anti-CD19/anti-CD47 bispecific antibody; NI-1801, anti-CD47/mesothelin bispecific antibody); VEGFR1D2-SIRPαD1. NI-1701 has three arms. The targeting arm binds CD19, a cell-surface antigen expressed by B-cell-origin tumors. The effector’s arm destroys the CD47-mediated anti-phagocytosis signal. The Fc arm of the antibody can recruit macrophages and other innate immune killer cells. NI-1801 destroy mesothelin-positive solid tumors through the innate immune system; VEGFR1D2-SIRPαD1 consists of the second extracellular domain of VEGFR1 (VEGFR1D2) and the first extracellular domain of SIRPα (SIRPαD1), which exerted potent anti-tumor effects via suppressing VEGF-induced angiogenesis and activating macrophage-mediated phagocytosis (68–70). Among the immunological checkpoint inhibitors, Hu5F9-G4, TTI-621, and TTI-622 are undergoing Phase I clinical trials, although the complete data have not been published (71).
Safety Assessment and Future Perspectives
The main concern of CD47 inhibitors is the risk of hematological toxicity such as anemia, thrombocytopenia, and leukopenia, given the high expression of CD47 on normal red blood cells and platelets (72, 73). Preclinical studies show that CD47 inhibitors in mice are well-tolerated, with no obvious signs of toxicity (50, 74). However, Arch Oncology and Celgene discontinued a clinical trial of the CD47 inhibitors because of possible off-target effects such as anemia (75). One of the most important issues is to reduce or avoid potential toxicity while preserving anti-tumor effects.
The toxicity of anti-CD47/SIRPα antibodies appears to be Fc-dependent. It may be desirable to block the SIRPα-CD47 interaction by antibodies devoid of the Fc portion or optimize the structure of the Fc portion. Meanwhile, targeting tumor cells for FcR-mediated phagocytosis using intact antibodies (31). For example, the macrophage checkpoint inhibitor 5F9 combined with rituximab showed promising activity in patients with aggressive and indolent lymphoma, with no clinically significant toxicity (65). SIRPα expression in normal cells is much narrower than CD47 and its targeting may result in more limited toxicity, such as recombinant fusion proteins TTI-621 and ALX148 and high-affinity monomeric SIRPα with lower affinities for normal red blood cells (67, 76, 77), which is also an ideal strategy. Red blood cells act as a “sink” binding to anti-CD47 antibodies and reduce the effective therapeutic dose. Hence, optimized initiation dose and maintenance dose to achieve an effective therapeutic blockade of CD47/SIRPα Axis is pivotal. For example, a non-human primate study revealed that the effector function competent mAb IgG1 C47B222-(CHO) showed antitumor activity in vitro and in vivo while decreased red blood cells (RBC), hematocrit and hemoglobin by >40% at 1 mg/kg (78). However, toxicokinetic studies suggest that alternative treatment regimens for Hu5F9-G4 (a low initiation dose and a higher maintenance dose) may contribute to achieving therapeutic efficacy with lower toxicity (71).
Conclusions
Preclinical studies have found that targeting the immunological checkpoint complex CD47-SIRPα can inhibit the development of glioblastoma, enhance the function of phagocytic cells, restore the function of dendritic cells and T lymphocytes, and exert anti-tumor effects by improving innate and adaptive immune responses. However, there are still a series of biosafety problems such as anemia that remain to be solved. Besides, it is incompletely understood how CD47-SIRPα blockade works at the molecular level. Further understanding of the mechanism of CD47-SIRPα inhibitors will help to improve the efficacy and reduce the side effects. Ongoing clinical trials will further clarify their efficacies as single agents or in combination therapies. Careful observations of cytotoxic T cell response, T cell exhaustion, immune gene expression signatures in GBM subtypes, immune suppression (predominant immunosuppressive cells such as TAMs) may aid in identifying patients suitable for this therapy, avoiding potential toxicities and designing optimal combination therapies.
Author Contributions
HJ collected the literature and drafted the manuscript. DM modified the paper format, and WB, WX, DG, and QX guided the writing and made significant revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81472364, 81702480, 81874086).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bakas S, Akbari H, Pisapia J, Martinez-Lage M, Rozycki M, Rathore S, et al. In Vivo Detection of EGFRvIII in Glioblastoma via Perfusion Magnetic Resonance Imaging Signature Consistent with Deep Peritumoral Infiltration: The phi-Index. Clin Cancer Res (2017) 23:4724–34. doi: 10.1158/1078-0432.ccr-16-1871
2. Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev (2012) 26:756–84. doi: 10.1101/gad.187922.112
3. Maxwell R, Jackson CM, Lim M. Clinical Trials Investigating Immune Checkpoint Blockade in Glioblastoma. Curr Treat Options Oncol (2017) 18:51. doi: 10.1007/s11864-017-0492-y
4. Kamran N, Calinescu A, Candolfi M, Chandran M, Mineharu Y, Asad AS, et al. Recent advances and future of immunotherapy for glioblastoma. Expert Opin Biol Ther (2016) 16:1245–64. doi: 10.1080/14712598.2016.1212012
5. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol (2018) 15:422–42. doi: 10.1038/s41571-018-0003-5
6. Chandramohan V, Mitchell DA, Johnson LA, Sampson JH, Bigner. Antibody DD. T-cell and dendritic cell immunotherapy for malignant brain tumors. Future Oncol (2013) 9:977–90. doi: 10.2217/fon.13.47
7. Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol (2015) 17:1064–75. doi: 10.1093/neuonc/nou307
8. Han S, Ma E, Wang X, Yu C, Dong T, Zhan W, et al. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett (2016) 12:2924–9. doi: 10.3892/ol.2016.4944
9. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res (2018) 24:4175–86. doi: 10.1158/1078-0432.Ccr-17-1846
10. Schupp J, Krebs FK, Zimmer N, Trzeciak E, Schuppan D, Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol (2019) 343:103713. doi: 10.1016/j.cellimm.2017.10.013
11. Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol (2019) 16:509–20. doi: 10.1038/s41571-019-0177-5
12. Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res (2017) 77:2266–78. doi: 10.1158/0008-5472.CAN-16-2310
13. Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol (2015) 17:170–82. doi: 10.1038/ncb3090
14. Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell (2015) 27:462–72. doi: 10.1016/j.ccell.2015.02.015
15. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
16. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41:49–61. doi: 10.1016/j.immuni.2014.06.010
17. Poon CC, Sarkar S, Yong VW, Kelly JJP. Glioblastoma-associated microglia and macrophages: targets for therapies to improve prognosis. Brain (2017) 140:1548–60. doi: 10.1093/brain/aww355
18. Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-like Cells via TGF-beta 1 Signaling Pathway. J Immunol (2012) 189:444–53. doi: 10.4049/jimmunol.1103248
19. Zhu CB, Mustafa D, Zheng PP, van der Weiden M, Sacchetti A, Brandt M, et al. Activation of CECR1 in M2-like TAMs promotes paracrine stimulation-mediated glial tumor progression. Neuro-Oncology (2017) 19:648–59. doi: 10.1093/neuonc/now251
20. Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro-Oncology (2012) 14:958–78. doi: 10.1093/neuonc/nos116
21. Wang N, Liang HW, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol (2014) 5:614. doi: 10.3389/fimmu.2014.00614
22. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med (2013) 19:1264–+. doi: 10.1038/nm.3337
23. Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol (2016) 18:797–806. doi: 10.1093/neuonc/nov272
24. Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science (2016) 352:aad3018. doi: 10.1126/science.aad3018
25. Zhu HY, Leiss L, Yang N, Rygh CB, Mitra SS, Cheshier SH, et al. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget (2017) 8:12145–57. doi: 10.18632/oncotarget.14553
26. Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A (2015) 112:E6215–6223. doi: 10.1073/pnas.1520032112
27. Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature (2016) 539:437–42. doi: 10.1038/nature19834
28. Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, et al. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol (2016) 18:790–802. doi: 10.1038/ncb3371
29. Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature (2017) 543:428–32. doi: 10.1038/nature21409
30. Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep (2016) 15:2000–11. doi: 10.1016/j.celrep.2016.04.084
31. Veillette A, Chen J. SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol (2018) 39:173–84. doi: 10.1016/j.it.2017.12.005
32. Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A (2013) 110:11103–8. doi: 10.1073/pnas.1305569110
33. von Roemeling CA, Wang Y, Qie Y, Yuan H, Zhao H, Liu X, et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun (2020) 11:1508. doi: 10.1038/s41467-020-15129-8
34. Tong B, Wang MZ. CD47 is a novel potent immunotherapy target in human malignancies: current studies and future promises. Future Oncol (2018) 14:2179–88. doi: 10.2217/fon-2018-0035
35. Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol (2001) 11:130–5. doi: 10.1016/s0962-8924(00)01906-1
36. van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol (2005) 175:7781–7. doi: 10.4049/jimmunol.175.12.7781
37. Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, et al. Signal-regulatory protein alpha (SIRP alpha) but not SIRP beta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(-) hematopoietic cells. Blood (2001) 97:2741–9. doi: 10.1182/blood.V97.9.2741
38. Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRP alpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev (2017) 276:145–64. doi: 10.1111/imr.12527
39. Li F, Lv B, Liu Y, Hua T, Han J, Sun C, et al. Blocking the CD47-SIRPalpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. OncoImmunology (2018) 7:139–49. doi: 10.1080/2162402X.2017.1391973
40. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A (2012) 109:6662–7. doi: 10.1073/pnas.1121623109
41. Zhao HJ, Pan F, Shi YC, Luo X, Ren RR, Peng LH, et al. Prognostic significance of CD47 in human malignancies: a systematic review and meta-analysis. Transl Cancer Res (2018) 7:609–74. doi: 10.21037/tcr.2018.05.31
42. Ghosh D, Funk CC, Caballero J, Shah N, Rouleau K, Earls JC, et al. A Cell-Surface Membrane Protein Signature for Glioblastoma. Cell Syst (2017) 4:516–529.e517. doi: 10.1016/j.cels.2017.03.004
43. Casey SC, Tong L, Li YL, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science (2016) 352:227–31. doi: 10.1126/science.aac9935
44. Gowda P, Patrick S, Singh A, Sheikh T, Sen E. Mutant Isocitrate Dehydrogenase 1 Disrupts PKM2-beta-Catenin-BRG1 Transcriptional Network-Driven CD47 Expression. Mol Cell Biol (2018) 38:18. doi: 10.1128/mcb.00001-18
45. Lo J, Lau EYT, Ching RHH, Cheng BYL, Ma MKF, Ng IOL, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology (2015) 62:534–45. doi: 10.1002/hep.27859
46. Chang WT, Huang AM. alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. J Biol Chem (2004) 279:14542–50. doi: 10.1074/jbc.M309825200
47. Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, et al. Activation of CD47 Receptors Causes Proliferation of Human Astrocytoma but Not Normal Astrocytes via an Akt-Dependent Pathway. Glia (2011) 59:308–19. doi: 10.1002/glia.21102
48. Boukhari A, Alhosin M, Bronner C, Sagini K, Truchot C, Sick E, et al. CD47 Activation-induced UHRF1 Over-expression Is Associated with Silencing of Tumor Suppressor Gene p16(INK4A) in Glioblastoma Cells. Anticancer Res (2015) 35:149–57. doi: 10.1002/glia.21102
49. Liu XJ, Wu X, Wang YM, Li YH, Chen XL, Yang WC, et al. CD47 Promotes Human Glioblastoma Invasion Through Activation of the PI3K/Akt Pathway. Oncol Res (2019) 27:415–22. doi: 10.3727/096504018x15155538502359
50. Li F, Lv BK, Liu Y, Hua T, Han JB, Sun CM, et al. Blocking the CD47-SIRP alpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology (2018) 7:12. doi: 10.1080/2162402x.2017.1391973
51. Huang YT, Ma YC, Gao P, Yao Z. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis (2017) 9:E168–74. doi: 10.21037/jtd.2017.02.30
52. Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, et al. Microglia are effector cells of CD47-SIRP alpha antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A (2019) 116:997–1006. doi: 10.1073/pnas.1721434116
53. Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS One (2016) 11:21. doi: 10.1371/journal.pone.0153550
54. Sakakura K, Takahashi H, Kaira K, Toyoda M, Murata T, Ohnishi H, et al. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab Invest (2016) 96:994–1003. doi: 10.1038/labinvest.2016.70
55. Gholamin S, Youssef OA, Rafat M, Esparza R, Kahn S, Shahin M, et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun (2020) 26:130–7. doi: 10.1177/1753425919876690
56. Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A (2016) 113:E2646–2654. doi: 10.1073/pnas.1604268113
57. McCracken MN, Cha AC, Weissman IL. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 “Don’t Eat Me” Signals. Clin Cancer Res (2015) 21:3597–601. doi: 10.1158/1078-0432.ccr-14-2520
58. Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep (2018) 23:3946–59.e3946. doi: 10.1016/j.celrep.2018.05.082
59. Hatterer E, Chauchet X, Richard F, Barba L, Moine V, Chatel L, et al. Targeting a membrane-proximal epitope on mesothelin increases the tumoricidal activity of a bispecific antibody blocking CD47 on mesothelin-positive tumors. mAbs (2020) 12:1739408. doi: 10.1080/19420862.2020.1739408
60. Chao MP, Weissman IL, Majeti R. The CD47-SIRP alpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol (2012) 24:225–32. doi: 10.1016/j.coi.2012.01.010
61. Kikuchi Y, Uno S, Kinoshita Y, Yoshimura Y, Iida S, Wakahara Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res (2005) 29:445–50. doi: 10.1016/j.leukres.2004.09.005
62. Kikuchi Y, Uno S, Yoshimura Y, Otabe K, Iida S, Oheda M, et al. A bivalent single-chain Fv fragment against CD47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun (2004) 315:912–8. doi: 10.1016/j.bbrc.2004.01.128
63. Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res (2004) 64:1026–36. doi: 10.1158/0008-5472.can-03-1708
64. Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol (2003) 170:3544–53. doi: 10.4049/jimmunol.170.7.3544
65. Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med (2018) 379:1711–21. doi: 10.1056/NEJMoa1807315
66. Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. Anti-SIRP alpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A (2017) 114:E10578–85. doi: 10.1073/pnas.1710877114
67. Petrova PS, Viller NN, Wong M, Pang XL, Lin GHY, Dodge K, et al. TTI-621 (SIRP alpha Fc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin Cancer Res (2017) 23:1068–79. doi: 10.1158/1078-0432.ccr-16-1700
68. Hatterer E, Barba L, Noraz N, Daubeuf B, Aubry-Lachainaye JP, von der Weid B, et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. Mabs (2019) 11:322–34. doi: 10.1080/19420862.2018.1558698
69. Dheilly E, Majocchi S, Moine V, Didelot G, Broyer L, Calloud S, et al. Tumor-Directed Blockade of CD47 with Bispecific Antibodies Induces Adaptive Antitumor Immunity. Antibodies (2018) 7:15. doi: 10.3390/antib7010003
70. Zhang XY, Wang SF, Nan YY, Fan JJ, Chen W, Luan JY, et al. Inhibition of autophagy potentiated the anti-tumor effects of VEGF and CD47 bispecific therapy in glioblastoma. Appl Microbiol Biotechnol (2018) 102:6503–13. doi: 10.1007/s00253-018-9069-3
71. Liu J, Wang LJ, Zhao FF, Tseng S, Narayanan C, Shura L, et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One (2015) 10:23. doi: 10.1371/journal.pone.0137345
72. Catani L, Sollazzo D, Ricci F, Polverelli N, Palandri F, Baccarani M, et al. The CD47 pathway is deregulated in human immune thrombocytopenia. Exp Hematol (2011) 39:486–94. doi: 10.1016/j.exphem.2010.12.011
73. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science (2000) 288:2051–62. doi: 10.1126/science.288.5473.2051
74. Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, et al. Disrupting the CD47-SIRP alpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med (2017) 9:13. doi: 10.1126/scitranslmed.aaf2968
75. Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front Immunol (2020) 11:18. doi: 10.3389/fimmu.2020.00018
76. Kauder SE, Kuo TC, Chen A, Harrabi O, Rocha SS, Doyle L, et al. ALX148 Is a High Affinity Sirp alpha Fusion Protein That Blocks CD47, Enhances the Activity of Anti-Cancer Antibodies and Checkpoint Inhibitors, and Has a Favorable Safety Profile in Preclinical Models. Blood (2017) 9:13. doi: 10.1126/scitranslmed.aaf2968
77. Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science (2013) 341:88–91. doi: 10.1126/science.1238856
Keywords: glioblastoma, immune checkpoint, CD47-SIRPα, tumor-associated macrophages/microglia, glioblastoma microenvironment
Citation: Hu J, Xiao Q, Dong M, Guo D, Wu X and Wang B (2020) Glioblastoma Immunotherapy Targeting the Innate Immune Checkpoint CD47-SIRPα Axis. Front. Immunol. 11:593219. doi: 10.3389/fimmu.2020.593219
Received: 10 August 2020; Accepted: 02 November 2020;
Published: 27 November 2020.
Edited by:
Jian Wang, University of Bergen, NorwayCopyright © 2020 Hu, Xiao, Dong, Guo, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Wu, wuxudong@tmu.edu.cn; Baofeng Wang, wbf620@163.com
 Jinyang Hu
Jinyang Hu Qungen Xiao
Qungen Xiao Minhai Dong1
Minhai Dong1 Dongsheng Guo
Dongsheng Guo